Introduction
Ovarian cancer is one of the most lethal
malignancies among all gynecological tumors and results in the
second highest number of cancer-related deaths among women
worldwide (1). Ovarian cancer is a
group of heterogeneous tumors, which comprise several histological
subtypes: serous, mucinous, endometrioid, clear cell and
undifferentiated carcinomas. High-grade serous carcinoma accounts
for ~80% of epithelial ovarian carcinomas and has the highest
recurrence rate. Due to advances in surgical management and
adjuvant chemotherapy, the survival rate of ovarian cancer patients
has been markedly increased, but ovarian cancer still remains the
most lethal cancer in gynecology with a 5-year overall survival of
only 25–35% and causes more than 15,000 deaths/year (2–4), which
is the focus of ovarian cancer study for decades (5). The high mortality of ovarian cancer is
partly due to clinical silence and high recurrence. Most patients
are diagnosed at an advanced stage and the majority of patients, up
to 75%, eventually suffer recurrence, although they receive
debulking surgery and standard chemotherapy (6). In conclusion, effective, predictive or
prognostic biomarkers are urgently needed for early diagnosis and
survival time prolongation.
In the human, fibroblast growth factors (FGFs) are
heparin-binding proteins consisting of a family of 18 members,
which are essential for signal transduction through interacting
with cell surface-associated heparan sulfate proteoglycans
(7,8). FGFs are involved in many cellular
processes such as proliferation, differentiation and angiogenesis,
and their ectopic overexpression has been discovered in many types
of cancers. FGFs function as signaling inducers by interacting with
FGFRs. Fibroblast growth factor receptors (FGFRs) comprise five
members, including four tyrosine kinase receptors (FGFR1, FGFR2,
FGFR3 and FGFR4) and a non-tyrosine kinase receptor (FGFR5, also
known as FGFRL1) (9). FGFR4 is
demonstrated to bind with FGF1, FGF2, FGF4, FGF6, FGF8, FGF9,
FGF16, FGF17, FGF18, FGF19, FGF21 and FGF23, but FGF19 and FGF21
have the highest affinity and specificity to FGFR4. Among the FGFR
family, FGFR4 is distinguished in ovarian cancer because previous
studies have determined that it is a prognostic biomarker and
potential therapeutic target for ovarian cancer (10). However, the underlying mechanism of
how FGFR4 leads to an unfavorable prognosis and how FGFR4 is
stimulated and triggers the downstream signaling network has not
been well elucidated.
In the present study, we hypothesized that FGFR4
functions in ovarian cancers as part of a signaling network in the
cancer microenvironment and thus we detected the expression of
FGFR4-specific ligand, FGF19, in advanced-stage serous ovarian
cancer with immunohistochemistry (IHC). Furthermore, we evaluated
the prognostic value of FGF19 with univariate and multivariate
analyses. Using experiments in vitro, we demonstrated that
FGF19 can be secreted and promotes ovarian cancer progression such
as proliferation and invasion by activating FGFR4, indicating that
the FGF19-FGFR4 paracrine pathway can be considered as a potential
drug target in ovarian cancer therapy.
Materials and methods
Patients and follow-up
A total of 134 patients were diagnosed with
advanced-stage serous ovarian cancer by routine pathology from 2002
to 2012 at Linyi Hospital and Yishui Central Hospital, which was
the primary cohort. Advanced stage was identified as stage IIIB-IV
in the International Federation of Gynecology and Obstetrics stage
system according to a previous study (10). The validation cohort consisting of
74 samples was selected from the primary cohort with criteria as
available tissue samples and follow-up of more than 3 months.
Samples (tissues and blood samples) were obtained from the
Pathology Department with prior consent of the patients or their
families and by approval of the Institutional Clinical Ethics
Review Board. The diagnoses of the validation cohort were confirmed
by two senior pathologists.
Additionally, 15 pairs of fresh frozen tumor tissues
and the corresponding adjacent non-tumor tissues were collected
immediately after surgery with prior consent of the patients and
preserved in liquid nitrogen. Real-time polymerase chain reaction
(PCR) with primers of FGF19 and FGFR4 was used to detect the mRNA
levels in the tumor and non-tumor tissues.
Cell culture and agents
Ovarian cancer cell lines SK-OV-3, HO8910, HO8910PM
and OVCAR3, hepatocellular carcinoma cell line HepG2, breast cancer
cell line MCF-7 and colorectal cell line SW480 were purchased from
the Chinese Academy of Sciences Cell Bank (Shanghai, China). The
cell line SK-OV-3 was cultured in McCoy's 5A medium (Sigma-Aldrich)
and HO8910, HO8910PM and OVCAR3 cells were cultured in RPMI-1640
medium. HepG2, MCF-7 and SW480 were cultured in Dulbecco's modified
Eagle's medium (DMEM), all of which were supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin, and cultured under 5% CO2 in a humidified
incubator at 37°C. Recombinant human FGF19 (RFGF19) protein was
purchased from Sino Biological, Inc. (Beijing China). Antibodies
used in our experiments were as follows: mouse monoclonal
anti-FGF19 antibody was from R&D Systems (Minneapolis, MN,
USA), anti-FGFR4 (Phospho-Tyr642) antibody was from Signalway
Antibody LLC. (College Park, MD, USA), anti-β-actin antibody was
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
remaining antibodies were all from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
IHC and evaluation
IHC was carried out by the streptavidin peroxidase
complex method. Followed by deparaffinization with xylene and
graded alcohol, endogenous peroxidase was inactivated by incubation
in 3% hydrogen peroxide for 10 min. After nonspecific blocking with
5% bovine serum albumin, the slides were incubated in the primary
antibody at a dilution of 1:100 at 4°C overnight. After the primary
antibody, samples were incubated with the corresponding secondary
antibody at 37°C for 1–2 h and then in 3,3′-diaminobenzidine (DAB)
solution until satisfactory staining was shown. Every slide was
evaluated by two senior pathologists unaware of the clinical
information. Cases without concurrence were re-evaluated by a third
pathologist.
The score of the IHC staining was based on
multiplication of the staining intensity and the positive cell
percentage. The staining intensity was scored as negative (0), weak
(1), moderate (2) and strong (3), and scores of the stained area were
defined as follows: 0, <10%; 1, 10–30%; 2, >30–50%; and 3,
>50% positive cells. The cut-off was arbitrarily defined as a
score ≥4 was considered as high FGF19 or FGFR4 expression; a score
<4 was considered as low FGF19 or FGFR4 expression.
Western blotting
Expression of FGF19 and FGFR4 was detected by
western blotting. Briefly, the cells were lysed in modified RIPA
buffer (Tris 50 mM, NaCl 150 mM, Triton X-100 1%, SDS 0.1%,
deoxycholate 0.5%, sodium orthovanadate 2 mM, sodium pyrophosphate
1 mM, NaF 50 mM, EDTA 5 mM, PMSF 1 mM and 1X protease inhibitor
cocktail) on ice for 10 min for complete lysis, and then
centrifuged at 10,000 × g for 30 min. Protein concentration of the
supernatant was measured by Bradford assay kit (Sangon Biotech Co.,
Ltd., Shanghai, China). An amount of 40 µg of the extracted
protein from each sample was used for electrophoresis in 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
then transferred to an NC membrane (Pall Corporation). The membrane
was incubated in 5% defatted milk for 1 h, in diluted primary
antibody overnight and in horseradish peroxidase-labeled secondary
antibody for 2 h in order. Proteins were finally visualized using
enhanced chemiluminescence western blotting detection reagents
(Thermo Fisher Scientific, Waltham, MA, USA).
Quantitative real-time polymerase chain
reaction (qRT-PCR)
RT-PCR was used to detect the mRNA levels of FGF19
and FGFR4. Primers were designed and purchased from Sigma-Aldrich
as follows: FGF19 forward, 5′-GCACAGTTTGCTGGAGATCA-3′ and reverse,
5′-ATCTCCTCCTCGAAAGCACA-3′; FGFR4 forward,
5′-AGCACCCTACTGGACACACC-3′ and reverse, 5′-ACGCTCTCCATCACGAGACT-3′.
Briefly, total RNA of ovarian cancer cells was first extracted from
the cell lines with the Rneasy kit (Qiagen) and the concentration
of total RNA was tested. Then qRT-PCR was achieved and relative
gene expression was measured by the StepOnePlus Real-Time PCR
system (Applied Biosystems) using the SYBR-Green method according
to the manual. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was
applied as an internal control.
Small interfering RNA and
transfection
Knockdown of FGF19 or FGFR4 was accomplished by
small interfering RNA (siRNA) purchased from Invitrogen (Carlsbad,
CA, USA). The siRNA sequences of FGF19 and FGFR4 were obtained from
Santa Cruz biotechnology, Inc. Transfection of siRNA was realized
with Lipofectamine 2000 (Invitrogen) according to the user guide.
Results of the knockdown were validated by qRT-PCR and western
blotting.
Cell proliferation assays
Cell proliferation was assessed by the MTT kit
(Sangon Biotech Co., Ltd.). Briefly, an equal number of cells was
planted into a 96-well plate and then starved for 6 h before
stimulation. After stimulation with recombinant FGF19 or
conditioned medium for the expected time, the proliferation was
terminated by addition of 10 mg/ml MTT and then incubated at 37°C
for 4–6 h. After removing the superior carefully, the crystals at
the bottom were resolved by 100 µl DMSO and the absorbance
was read at 490 nm. The optical density (OD) at 490 nm was set as
the baseline, and the proliferation index of the other groups was
calculated by the OD490 ratio to the baseline. Every group had at
least eight parallel wells, and analyzed data were from at least
three independent experiments.
Matrigel invasion assays
Matrigel invasion assays were performed to evaluate
the effect of FGF19 on cell invasion using 8-µm pore size BD
BioCoat Matrigel invasion chambers (Becton-Dickinson, Franklin
Lakes, NJ, USA). After being planted into the upper chambers and
starved for 6 h, the cells were incubated in 10 ng/ml recombinant
FGF19 protein of conditioned medium for 24 h and then fixed and
stained with Diff-Quik stain. Non-invading cells in the upper
chambers were wiped off while the cells in the lower chamber were
counted using at least 8 random fields. The invaded number of cells
without stimulation was counted and set as the baseline and the
invasion index of the other groups was calculated as the ratio to
the baseline. Analyzed data were from at least three independent
experiments.
Enzyme-linked immunosorbent assay (ELISA)
and conditioned medium
Serum FGF19 levels were measured with sandwich ELISA
by the FGF19 Quantikine ELISA kit (R&D Systems), following the
manufacturer's instructions and previous studies (11,12).
FGF19 concentration in the cell medium was tested after treatment
with 50 µM chenodeoxycholic acid (CDCA) for 48 h, and was
then detected by an ELISA kit according to previous studies
(13,14). All samples from the cell culture
media or serum were detected in duplicate.
To obtain efficient conditioned medium (CM), OVCAR3
cells were cultured in normal medium with 10% FBS for the expected
time. Then CM was collected and filtered with a low protein-binding
filter (0.22 µm) (Millipore, Billerica, MA, USA). Amicon
Ultra 15 ml filters were used to concentrate the CM at 4,000 g when
necessary. The same procedures were performed using SK-OV-3 cells
as a control group. The CM was used immediately, stored at 4°C for
a week or frozen at −80°C.
Statistical analysis
All data were analyzed by SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Correlations between FGF19 and
clinicopathological features were analyzed by χ2 test,
and correlations between FGF19, FGFR4 and overall survival rates
were calculated by the Kaplan-Meier method, and the difference
between the high-expression and low-expression group was analyzed
by log-rank test. In experiments in vitro, differences
between the control group and tested group were analyzed by the
Student's unpaired t-test. P<0.05 was considered to indicate a
statistically significant result.
Results
Expression of FGF19 and FGFR4 in ovarian
cancer
Expression of FGF19, the known specific ligand of
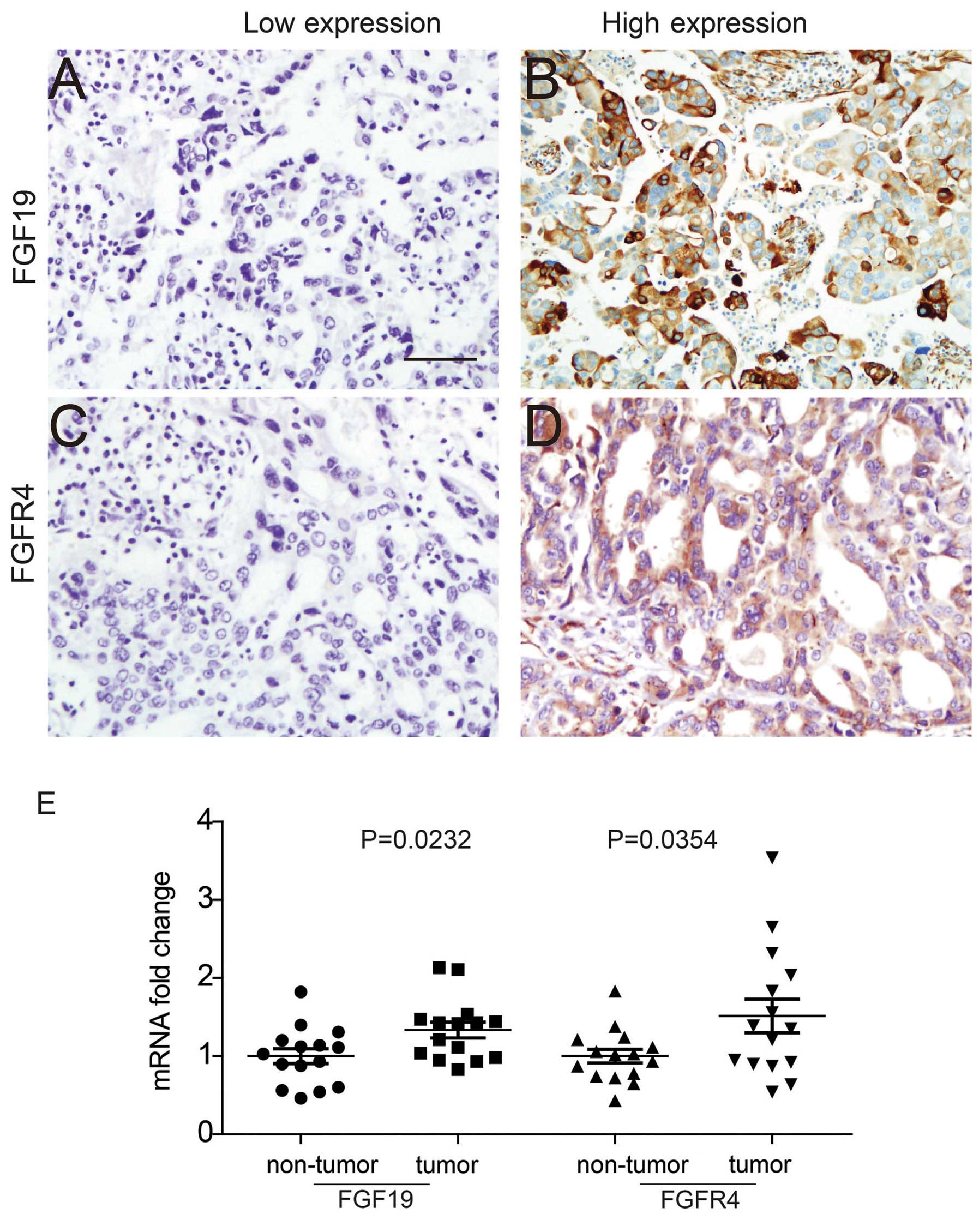
FGFR4, was first detected in ovarian cancer samples with IHC. FGF19
expression was mainly observed in the cytoplasm, where it may be
secreted out and function as a growth factor (Fig. 1A and B). FGFR4 expression was
observed in both the membrane and cytoplasm (Fig. 1C and D). According to the criteria
described in Materials and methods, expression of FGF19 and FGFR4
was divided into a high expression and low expression group. The
percentage of samples with high-FGF19 and high-FGFR4 was 41.89 and
39.19%, respectively (Table I).
Moreover, to evaluate the significance of FGF19 and FGFR4
co-expression, we further defined FGF19-FGFR4 double high
expression as cases having high expression for both FGF19 and FGFR4
expression, with the rest of the cases defined as the control
group. The percentage of cases with FGF19-FGFR4 double high
expression was 21.62% in our cohort.
 | Table ICharacteristics of the patients with
advanced-stage serous ovarian cancer. |
Table I
Characteristics of the patients with
advanced-stage serous ovarian cancer.
| Characteristics | No. of patients | % |
|---|
| Age (years) | | |
| <50 | 17 | 22.97 |
| ≥50 | 57 | 77.03 |
| Lymph node
metastasis | | |
| No | 37 | 50.00 |
| Yes | 37 | 50.00 |
| Histological
grade | | |
| I | 11 | 14.86 |
| II | 24 | 32.43 |
| III | 39 | 52.70 |
| FGF19 | | |
| Low | 43 | 58.11 |
| High | 31 | 41.89 |
| FGFR4 | | |
| Low | 45 | 60.81 |
| High | 29 | 39.19 |
| FGF19+FGFR4 | | |
| Low | 58 | 78.38 |
| High | 16 | 21.62 |
Moreover, FGF19 and FGFR4 mRNA expression levels in
tumor tissues and adjacent non-tumor tissues were detected and
compared (Fig. 1E). The mRNA levels
of FGF19 and FGFR4 in tumor tissues were demonstrated to be much
higher than levels in the non-tumor tissues.
Clinical significance of FGF19
The correlation between FGF19 and clinicopathologic
factors and the 5-year overall survival rates were analyzed to
evaluate the clinical significance of FGF19 in ovarian cancer.
Based on the χ2 test, we demonstrated that high
expression of FGF19 was significantly associated with positive
lymph node metastasis (P=0.033), indicating that FGF19 may play an
important role in tumor invasion and metastasis. High FGF19
expression tended to be correlated with high FGFR4 expression, but
the tendency was not statistically significant (P=0.063) (Table II).
 | Table IICorrelation between FGF19 and
clinicopathological features. |
Table II
Correlation between FGF19 and
clinicopathological features.
|
Characteristics | FGF19
| P-valuea |
|---|
| Low | High |
|---|
| Age (years) | | | |
| <50 | 10 | 7 | 0.946 |
| ≥50 | 33 | 24 | |
| Lymph node
metastasis | | | |
| No | 26 | 11 | 0.033 |
| Yes | 17 | 20 | |
| Histological
grade | | | |
| I | 8 | 3 | 0.122 |
| II | 10 | 14 | |
| III | 25 | 14 | |
| FGFR4 | | | |
| Low | 30 | 15 | 0.063 |
| High | 13 | 16 | |
Kaplan-Meier method was performed to further
evaluate the prognostic value of detected clinicopathological
parameters (Table III). High
FGFR4 expression was demonstrated to be significantly associated
with a poorer overall survival rate (P= 0.018), which was
consistent with a previous study (Fig.
2A) (10). In addition, we
first proved that high FGF19 expression was closely correlated with
unfavorable prognosis (P=0.033), suggesting that FGF19 could be
considered as a prognostic biomarker and potential drug target
(Fig. 2b). Notably, double high
expression of FGF19 and FGFR4 was a better biomarker and predicted
prognosis more reliably than FGF19 or FGFR4 alone (P<0.001)
(Fig. 2C). FGF19-FGFR4 double high
expression can predict poorer prognosis more accurately and
sensitively than FGF19 or FGFR4 alone, indicating the promising and
potential clinical significance of considering FGF19-FGFR4 double
positivity as a biomarker. Moreover, the association between
FGF19-FGFR4 and poor prognosis indicated that the FGF19-FGFR4
signaling pathway may promote cancer progression by a
self-sufficient autocrine or paracrine pathway.
 | Table IIICorrelation between
clinicopathological features and the overall survival rate. |
Table III
Correlation between
clinicopathological features and the overall survival rate.
|
Characteristics | 5-year survival
rate (%) | P-valuea |
|---|
| Age (years) | | |
| <50 | 39 | 0.967 |
| ≥50 | 31.7 | |
| Lymph node
metastasis | | |
| No | 54.3 | 0.002 |
| Yes | 7.8 | |
| Histological
grade | | |
| I | 38.1 | 0.401 |
| II | 37 | |
| III | 31.4 | |
| FGF19 | | |
| Low | 41.6 | 0.033 |
| High | 20.6 | |
| FGFR4 | | |
| Low | 41.8 | 0.018 |
| High | 0 | |
| FGF19+FGFR4 | | |
| Low | 37.9 | <0.001 |
| High | 13.4 | |
FGF19 and FGFR4 in ovarian cancer cells,
medium and blood
Using experiments in vitro, we performed a
series of functional assays to explore the mechanism explaining why
high FGF19 and FGFR4 expression imply a much poorer prognosis.
Expression levels of FGF19 and FGFR4 in ovarian cancers were first
detected by western blotting. As shown in Fig. 3A, FGF19 and FGFR4 were widely
expressed in ovarian cancer cell lines. SK-OV-3 had the lowest
FGFR4 expression and HO8910 had the lowest FGF19 expression. OVCAR3
had relatively high FGF19 and FGFR4 expression, suggesting OVCAR3
as a suitable model for a knockdown assay. To evaluate the role of
FGF19 and FGFR4 in ovarian cancer progression, we regulated FGF19
and FGFR4 expression in OVCAR3 cells by siRNA transfection and
validated the successful knockdown of FGF19 (Fig. 3B and C) and FGFR4 (Fig. 3D and E) by qRT-PCR and western
blotting. As a secreted growth factor, FGF19 was suspected to be
secreted out from ovarian cancer cells. Thus medium of the SK-OV-3
and OVCAR3 cells, which had the lowest and highest FGF19 expression
respectively, was used to detect the FGF19 concentration. After
treatment with 50 µM CDCA for 72 h, FGF19 in both the
SK-OV-3 and OVCAR3 medium was detectable (Fig. 3F). The FGF19 concentration in the
OVCAR3 medium was markedly higher than that in the SK-OV-3 medium,
which was consistent with the intracellular FGF19 content. After
FGF19 was knocked down by siRNA, the concentration of FGF19 in the
medium was significantly decreased compared to the control group,
suggesting that its concentration in medium was directly related to
the intracellular content. Based on the finding that the FGF19 is
associated with prognosis and is secreted out from ovarian cancer,
we hypothesized that FGF19 concentration in blood may predict
earlier ovarian cancer progression and poorer prognosis. Thus, we
detected the FGF19 concentration in 12 healthy individuals and 12
pre-operational patients with advanced-stage ovarian cancer by
ELISA method. However, the FGF19 concentration between the healthy
individuals and the ovarian cancer patients had no significant
difference (P=0.492) (Fig. 3G).
Additionally, we detected the expression and
function of FGF19 and FGFR4 in control cell lines MCF7, SW480 and
HepG2 to determine the exclusive effect of FGF19 on FGFR4. HepG2
had the highest FGFR4 expression and SW480 exhibited the most
abundant FGF19 expression (Fig.
4A). Under 10 ng/ml recombinant FGF19 stimulation for 48 h,
these three cell lines exhibited different proliferative rates
(HepG2 had the highest and MCF7 had the lowest rate) (Fig. 4b). Moreover, this variation to FGF19
stimulation faded away after FGFR4 was knocked down in these cell
lines (Fig. 4C), indicating that
FGF19 accelerated the proliferation dependent on FGFR4
existence.
FGF19 promotes ovarian cancer progression
in a paracrine manner
To explore the role of FGF19 in ovarian cancer
progression, we further examined the effect of exogenous FGF19 on
the AKT-MAPK signaling pathway in OVCAR3 cells. Proteins in the
AKT-MAPK signaling pathway were detected after 10 ng/ml RFGF19
stimulation for 0–30 min (Fig. 5A).
The phosphorylation level of FGFR4, AKT, Erk and p38 increased
notably along with the FGF19 stimulation, indicating that FGF19
promoted FGFR4 phosphorylation and activated the AKT-MAPK signaling
pathway. Although considered as a specific ligand of FGFR4, FGF19
was also proven to interact with FGFR1, FGFR2 and FGFR3 in the
presence of β-klotho (15–17). To evaluate whether FGFR4 was
essential in the FGF19 activation process, FGFR4 was knocked down
in OVCAR3 cells and the AKT-MAPK signaling pathway with recombinant
FGF19 stimulation was subsequently detected (Fig. 5b). After FGFR4 knockdown, the
phosphorylation levels of AKT, Erk and p38 were significantly
reduced due to the decrease in FGFR4 phosphorylation, which
demonstrated that FGFR4 was essentially required in the
FGF19-induced AKT-MAPK activation.
To explore FGF19-FGFR4 signaling in tumor
progression, 10 ng/ml FGF19 was used to stimulate OVCAR3 cells for
0–72 h after FGFR4 knockdown, and MTT assay was performed to
evaluate cell proliferation (Fig.
5C). Cells with FGF19 stimulation had an obviously higher
proliferation rate than the control group, and FGFR4 knockdown
reversed this FGF19-induced acceleration of proliferation,
indicating that FGF19 promoted ovarian cancer cell proliferation by
activating FGFR4. To estimate FGF19 influence on invasion, OVCAR3
cells were cultured in 10 ng/ml RFGF19 for 24 h, at which time the
proliferation did not differ, and then the Matrigel assay was
carried out to detect cell invasion (Fig. 5D). In our experiment, FGF19 markedly
promoted cell invasion and FGFR4 knockdown impaired this tendency,
which confirmed that FGFR4 was also required in the FGF19-induced
invasion. Based on our findings that FGF19 was secreted by ovarian
cancer cells, we suspected that the FGF19-FGFR4 signaling pathway
may be stimulated ectopically by an autocrine or paracrine pathway.
Thus, we used the conditioned medium of OVCAR3, the cell line with
high FGF19 expression, to stimulate SK-OV-3 cells to confirm our
hypothesis. In our experiments, the conditioned medium of OVCAR3
cells notably accelerated the proliferation of SK-OV-3 cells at 72
h and the invasion of SK-OV-3 at 24 h (Fig. 5E and F), demonstrating that the
FGF19 concentration in the ovarian cancer microenvironment was high
enough to activate FGFR4 and downstream signaling pathways.
Discussion
In our experiments, we detected the expression of
FGF19, the FGFR4 specific ligand, in advanced-stage ovarian cancer
tissues, and systemically evaluated the correlation between FGF19
and clinicopathological factors and the overall survival for the
first time according to our knowledge. Moreover, we performed a
series of experiments in vitro to estimate the role of the
FGF19-FGFR4 signaling pathway in ovarian cancer progression.
Consequently, we found that FGF19 expression was significantly
associated with lymphatic metastasis (P=0.033). FGF19 and FGFR4
expression could predict poorer prognosis (P=0.033 and 0.018,
respectively), and FGF19-FGFR4 double high expression was a more
accurate and sensitive prognostic factor in ovarian cancer
(P<0.001). With experiments in vitro, we found that FGF19
existed in ovarian cancer cells and was secreted out. In addition,
FGF19 stimulated the AKT-MAPK signaling pathway via activating
FGFR4, by which FGF19 accelerated proliferation and invasion.
Moreover, we proved that the FGF19 concentration in cell culture
medium was high enough to promote cell proliferation and invasion,
indicating that the FGF19-FGFR4 signaling pathway may be activated
ectopically by an autocrine or paracrine pathway.
FGFR4 overexpression has been previously proven to
be associated with progression and prognosis in many types of
cancers, including gastric cancer, hepatocellular carcinoma,
cholangiocarcinoma, colorectal and oropharynx cancer (18–22).
As the known specific ligand to FGFR4, FGF19 was also demonstrated
to be involved in progression of cancers such as hepatocellular
carcinoma, prostate cancer (12,18,22,23)
and colon cancer. The amplification of the FGFR4 gene was
discovered in gynecological cancers by Jaakkola et al in
1993 (24). A previous study
pointed out that FGFR4 could predict prognosis and may be a
potential therapeutic target in high-advanced ovarian cancer
(10). However, the FGFR4 ligand
was not detected and the underlying molecular mechanism was not
well elucidated, which prompted us to explore the correlation
between FGF19, FGFR4, progression and prognosis in ovarian cancer.
Moreover, FGFR4 is widely expressed in many types of tumors and we
demonstrated that not only ovarian cancer cell lines but also other
cell lines exhibited a different response to FGF19-induced
proliferation, which required the participation of FGFR4. Thus,
FGF19-induced proliferation may be a general phenomenon regarding
all cell lines with abundant FGFR4, indicating that the FGF19-FGFR4
signaling pathway may be a potential molecular target for ovarian
cancer as well as other tumors with FGFR4 overexpression.
Among the human FGF factors, FGF19 is distinguished
by its function as a hormone, regulating bile acid synthesis, with
effects on glucose and lipid metabolism (25,26).
Interestingly, ovarian cancer risk is believed to be positively
correlated with glucose and lipid metabolism disorders such as
obesity and diabetes (27,28). Recent evidence indicates that
obesity, diabetes and ovarian cancer are more intricately related
(29,30). Previous studies reported that diet
and diabetes are associated with mortality and the prognosis of
ovarian cancer (28,31). Based on our new findings, we boldly
hypothesize that FGF19 ectopic expression and function may be one
explanation why obesity and diabetes are related to high-risk or
even a poorer prognosis of ovarian cancer. In our study, the FGF19
concentration of blood samples was detected but no significant
difference between the patients and healthy individuals was
observed. We believe that more cases should be enrolled and the
cohort should be further divided into subgroups according to
suspicious parameters such as diabetes and BMI to detect the role
of FGF19 in the correlation between ovarian cancer and lipid
metabolism.
The FGFR family has five members and can interact
with several FGFs with different affinity, which may trigger
different downstream signaling pathways and regulate different
cellular biological processes (32,33).
Moreover, the FGFR family is also proven to undergo crosstalk with
other receptors such as the epidermal growth factor receptor
family, which may increase the complexity of the FGFR signaling
network exponentially (34). Even
in ovarian cancer, the FGF19-FGFR4 signaling pathway is associated
with obvious conundrums to solve, such as the difference in FGF19
secretion between biological and pathological patterns, and how
FGF19 interacts with FGFR4 and which downstream pathways are
dominantly triggered. These questions require further fundamental
experiments, especially using animal models. Moreover, inhibitors
mimicking the FGF19 structure are potential molecular drugs
targeting FGFR4 in ovarian cancer based on our finding that FGF19
is also a prognostic marker. We hope our findings stimulate more
research on the function of FGF19 in ovarian cancer, which may
further help identify effective therapeutic drugs and increase the
survival of patients with ovarian cancer.
In conclusion, we demonstrated that both FGF19 and
FGFR4 overexpression predict an unfavorable prognosis in ovarian
cancer, while FGF19-FGFR4 double high expression is a better and
more sensitive prognostic biomarker. Moreover, either recombinant
FGF19 or FGF19 secreted by ovarian cancer cells promotes
proliferation and invasion by FGFR4 activation and the subsequent
AKT-MAPK signaling pathway. This indicates that FGF19-FGFR4
signaling may be auto-activated in a paracrine or autocrine manner
and that FGF19 could be a potential drug target for ovarian
cancer.
References
|
1
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics, 2002. CA Cancer J Clin. 52:23–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colombo PE, Fabbro M, Theillet C, Bibeau
F, Rouanet P and Ray-Coquard I: Sensitivity and resistance to
treatment in the primary management of epithelial ovarian cancer.
Crit Rev Oncol Hematol. 89:207–216. 2014. View Article : Google Scholar
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seltzer V; NIH Consensus Development Panel
on Ovarian Cancer: NIH consensus conference. Ovarian cancer.
Screening, treatment, and follow-up. JAMA. 273:491–497. 1995.
View Article : Google Scholar
|
|
5
|
Kang KW, Lee MJ, Song JA, Jeong JY, Kim
YK, Lee C, Kim TH, Kwak Kb, Kim OJ and An HJ: Overexpression of
goosecoid homeobox is associated with chemoresistance and poor
prognosis in ovarian carcinoma. Oncol Rep. 32:189–198.
2014.PubMed/NCBI
|
|
6
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner N and Grose R: Fibroblast growth
factor signalling: From development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eswarakumar VP, Lax I and Schlessinger J:
Cellular signaling by fibroblast growth factor receptors. Cytokine
Growth Factor Rev. 16:139–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiedemann M and Trueb B: Characterization
of a novel protein (FGFRL1) from human cartilage related to FGF
receptors. Genomics. 69:275–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaid TM, Yeung TL, Thompson MS, Leung CS,
Harding T, Co NN, Schmandt RS, Kwan SY, Rodriguez-Aguay C,
Lopez-Berestein G, et al: Identification of FGFR4 as a potential
therapeutic target for advanced-stage, high-grade serous ovarian
cancer. Clin Cancer Res. 19:809–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D, Zhu W, Li J, An C and Wang Z:
Serum concentrations of fibroblast growth factors 19 and 21 in
women with gestational diabetes mellitus: Association with insulin
resistance, adiponectin, and polycystic ovary syndrome history.
PLoS One. 8:e811902013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng S, Dakhova O, Creighton CJ and
Ittmann M: Endocrine fibroblast growth factor FGF19 promotes
prostate cancer progression. Cancer Res. 73:2551–2562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song KH, Li T, Owsley E, Strom S and
Chiang JY: Bile acids activate fibroblast growth factor 19
signaling in human hepatocytes to inhibit cholesterol
7alpha-hydroxylase gene expression. Hepatology. 49:297–305. 2009.
View Article : Google Scholar :
|
|
14
|
Stejskal D, Karpísek M, Hanulová Z and
Stejskal P: Fibroblast growth factor-19: Development, analytical
characterization and clinical evaluation of a new ELISA test. Scand
J Clin Lab Invest. 68:501–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie MH, Holcomb I, Deuel B, Dowd P, Huang
A, Vagts A, Foster J, Liang J, Brush J, Gu Q, et al: FGF-19, a
novel fibroblast growth factor with unique specificity for FGFR4.
Cytokine. 11:729–735. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Ibrahimi OA, Olsen SK, Umemori H,
Mohammadi M and Ornitz DM: Receptor specificity of the fibroblast
growth factor family. The complete mammalian FGF family. J Biol
Chem. 281:15694–15700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurosu H, Choi M, Ogawa Y, Dickson AS,
Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA and
Kuro-o M: Tissue-specific expression of betaKlotho and fibroblast
growth factor (FGF) receptor isoforms determines metabolic activity
of FGF19 and FGF21. J Biol Chem. 282:26687–26695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miura S, Mitsuhashi N, Shimizu H, Kimura
F, Yoshidome H, Otsuka M, Kato A, Shida T, Okamura D and Miyazaki
M: Fibroblast growth factor 19 expression correlates with tumor
progression and poorer prognosis of hepatocellular carcinoma. BMC
Cancer. 12:562012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye YW, Zhou Y, Yuan L, Wang CM, Du CY,
Zhou XY, Zheng BQ, Cao X, Sun MH, Fu H, et al: Fibroblast growth
factor receptor 4 regulates proliferation and antiapoptosis during
gastric cancer progression. Cancer. 117:5304–5313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu YF, Yang XQ, Lu XF, Guo S, Liu Y, Iqbal
M, Ning SL, Yang H, Suo N and Chen YX: Fibroblast growth factor
receptor 4 promotes progression and correlates to poor prognosis in
cholangiocarcinoma. Biochem Biophys Res Commun. 446:54–60. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dutra RL, de Carvalho MB, Dos Santos M,
Mercante AM, Gazito D, de Cicco R, Group G, Tajara EH, Louro ID and
da Silva AM: FGFR4 profile as a prognostic marker in squamous cell
carcinoma of the mouth and oropharynx. PLoS One. 7:e507472012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li CS, Zhang SX, Liu HJ, Shi YL, Li LP,
Guo XB and Zhang ZH: Fibroblast growth factor receptor 4 as a
potential prognostic and therapeutic marker in colorectal cancer.
Biomarkers. 19:81–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawey ET, Chanrion M, Cai C, Wu G, Zhang
J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L, et al:
Identification of a therapeutic strategy targeting amplified FGF19
in liver cancer by oncogenomic screening. Cancer Cell. 19:347–358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaakkola S, Salmikangas P, Nylund S,
Partanen J, Armstrong E, Pyrhönen S, Lehtovirta P and Nevanlinna H:
Amplification of fgfr4 gene in human breast and gynecological
cancers. Int J Cancer. 54:378–382. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones SA: Physiology of FGF15/19. Adv Exp
Med Biol. 728:171–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Potthoff MJ, Kliewer SA and Mangelsdorf
DJ: Endocrine fibroblast growth factors 15/19 and 21: From feast to
famine. Genes Dev. 26:312–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doll KM, Kalinowski AK, Snavely AC, Irwin
DE, Bensen JT, Bae-Jump VL, Kim KH, Van Le L, Clarke-Pearson DL and
Gehrig PA: Obesity is associated with worse quality of life in
women with gynecologic malignancies: An opportunity to improve
patient-centered outcomes. Cancer. 121:395–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shah MM, Erickson BK, Matin T, McGwin G
Jr, Martin JY, Daily LB, Pasko D, Haygood CW, Fauci JM and Leath CA
III: Diabetes mellitus and ovarian cancer: More complex than just
increasing risk. Gynecol Oncol. 135:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomson CA, E Crane T, Wertheim BC,
Neuhouser ML, Li W, Snetselaar LG, Basen-Engquist KM, Zhou Y and
Irwin ML: Diet quality and survival after ovarian cancer: Results
from the Women's Health Initiative. J Natl Cancer Inst.
106:1062014. View Article : Google Scholar
|
|
30
|
Olsen CM, Nagle CM, Whiteman DC, Ness R,
Pearce CL, Pike MC, Rossing MA, Terry KL, Wu AH, Risch HA, et al
Australian Cancer Study (Ovarian Cancer); Australian Ovarian Cancer
Study Group: Ovarian Cancer Association Consortium: Obesity and
risk of ovarian cancer subtypes: Evidence from the Ovarian Cancer
Association Consortium. Endocr Relat Cancer. 20:251–262. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bae HS, Kim HJ, Hong JH, Lee JK, Lee NW
and Song JY: Obesity and epithelial ovarian cancer survival: A
systematic review and meta-analysis. J Ovarian Res. 7:412014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katoh M and Nakagama H: FGF receptors:
Cancer biology and therapeutics. Med Res Rev. 34:280–300. 2014.
View Article : Google Scholar
|
|
33
|
Kotsopoulos J, Moody JR, Fan I, Rosen B,
Risch HA, McLaughlin JR, Sun P and Narod SA: Height, weight, BMI
and ovarian cancer survival. Gynecol Oncol. 127:83–87. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding W, Shi W, Bellusci S, Groffen J,
Heisterkamp N, Minoo P and Warburton D: Sprouty2 downregulation
plays a pivotal role in mediating crosstalk between TGF-beta1
signaling and EGF as well as FGF receptor tyrosine kinase-ERK
pathways in mesenchymal cells. J Cell Physiol. 212:796–806. 2007.
View Article : Google Scholar : PubMed/NCBI
|