Introduction
Ovarian cancer is a common gynecological tumor and
is one of the leading causes of death among women with
gynecological tumors. Although positive surgical treatment and
chemotherapy with postoperative joint application of platinum and
new drugs have improved the prognosis of patients, in 70% of the
patients ovarian cancer recurs in 2 years with a very poor
prognosis (1). Recently, the theory
of cancer stem cells (CSCs) presents that CSC is similar to normal
stem cells with regard to self-renewal, unlimited proliferation and
multidirectional differentiation potential, and express the
pluripotent stem cell-specific transcription factors
octamer-binding protein 4 (Oct-4) and Nanog (2,3). It is
suggested that CSCs are the key to the transfer of tumor recurrence
and the root of the chemotherapeutic drug resistance.
Ursolic acid (UA, molecular weight=456) is a
pentacyclic triterpene acid, present in apples, basil, bilberries,
cranberries, elder flower, peppermint, rosemary, lavender, oregano,
thyme, hawthorn, prunes and medicinal plants such as Oldenlandia
diffusa, Eriobotrya japonica, Rosmarinus
officinalis and Glechoma hederacea (4), and has recently been found to be
capable of inhibiting various types of cancer cells (5–7). Yet,
no studies on the inhibitory effects of UA on the ovarian CSCs are
available.
The epithelial-mesenchymal transition (EMT) is a
transdifferentiation process by which cells undergo a morphological
switch from the epithelial polarized phenotype to the mesenchymal
fibroblastoid phenotype and involves loss of cell polarity,
decreased cell-to-cell adhesion, and increased motility and
capacity for migration (8).
Emerging evidence suggests an intricate role of CSCs and EMT-type
cells in anticancer drug resistance. Luo et al (9) demonstrated that EMT contributed to the
enrichment of ovarian CSCs in vitro, making targeting of EMT
in epithelial ovarian cancer a novel therapeutic option. The link
between EMT and acquisition of stem cell-like properties by cancer
cells may explain the reason for EMT inducing tumor progression. In
addition, drug resistance of cancer cells was also regarded to be
associated with EMT (10). However,
EMT in ovarian CSCs and its effects on drug resistance are still
undiscovered.
Our experiments revealed that EMT, CSCs and UA are
involved in anticancer drug resistance, indicating that the
involvement of UA in the regulation of EMT may lead to the
elimination of CSCs or EMT-type cells that are typically drug
resistant.
Materials and methods
Cell culture
The SKOV3 ovarian cancer cell line was obtained from
the Shanghai Cell Bank of Chinese Academy of Sciences and
maintained in McCoy's medium (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum (FBS). Cells were
incubated at 37°C in a humidified atmosphere containing 5%
CO2. Later cells were dissociated using 0.25%
trypsin-ethylenediaminetetraacetic acid (EDTA) for 1–2 min at 37°C
and maintained under stem cell conditions using serum-free
Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 5
mg/ml insulin (Sigma-Aldrich), 10 ng/ml human recombinant epidermal
growth factor (Invitrogen, Carlsbad, CA, USA), 10 ng/ml basic
fibroblast growth factor (Invitrogen), 12 ng/ml leukemia inhibitory
factor (Gibco, Paisley, UK) and 0.3% bovine serum albumin
(Sigma-Aldrich). The selected cancer cells formed non-adherent
spheres grown in this condition. The medium was changed every 2
days by centrifuging at 800 rpm for 5 min to remove the dead cell
debris. Regular cell culture plates were used for the experiment.
The other tumor cells were maintained under standard conditions
(DMEM/F12 supplemented with 10% FBS without growth factors) and
formed attached differentiated cells.
MTT assay
Appropriate number of the UA or UA in combination
with cisplain in SKOV3 cells and SKOV3 sphere cells along with
equal number of their respective controls were cultured in 96-well
plates at 37°C in 5% CO2 incubator for 48 h. The
experimental concentration of UA and cisplatin was 3.125, 6.25,
12.5, 25, 50 and 100 µg/ml, respectively. At the endpoints,
cells were incubated with thiazolyl blue tetrazolium bromide
(Sigma-Aldrich) at a concentration of 0.5 mg/ml for further 4 h.
Resulting formazan crystals were dissolved with 100 µl of
dimethyl sulfoxide, and proliferation was monitored by the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay and optical density (OD) reading at 490 nm. Then the
inhibition rate (IR) and half maximal inhibitory concentration
(IC50) of the two kinds of cells were calculated.
Cell cycle analysis
SKOV3 sphere cells were treated with UA and UA
combined with cisplain at IC50 concentrations for 48 h.
The experiment was divided into four groups: SKOV3 cells, SKOV3
sphere cells, SKOV3 sphere cells with UA and SKOV3 sphere cells
with UA plus cisplatin. Then, cells in each group were collected
and fixed in 70% cold ethanol at −20°C overnight. After washing
twice with PBS, cells were resuspended in PBS. RNaseA (0.02 mg/ml)
and propidium iodide (PI) (0.02 mg/ml) were added to the fixed
cells for 1 h at 4°C. The DNA content of cells was then analyzed
using a flow cytometer. The percentage of cells in the different
cell cycle phases was calculated using BD FACSDiva™ software (BD
Biosciences, San Jose, CA, USA).
Cell apoptosis assay
SKOV3 sphere cells were treated with UA at
IC50 concentrations and UA plus cisplatin for 48 h.
Then, cells in four groups were digested with 0.25% trypsin without
EDTA and washed twice with PBS and then re-suspended in the binding
buffer, with the cell density adjusted to 2×105/ml. The
cell suspension (195 µl) was obtained, and Annexin
V-fluorescein isothiocyanate (FITC) (5 µl) and PI (10
µl) were added, respectively. The mixture was kept at room
temperature for 30 min and then measured for apoptosis using flow
cytometry.
Cell migration and invasion assays
SKOV3 sphere cells were treated with UA and combined
with cisplain at IC50 concentrations for 48 h. The
experiment was divided into four groups as above. Invasion assays
were performed in triplicate using 8.0 µm Transwell invasion
chambers (Corning Costar, Rochester, NY, USA) coated with Matrigel
(100 µg per filter) (Becton-Dickinson, Franklin Lakes, NJ,
USA) as described in the manufacturer's instructions. Cells
(1×105/well; 200 µl per chamber) in serum-free
media were seeded onto top chambers. Complete medium (600
µl) with 10% FBS was added to the lower chambers. Following
48-h incubation, cells that had invaded through the surface of the
membrane were fixed in methanol and stained with crystal violet.
Cells that did not invade into the lower chamber were scraped from
the top of the Transwell plate with a cotton swab. Invading cells
from three random microscopic fields per filter were selected for
cell counting. Procedure for the migration assay was similar to
that for the invasion assay, differing in that for the migration
assay the Transwell chambers were not coated with Matrigel and
5×104 cells/well were seeded onto top chambers and
incubated for 16 h.
RNA extraction and real-time quantitative
polymerase chain reaction analysis
Before and after the treatment of IC50
concentration of UA and cisplain in vitro, the expression of
marker gene mRNA of SKOV3 cells and sphere cells was measured.
Total RNA was extracted from SKOV3 sphere cells and SKOV3 cells
using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). In total,
500 ng of total RNA from each sample was utilized for reverse
transcription using the iScript cDNA synthesis kit (Bio-Rad
Laboratories, Hercules, CA, USA). Real-time PCR was performed on
cDNA using iQ SYBR-Green with Mastercycler ep realplex real-time
PCR system (Eppendorf, Hamburg, Germany). All reactions were
performed in a 25-ml volume. PCR was performed by an initial
denaturation at 95°C for 5 min, followed by 40 cycles for 30 sec at
95°C, 30 sec at 60°C and 30 sec at 72°C. PCR using water instead of
the template was used as a negative control. Specificity was
verified by melting curve analysis and agarose gel electrophoresis.
The threshold cycle (Ct) values of each sample were used in the
post-PCR data analysis. 18S RNA was used as an internal control for
mRNA-level normalization. The primer sequences for each gene
analyzed are summarized in Table
I.
 | Table ISequences of the primers used for
qPCR. |
Table I
Sequences of the primers used for
qPCR.
| Gene | Sequence | Product (bp) |
|---|
| NANOG | F:
TTCCTTCCTCCATGGATCTG | 213 |
| R:
TCTGCTGGAGGCTGAGGTAT | |
| OCT4 (POU5F1) | F:
GGCCCGAAAGAGAAAGCGAACC | 224 |
| R:
ACCCAGCAGCCTCAAAATCCTCTC | |
| ABCG2 | F:
TGAGCCTTTGGTTAAGACCG | 107 |
| R:
TGGTGTTTCCTTGTGACACTG | |
| CD133 (PROM1) | F:
TGGATGCAGAACTTGACAACGT | 133 |
| R:
ATACCTGCTACGACAGTCGTGGT | |
| CD117 (KIT) | F:
CAAGGAAGGTTTCCGAATGC | 74 |
| R:
CCCAGCAGGTCTTCATCATGT | |
| SOX2 | F:
GCGCGGGCGTGAACCAG | 396 |
| R:
CGGCGCCGGGGAGATACA | |
| CK19 (KRT19) | F:
TTTGAGACGGAACAGGCTCT | 211 |
| R:
AATCCACCTCCACACTGACC | |
| Fibronectin
(FN1) | F:
CATGTCTCTCTGCCAAGATCCATCT | 390 |
|
R:TTGTTCCTACAGTATTGCGGGCCAG | |
| Vimentin (VIM) | F:
ACAGGCTTTAGCGAGTTATT | 182 |
| R:
GGGCTCCTAGCGGTTTAG | |
| Snail (SNAI1) | F:
ATCCGAAGCCACACGCTGCC | 130 |
| R:
CACGGCTGCAGTGGGGACAG | |
| Slug (SNAI2) | F:
AGATGCATATTCGGACCCAC | 258 |
| R:
CCTCATGTTTGTGCAGGAGA | |
| Twist (TWIST1) | F:
TGTCCGCGTCCCACTAGC | 93 |
| R:
TGTCCATTTTCTCCTTCTCTGGA | |
| N-cadherin
(CDH2) | F:
GACGGTTCGCCATCCAGAC | 67 |
| R:
TCGATTGGTTTGACCACGG | |
| E-cadherin
(CDH1) | F:
CTGGACGCTCGGCCTGAAGT | 140 |
| R:
GGGTCAGTATCAGCCGCTTT | |
|
18sRNA(RNA18S5) | F:
CGTTGATTAAGTCCCTGCCCTT | 202 |
| R:
TCAAGTTCGACCGTCTTCTCAG | |
In vivo xenograft experiments
The in vivo evaluation of UA was performed
using a xenograft model of ovarian cancer SKOV3 sphere cells.
Athymic BALB/c-nu female nude mice (5–6 weeks old, obtained from
Beijing HFK Bioscience Co., Ltd., Beijing, China) were housed in a
specific pathogen-free room within the animal facilities at the
Laboratory Animal Center. Animals were allowed to acclimatize to
their new environment for 1 week prior to use. The dissociated
sphere SKOV3 cells (5×106) were resuspended in PBS, and
injected s.c. into the left side of flank of nude mice. Engrafted
mice were inspected for the appearance of tumor by visual
observation and palpation until the tumor formed. From the 20th day
of injection, mice were randomly assigned to three treatment groups
(n=4 for each group) and injected intraperitoneally (i.p.) with
normal saline, UA (60 mg/kg body weight, daily) and UA combined (60
mg/kg body weight, daily) with cisplatin (2.5 mg/kg body weight,
daily) treatment for 14 consecutive days. Body weight and tumor
mass were measured every 2 days. Tumor volume was determined using
a caliper and calculated according to the formula
(width2 × length × π)/6. Mice were sacrificed by
cervical dislocation under anesthesia after 2 weeks of treatment.
Animal welfare and experimental procedures were performed strictly
in accordance with high standards for animal welfare and other
related ethical regulations approved by the Shanghai University of
Traditional Chinese Medicine.
Immunohistochemical analysis
Immunohistochemical studies were performed on the
xenograft tumors after they were removed from nude mice. The tumors
were fixed in 40 mg/ml paraformaldehyde, paraffin-embedded and cut
into 4µm serial sections. Next, endogenous peroxidases were
quenched and the sections were washed carefully with
phosphate-buffered saline (PBS) three times. The sections were
blocked with 2% goat serum and rabbit serum, respectively, in PBS
at 37°C for 45 min, then incubated with mouse anti-proliferating
cell nuclear antigen (PCNA) antibody (1:200 dilution; Abcam),
rabbit anti-Ki-67 antibody (1:200 dilution; Millipore), mouse
anti-vimentin antibody (1:300 dilution; Abcam), mouse
anti-fibronection antibody (1:300 dilution; Abcam) overnight at
4°C. Later, the sections were incubated with horseradish
peroxidase-conjugated secondary antibodies separately and
avidin-biotin complex followed by diaminobenzidine (Vectastain ABC;
Vector Laboratories Burlingame, CA, USA). The sections were
immersed in 2% ammonia water and hematoxylin was used for
counterstaining.
Positive PCNA and Ki-67 staining was mainly in the
nuclei, while positive expression of vimentin and fibronection was
primarily a cytoplasmic pattern in tumor cells. For evaluation of
positive expression, staining intensity was scored as 0 (negative),
1 (weak), 2 (medium) or 3 (strong). Strong positive (scored as 3),
strong staining intensity (90% of positive cells); moderate
positive (scored as 2), moderate staining intensity (50–89% of
positive cells); weak positive (scored as 1), weak staining
intensity (10–49% of positive cells); absent (scored as 0), no
staining intensity and no positive or only a few positive cells
(11).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-test was performed to evaluate the difference between
mean values. P<0.05 was considered to indicate a statistically
significant result. All experiments were performed in
triplicate.
Results
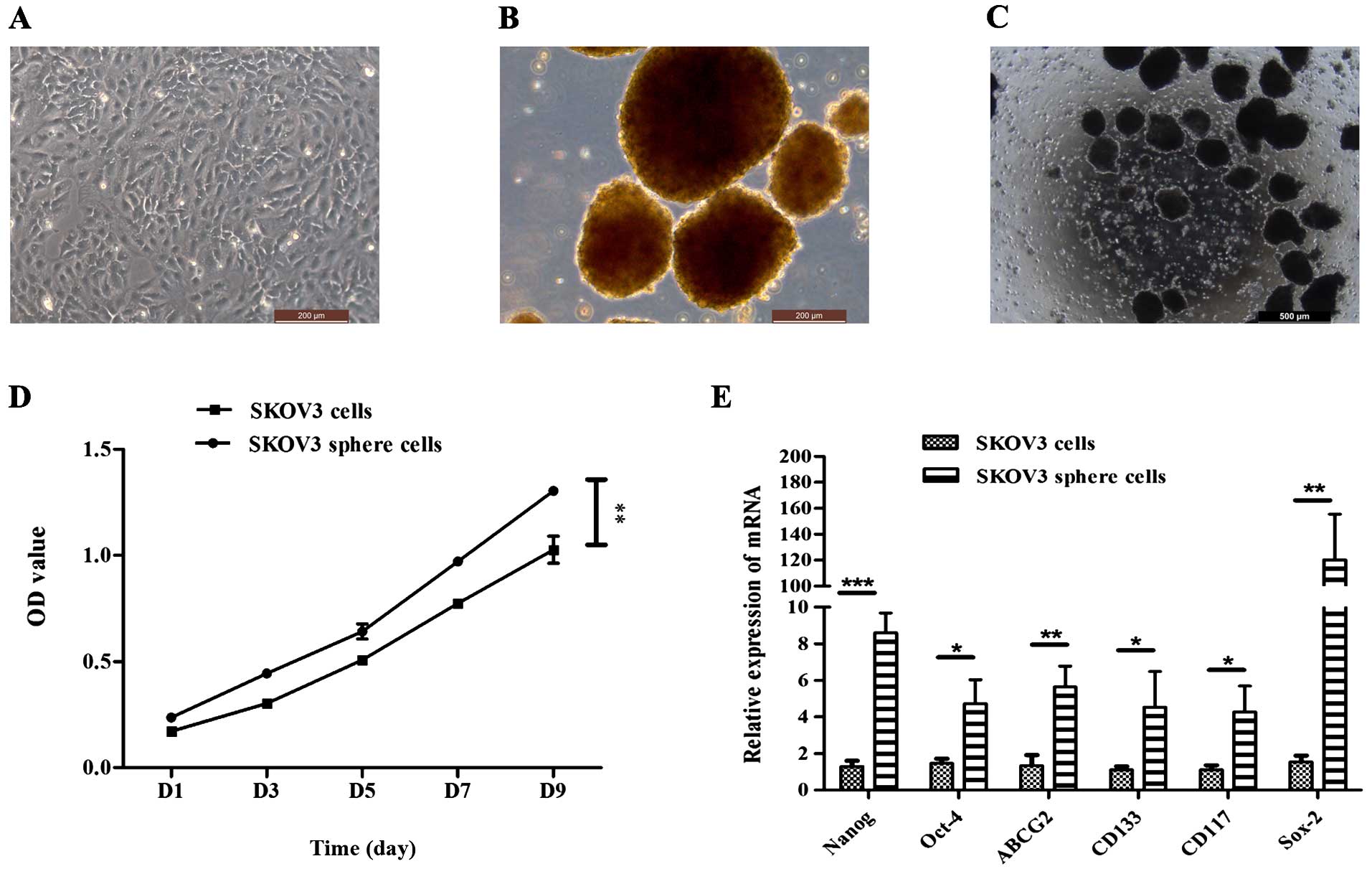
Sphere cell formation under stem
cell-selective conditions
It has been reported that ovarian cancer stem-like
cells could be enriched and exhibit characteristics expected of
CSCs (12–15). In the present study, attempts were
made to isolate a self-renewing stem cell population from the SKOV3
cell line. The SKOV3 cell line was cultured with McCoy's medium
supplemented with 10% FBS (Fig.
1A). Under serum-free condition, the SKOV3 cells were able to
form non-adherent spheres. The formation of sphere cells was
observed on day 3 after plating (Fig.
1B and C). These cluster cells were small, non-adherent and
non-symmetric. Primary spheres could be enzymatically dissociated
to single cells, which in turn give rise to secondary spheres. This
procedure could be repeated, and the tumorigenic spheres grow
faster than the cells under differentiating conditions (Fig. 1D). The stem/progenitor cell
phenotype of the sphere cells was further confirmed by the
expression of putative stem cell markers. Quantitative real-time
PCR showed that the expression of Nanog, Oct-4, Sox-2, CD133, CD117
and ABCG2 in sphere cells was higher than that in differentiated
cells (Fig. 1E; P<0.01).
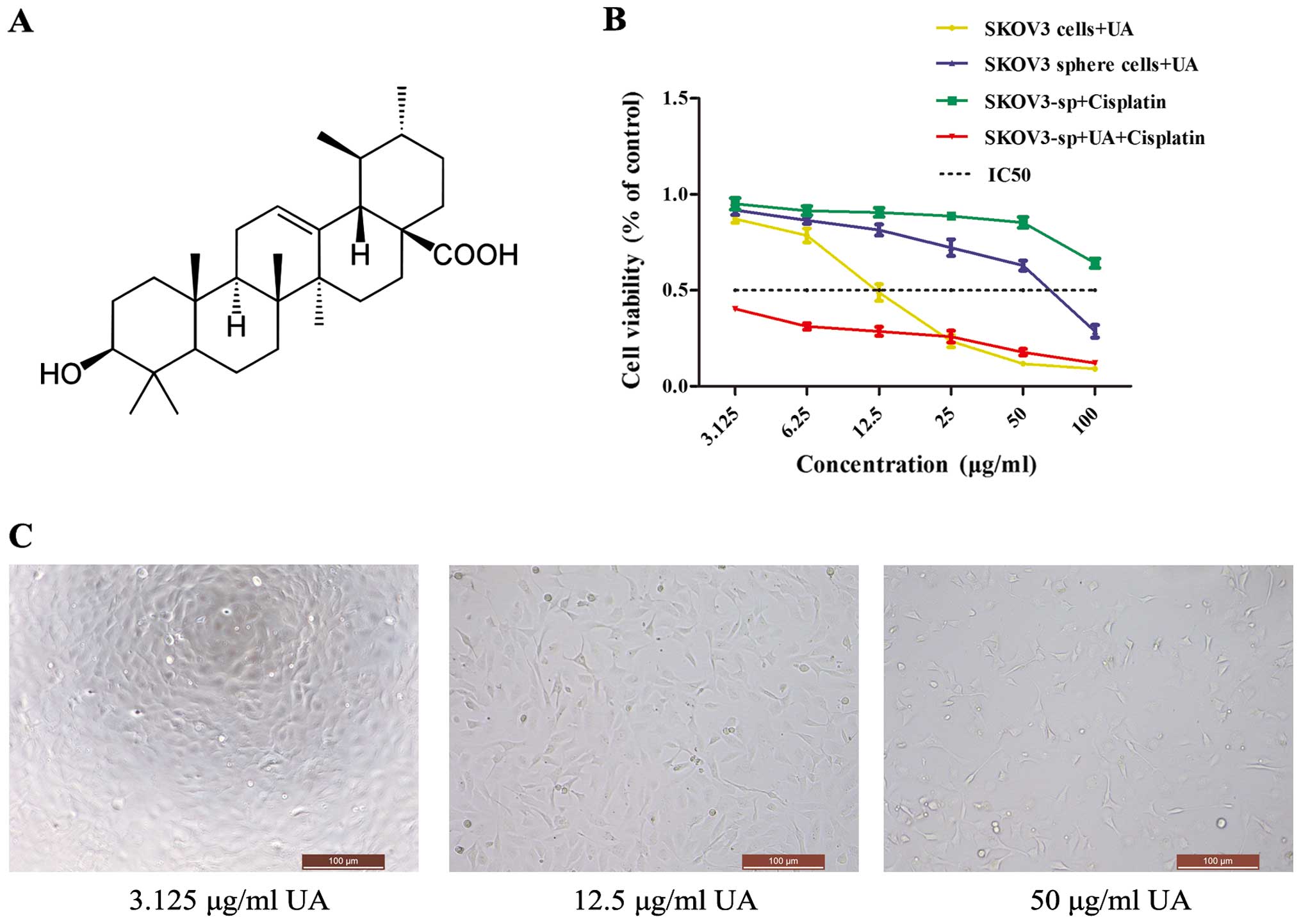
Proliferation of SKOV3 cells and SKOV3
sphere cells is inhibited by UA
UAs are triterpenoid compounds that exist widely in
food, medicinal herbs and other plants (Fig. 2A). To demonstrate its effects on
ovarian cancer cells, MTT assay was performed, which showed that
the proliferation rate was significantly decreased in the
UA-treated cells when compared with the non-treated cells
(P<0.05 or P<0.01). UA inhibited the proliferation of the
cells in a concentration-dependent manner (Fig. 2C). The IR of UA on the SKOV3 cells
is much higher than that of the sphere cells, while IC50
of SKOV3 cells is 12.04 mg/l, whereas that of sphere cells is 74.54
mg/l. To determine whether UA could enhance the cisplatin
cytotoxicity to ovarian cancer cells, SKOV3 sphere cells were
exposed to different concentrations of UA, cisplatin and a
combination of UA and cisplatin. The IC50 of
cisplatin-treated SKOV3 and SKOV3 sphere cells was 70.38 and 217.73
mg/l, respectively. When SKOV3 sphere cells were co-treated with 75
mg/l of UA, the cell viability was significantly decreased compared
with treatment with UA alone or cisplatin alone (Fig. 2B). Thus, UA may regulate cisplatin
chemosensitivity in ovarian cancer sphere cells.
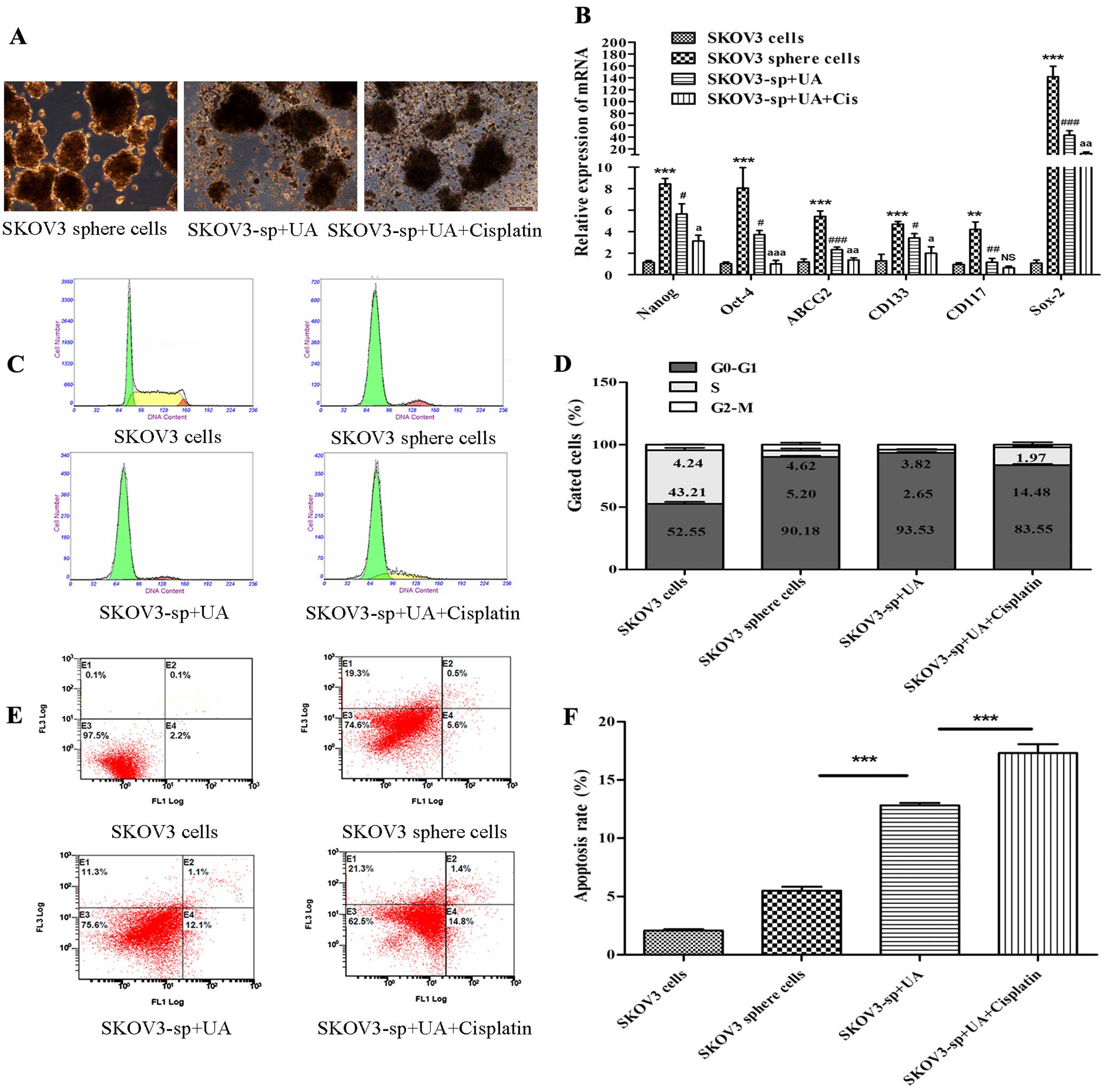
UA induces apoptosis of SKOV3 sphere
cells
To further demonstrate whether UA induces apoptosis
of SKOV3 sphere cells, SKOV3 sphere cells were treated with
control, UA, and combinations of UA (75 mg/l) with cisplatin (70
mg/l), respectively. Fig. 3A shows
that UA obviously destructed the morphology of sphere cells.
Real-time PCR showed that after treatment with UA and cisplatin,
stem cell genetic marker mRNA expression quantity of SKOV3 sphere
cells was reduced; stem cell marker mRNA expression of UA combined
with cisplain group reduced more obviously (P<0.05 or P<0.01
or P<0.001) (Fig. 3B). To
further quantify the apoptotic effects of treatment with UA and
cisplatin, SKOV3 cells and sphere cells were stained with Annexin
V-FITC and PI, and subsequently analyzed using flow cytometry for
cell apoptosis. Consistent with growth inhibitory effects, UA
combined with cisplatin caused a significant increase in the
distribution of cells at the S phase in a dose-dependent manner.
Besides evident S arrest, distinct G0–G1 peaks were observed in
SKOV3 sphere cells after treatment (Fig. 3C and D). Proportions of Annexin
V-stained cells were higher in cisplatin- and UA-treated cells than
in the control SKOV3 sphere cells. Obvious increase in the number
of apoptotic cells was detected for cells treated with cisplatin
and UA compared to UA alone (Fig. 3E
and F).
UA diminishes migration and invasion of
SKOV3 sphere cells via downregulated expression of EMT
characteristic
In our previous study, we reported that enrichment
of ovarian CSCs is accompanied by EMT (9). To investigate the influence of UA and
cisplatin on other mitogen-dependent processes, two assays were
employed in the next step to compare the motility and invasion of
the SKOV3 sphere cells with those of the negative control group.
The EMT markers were also examined to demonstrate biological
changes after treatment with UA and cisplatin. The Transwell
invasion assay (Fig. 4B) showed
that sphere cells had significant elevation in their invasive
ability, and Transwell migration assay (Fig. 4A) was further used to assess their
motility. Results from the migration assay (Fig. 4A) indicated that the migration of
SKOV3 sphere cells treated with UA combined with cisplatin was
significantly lower than their control counterparts 16 h after
plating. The invasion assay (Fig.
4B) similarly showed reduced number of invaded cells in the UA
combined with cisplatin group examined at 48 h. In summary,
treatment with UA and cisplatin was associated with attenuation of
the motility and invasion of SKOV3 sphere cells, in vitro.
As shown in (Fig. 4C), mesenchymal
markers of SKOV3 sphere cells such as Snail, Slug, Twist, vimentin,
N-cadherin and fibronectin were expressed significantly higher than
SKOV3 cells. Twist, vimentin, N-cadherin and fibronectin of UA
alone group and UA combined with cisplatin group were significantly
reduced compared to the sphere cells group, while epithelial
markers CK19 and E-cadherin did not change significantly.
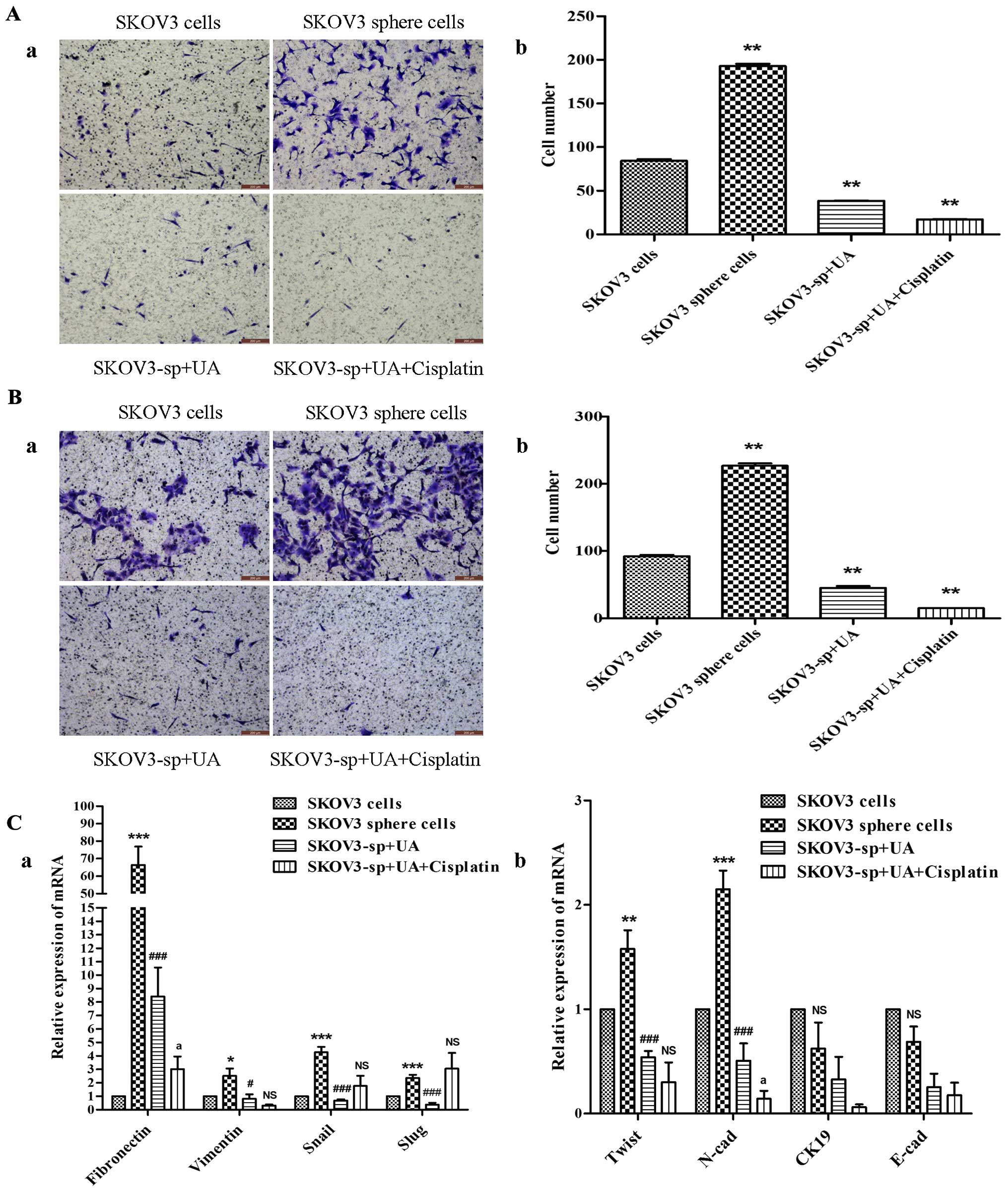 | Figure 4Ursolic acid inhibits the migration
and invasion of SKOV3 sphere cells. (A-a) Representative images of
migrating SKOV3 cells and sphere cells, both UA alone and UA plus
cisplatin decreases the migratory capabilities of sphere cells.
Corresponding quantitative data are shown (b).
**P<0.01. (B-a) Representative images of invading
SKOV3 cells and sphere cells. Both UA alone and UA plus cisplatin
decrease the invasion capabilities of the sphere cells showing
weaker invasion capability in the UA combined with cisplatin group.
Corresponding quantitative data depicting the cell number/field are
shown (b). **P<0.01. (C) As shown by qRT-PCR, SKOV3
sphere cells, under stem cell-selective conditions treated with UA
and cisplatin, exhibited lower expression of mesenchymal markers
(Twist, vimentin, N-cadherin and fibronectin) compared with control
SKOV3 sphere cells, *P<0.05, vs. SKOV3 cells group;
#P<0.05, vs. SKOV3 sphere cells group;
aP<0.05, vs. SKOV3-sp+UA group, while epithelial
markers (CK19 and E-cadherin) did not change significantly. |
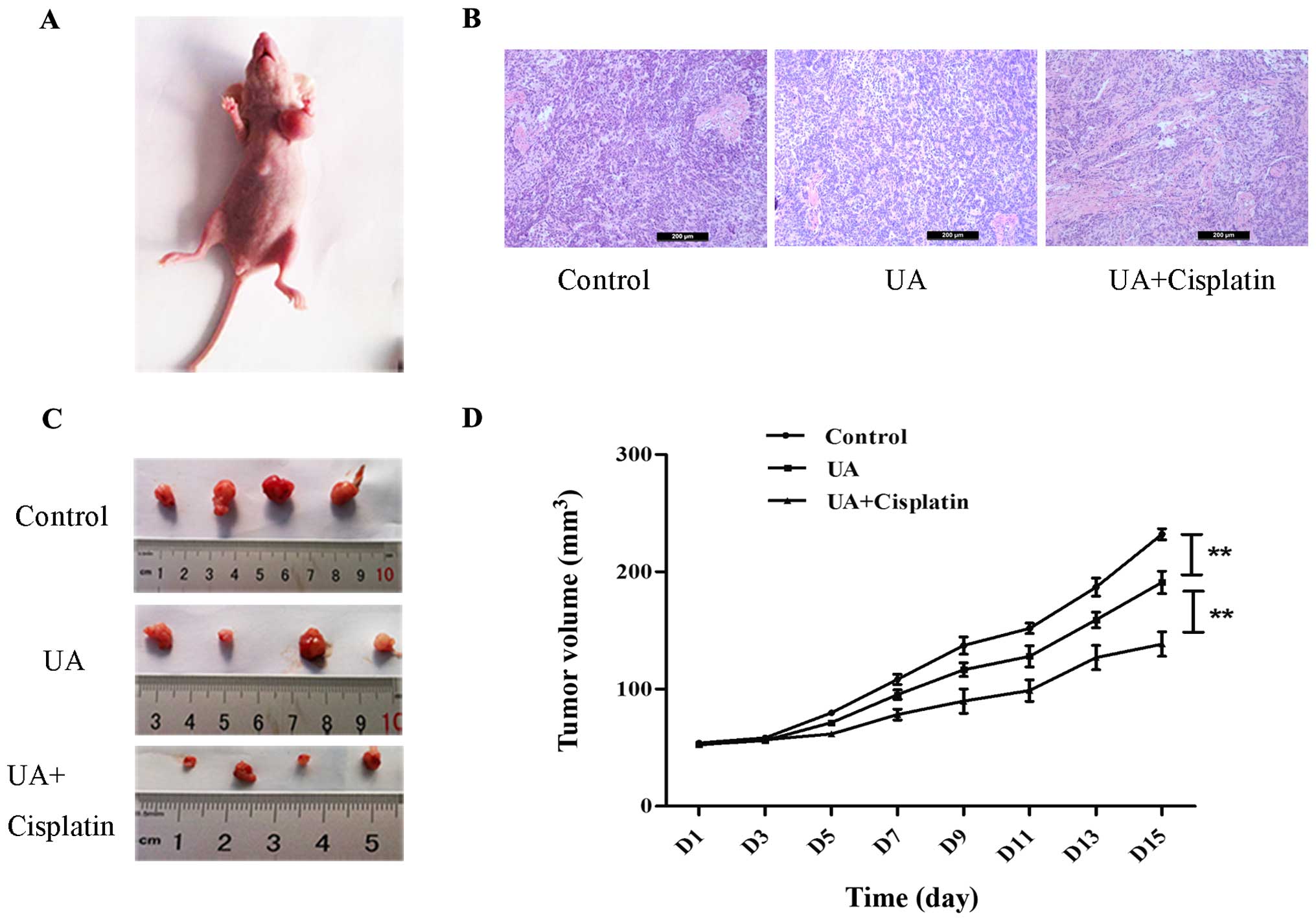
UA promotes cisplatin inhibition of
ovarian cancer growth in vivo
SKOV3 sphere cells were used to generate xenograft
tumors in athymic nude BALB/c-nu mice to determine whether UA could
strengthen the effects of chemotherapy in vivo. SKOV3 sphere
cells (5×106) formed tumors with a 13 day tumor latency
(Fig. 5A). Representative
hematoxylin and eosin staining of xenograft ovarian cancer of each
group is shown in Fig. 5B. As
expected, treatment with UA alone suppressed tumor growth compared
with normal saline control. Tumors from mice treated with cisplatin
in combination with UA were smaller at day 28 than those treated
with UA alone. Furthermore, when measured both in tumor size and in
tumor weight, the combined treatment of cisplatin and UA displayed
a cancer prohibition effect when compared to the control group
(Fig. 5C and D).
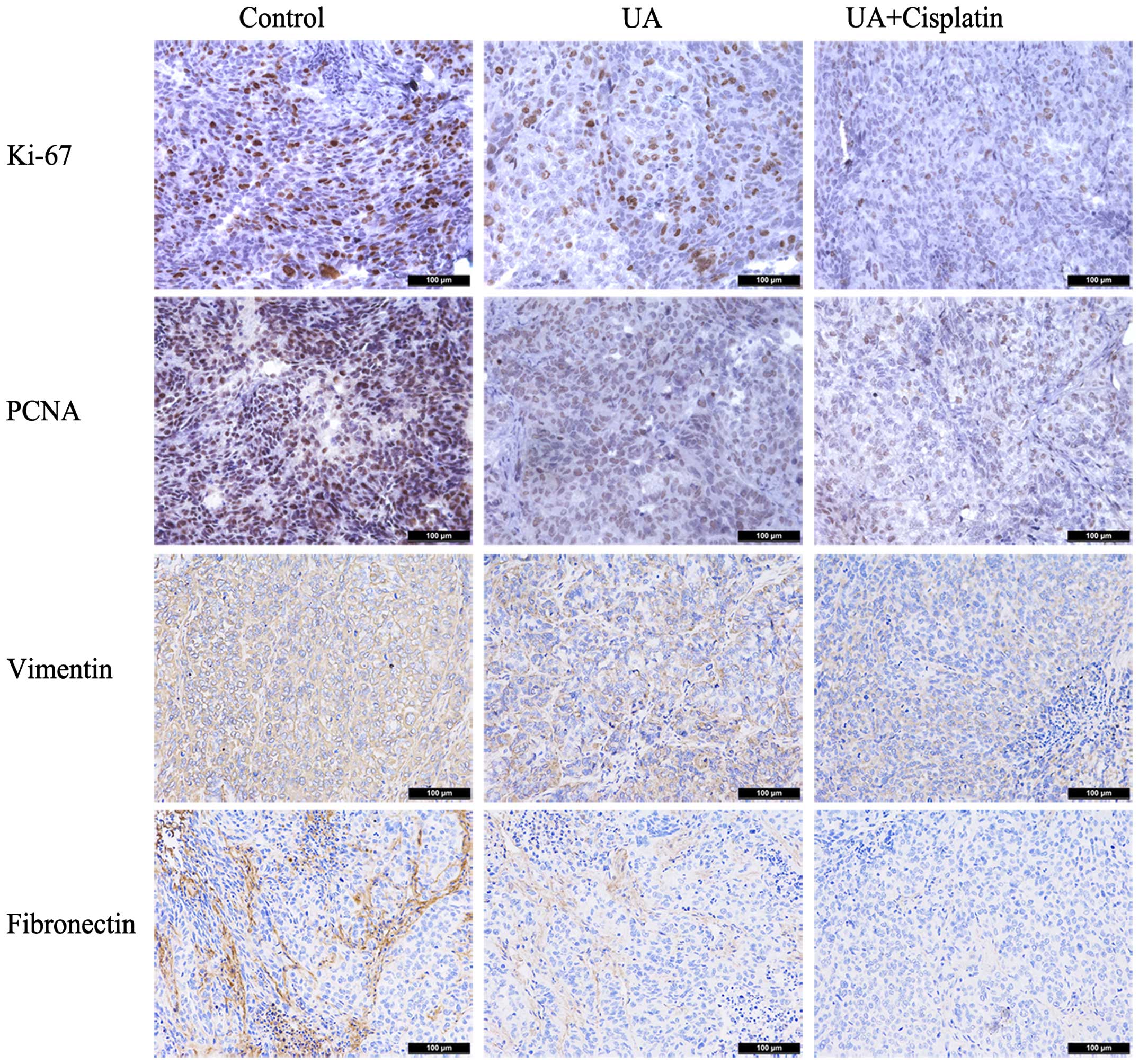
UA downregulates the expression of Ki-67,
PCNA, vimentin and fibronection of ovarian cancer in vivo
Immunohistochemical assays were further performed in
tumors removed from the nude mouse xenograft model. In tumors
treated with UA alone, Ki-67 and PCNA staining, respectively,
showed moderate intensity with the scores of 1.6 and 1.4. As
expected, mouse group treated with cisplatin in combination with UA
had a much lower level of Ki-67 and PCNA staining (score 0.8 and
1.0). In the group treated with UA alone, almost all cancer cells
displayed weak vimentin and fibronection staining with scores of
1.8 and 1.2, while treatment with cisplatin plus UA displayed
scores of 0.8 and 0.4 (Fig. 6).
These results demonstrated that UA downregulates the expression of
Ki-67, PCNA, vimentin and fibronection of ovarian cancer in
vivo.
Discussion
There is increasing evidence that cancer cells from
both ovarian cancer cell lines and primary ovary tumor samples can
survive and grow in serum-free suspensions, forming non-adherent
spheres and display remarkable stem-like properties (16,17).
As shown in the present study, the sphere cells isolated from the
SKOV3 cell line, which form non-adherent spheres and display
remarkable stem cell properties (Fig.
1), have higher drug resistance characteristics and are more
tumorigenic.
UA, a pentacyclic triterpenoid found in most plant
species, has recently drawn a great deal of attention for its
effects on cancer cells including inhibition of tumor cell growth
and induction of apoptosis (18–23).
Our studies showed that UA inhibited proliferation and metastasis
in a dose-dependent manner in human ovarian cancer SKOV3 cells and
SKOV3 sphere cells. Furthermore, it was found that sphere cells
treated with UA expressed lower level of mesenchymal gene marker
than sphere cells. UA combined with cisplatin downregulated the
expression of vimentin and N-cadherin. Moreover, UA and UA plus
cisplatin decelerated cell viability and migration ability and
accelerated apoptosis compared with the negative control group
(Figs. 2 and 4). In a nude mouse xenograft model
injected with SKOV3 sphere cells, daily i.p. injection of UA at 60
mg/kg led to the enhancement of therapeutic efficacy of cisplatin
(Fig. 5). Tumors in mice treated
with UA in combination with cisplatin displayed decreased
expression of Ki-67, PCNA, vimentin and fibronectin staining
compared to mice treated with UA alone (Fig. 6). Thus, the data suggest that UA
inhibits SKOV3 sphere cells by reversing the mesenchymal feature of
ovarian CSC-like cells in EMT.
EMT is a necessary physical phenomenon of mammalian
embryonic development process, and it has been verified that EMT is
the manner by which embryonic stem cells mainly obtain migrating
ability (24). Accumulated evidence
has also revealed that EMT is the critical process for ovarian
cancer migration (25), and is
described as certain tumor cells acquiring new characteristics such
as expression of mesenchymal markers and loss of epithelial markers
and undergo profound morphogenetic changes during cancer
progression (26). Moreover, the
ovarian cancer cells undergoing EMT have been found to show
increased resistance to apoptosis and chemotherapeutic drugs and to
acquire traits reminiscent of those expressed by stem cells
(27). In our previous study we
reported that enrichment of ovarian CSCs is accompanied by EMT.
Compared to adherent cells, the sphere cells highly expressed
mesenchymal markers and exhibited significantly more motility
(9). UA was found to make the
cancer cells more sensitive to the chemotherapeutic drugs (28). It could be speculated that the
effect of UA on the EMT might partly contribute to the
anti-multidrug resistance. As cancer metastasis and resistance to
treatment are two major causes for the poor survival of patients
with ovarian cancer, UA is a potential anticancer drug for ovarian
cancer therapy, benefiting from its multiple effects such as
proapoptosis, antimetastasis and anti-multidrug resistance.
Cisplatin is the first-line chemotherapy drug for
many malignancies including ovarian cancer. In advanced ovarian
cancer, the first-line drugs of chemotherapy are the combination of
cisplatin/carboplatin with paclitaxel. With this regimen, ~20% of
patients do not respond at the first cycle and are characterized by
progression upon treatment in the first year and poor outcome
(29,30). In this study, it was found that UA
enhanced cisplatin chemosensitivity in SKOV3 sphere cells. These
findings suggested that UA could regulate cisplatin
chemosensitivity in ovarian CSCs. These results may be applied to
treat cisplatin resistance in patients with ovarian cancer.
Identification of an antitumor agent with low
toxicity has long been a hot research topic in oncological field.
The results of the present study suggest that UA may be used as a
drug against ovarian cancer in the future clinical practice. In the
present study, UA was found to be able to inhibit the proliferation
of SKOV3 sphere cells, a human ovarian cancer cell line, and the
mechanism is speculated to involve the inhibition of EMT activity,
development of cell apoptosis, as evidenced by the MTT assay and
flow cytometry. However, these results do not rule out the
possibility of other signaling pathways through which UA exerts its
inhibitory effects on tumor cells. More investigations in
vivo and in vitro, on the many aspects of UA, are still
warranted for further clarification.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (81173291). We are
grateful to the Animal Experimental Center of Shanghai University
of Traditional Chinese Medicine for housing the mice.
References
|
1
|
Tummala MK and McGuire WP: Recurrent
ovarian cancer. Clin Adv Hematol Oncol. 3:723–736. 2005.PubMed/NCBI
|
|
2
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsui W, Huff CA, Wang Q, Malehorn MT,
Barber J, Tanhehco Y, Smith BD, Civin CI and Jones RJ:
Characterization of clonogenic multiple myeloma cells. Blood.
103:2332–2336. 2004. View Article : Google Scholar
|
|
4
|
Taniguchi S, Imayoshi Y, Kobayashi E,
Takamatsu Y, Ito H, Hatano T, Sakagami H, Tokuda H, Nishino H,
Sugita D, et al: Production of bioactive triterpenes by Eriobotrya
japonica calli. Phytochemistry. 59:315–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu YL, Kuo PL and Lin CC: Proliferative
inhibition, cell-cycle dysregulation, and induction of apoptosis by
ursolic acid in human non-small cell lung cancer A549 cells. Life
Sci. 75:2303–2316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hollósy F, Idei M, Csorba G, Szabó E,
Bökönyi G, Seprödi A, Mészáros G, Szende B and Kéri G: Activation
of caspase-3 protease during the process of ursolic acid and its
derivative-induced apoptosis. Anticancer Res. 21:3485–3491.
2001.
|
|
7
|
Zhang YY, Deng T, Hu ZF, Zhang QP, Zhang J
and Jiang H: Mechanisms of inhibiting proliferation and inducing
apoptosis of human gastric cancer cell line SGC7901 by ursolic
acid. Ai Zheng. 25:432–437. 2006.In Chinese. PubMed/NCBI
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo X, Dong Z, Chen Y, Yang L and Lai D:
Enrichment of ovarian cancer stem-like cells is associated with
epithelial to mesenchymal transition through an miRNA-activated AKT
pathway. Cell Prolif. 46:436–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu P, Qiao J, He W, Wang J, Jia Y, Sun Y,
Tang S, Fu L and Qin Y: Genome-wide gene expression profile
analyses identify CTTN as a potential prognostic marker in
esophageal cancer. PLoS One. 9:e889182014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uchida N, Buck DW, He D, Reitsma MJ, Masek
M, Phan TV, Tsukamoto AS, Gage FH and Weissman IL: Direct isolation
of human central nervous system stem cells. Proc Natl Acad Sci USA.
97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
17
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F,
Maclaughlin DT and Donahoe PK: Ovarian cancer side population
defines cells with stem cell-like characteristics and Mullerian
Inhibiting Substance responsiveness. Proc Natl Acad Sci USA.
103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harmand PO, Duval R, Delage C and Simon A:
Ursolic acid induces apoptosis through mitochondrial intrinsic
pathway and caspase-3 activation in M4Beu melanoma cells. Int J
Cancer. 114:1–11. 2005. View Article : Google Scholar
|
|
19
|
Duval RE, Harmand PO, Jayat-Vignoles C,
Cook-Moreau J, Pinon A, Delage C and Simon A: Differential
involvement of mitochondria during ursolic acid-induced apoptotic
process in HaCaT and M4Beu cells. Oncol Rep. 19:145–149. 2008.
|
|
20
|
Kassi E, Sourlingas TG, Spiliotaki M,
Papoutsi Z, Pratsinis H, Aligiannis N and Moutsatsou P: Ursolic
acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast
cancer cells. Cancer Invest. 27:723–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang C, Lu YH, Xie JH, Wang F, Zou JN,
Yang JS, Xing YY and Xi T: Downregulation of survivin and
activation of caspase-3 through the PI3K/Akt pathway in ursolic
acid-induced HepG2 cell apoptosis. Anticancer Drugs. 20:249–258.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng YQ, Liu D, Cai LL, Chen H, Cao B and
Wang YZ: The synthesis of ursolic acid derivatives with cytotoxic
activity and the investigation of their preliminary mechanism of
action. Bioorg Med Chem. 17:848–854. 2009. View Article : Google Scholar
|
|
23
|
Huang HC, Huang CY, Lin-Shiau SY and Lin
JK: Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma
invasion through suppressing the association ZIP/p62 with PKC-zeta
and downregulating the MMP-9 expression. Mol Carcinog. 48:517–531.
2009. View
Article : Google Scholar
|
|
24
|
Molenaar JJ, Ebus ME, Koster J, van Sluis
P, van Noesel CJ, Versteeg R and Caron HN: Cyclin D1 and CDK4
activity contribute to the undifferentiated phenotype in
neuroblastoma. Cancer Res. 68:2599–2609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takai M, Terai Y, Kawaguchi H, Ashihara K,
Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M,
et al: The EMT (epithelial-mesenchymal-transition) related protein
expression indicates the metastatic status and prognosis in
patients with ovarian cancer. J Ovarian Res. 27:7:762014.
View Article : Google Scholar
|
|
26
|
Tomaskovic-Crook E, Thompson EW and Thiery
JP: Epithelial to mesenchymal transition and breast cancer. Breast
Cancer Res. 11:2132009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiu WT, Huang YF, Tsai HY, Chen CC, Chang
CH, Huang SC, Hsu KF and Chou CY: FOXM1 confers to
epithelial-mesenchymal transition, stemness and chemoresistance in
epithelial ovarian carcinoma cells. Oncotarget. 6:2349–2365. 2015.
View Article : Google Scholar :
|
|
28
|
Shan JZ, Xuan YY, Ruan SQ and Sun M:
Proliferation-inhibiting and apoptosis-inducing effects of ursolic
acid and oleanolic acid on multi-drug resistance cancer cells in
vitro. Chin J Integr Med. 17:607–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kyrgiou M, Salanti G, Pavlidis N,
Paraskevaidis E and Ioannidis JP: Survival benefits with diverse
chemotherapy regimens for ovarian cancer: Meta-analysis of multiple
treatments. J Natl Cancer Inst. 98:1655–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itamochi H: Targeted therapies in
epithelial ovarian cancer: Molecular mechanisms of action. World J
Biol Chem. 1:209–220. 2010. View Article : Google Scholar
|