Introduction
Worldwide, breast cancer is the most common
malignancy in women and also the leading cause of cancer mortality.
Resection of tumors with or without adjunctive chemotherapy is the
only00000 known strategy for long-term survival. More than 50% of
breast cancer cases are either intrinsically resistant or rapidly
acquire resistance to various anticancer drugs (1). Currently, drug resistance or drug
inefficacy are major obstacles in the successful treatment of
breast cancer (2). New
chemotherapeutic strategies are therefore needed.
Ferritin is a protein that binds to and stores iron.
It is composed of two subunits, the ferritin light chain (FTL,
L-subunit, 19 kDa) and the ferritin heavy chain (FTH, H-subunit 21
kDa) (3,4). Ferritin serves as a critical component
of iron homeostasis and requires the participation of the two
subunits (5–8). The H-subunit has ferroxidase activity,
it is essential and sufficient for rapid iron uptake (9), while the L-subunit facilitates stable
iron storage in the ferritin core (5). Recent findings have shown that
ferritin is involved in cell proliferation, angiogenesis,
immunosuppression, iron delivery and iron storage (10–13).
Elevated serum and tissue ferritin are associated with cancer
(14) and may contribute to cancer
cell survival and drug resistance.
Ferritin is detected in the serum of cancer
patients, with higher levels correlating with more aggressive
disease and poorer clinical outcomes. It is differentially
overexpressed in several malignancies including breast cancer,
liver cancer, lymphoma, and pancreatic cancer (15). In breast tumor tissues, L-ferritin
levels are ~6-fold higher than surrounding benign breast tissue
(10,16,17).
This increase correlates with greater epithelial cell
proliferation, histopathological dedifferentiation, shorter
survival rate and chemotherapeutic resistance (18). Alkhateeb et al have shown
that ferritin stimulates proliferation of the MCF-7 and T47D breast
cancer cell lines (10).
The source of serum ferritin remains to be
determined. Previously conducted studies have indicated that
ferritin is generated and secreted from tumor-associated
macrophages (TAMs) (10,16). The high levels of ferritin in TAMs
may protect them from iron-induced damage, stimulate survival,
proliferation, and angiogenesis (11). Moreover, the secretion of ferritin
from TAMs may have a direct role in promoting and maintaining tumor
proliferation.
It has recently been shown that the repression of
ferritin by siRNA increases the chemosensitivity of HeLa cells
(11) and human glioma (19). Of note, the downregulation of
ferritin by miR-200b is also associated with an increased
sensitivity of the MDA-MB-231 breast cancer cell line to
doxorubicin (20). These results
suggested that ferritin may be an attractive target for cancer
treatment because its suppression may induce cancer cell death and
increase the efficacy of chemotherapy.
However, to the best of our knowledge, there have
been few reports on the effects of ferritin in breast cancer cells.
In the present study, we investigated whether ferritin confers
protection against chemotherapeutic drugs in the MCF-7 breast
cancer cell line. We hypothesized that the inhibition of ferritin
was able to sensitize MCF-7 cells to doxorubicin and may be a
viable strategy for improving the efficacy of other anticancer
drugs against breast cancer.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) and other cell culture ingredients were
purchased from Life Technologies (Grand Island, NY, USA). PCR array
ingredients were supplied by SABiosciences (Frederick, MD, USA).
Human liver ferritin was obtained from EMD (Darmstadt, Germany) and
doxorubicin from Sigma Co. (St. Louis, MO, USA).
Cell viability assay
The human breast cancer cell line MCF-7 was obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and maintained according to the manufacturer's instructions at
37°C and 5% CO2 in DMEM (Gibco, Grand Island,
NY, USA). DMEM was supplemented with 10% FBS and renewed every 3
days, trypsinized with 0.05% trypsin-EDTA and subcultured in the
same medium.
The effects of human liver ferritin and doxorubicin
on the MCF-7 cell line were then determined. Briefly, MCF-7 cells
were seeded into 96-well culture plates at a density of
2.5×104 cells/well for 24 h, and cells were starved with
FBS-free medium for 24 h. Non-complete fresh media containing test
compound, human liver ferritin and doxorubicin were then incubated
at the indicated times. Cytotoxicity was measured using the
CellTiter 96 AQueous Cell Proliferation Assay kit (MTS; Promega,
Madison, WI, USA) and MTT Cell Proliferation kit I (Roche Molecular
Biochemicals, Mannheim, Germany) according to the manufacturer's
instructions.
Transient transfection of FTL and/or FTH
small-interfering RNA
Pre-designed FTL siRNA (siGENOME SMARTpool siRNA no.
E-016214-00-0005) and control siRNA (siGENOME non-targeting siRNA
no. D-001210-02-20) were purchased from Thermo Scientific (Waltham,
MA, USA). FTH siRNA (sc-40575) was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
The cells were grown in a 6-well plate to a
confluence of 70%. For each well, 100 nM of FTL siRNA and 400 nM of
FTH siRNA were mixed with 2 µl of Lipofectamine 2000 and 1
ml of OptiMEM® medium was added. The cells were exposed
to the transfection mixture for 4 h. At the end of the incubation
period, the medium was discarded, 2 ml of antibiotic-free DMEM
complete medium was added, and the cells were cultured for an
additional 20 h. The siGENOME non-targeting siRNA (D-001210-02-05;
Dharmacon) was used as a negative control, and was introduced to
the cells using the same protocol. Total RNA was extracted from
MCF-7 cells after transfection. Efficiency of the transient
transfection was determined by reverse transcription-polymerase
chain reaction (RT-PCR) using TaqMan® primers.
To assess the cytotoxicity of doxorubicin against
the FTL and FTH knockdown of MCF-7 cells, MCF-7 cells
(2.5×104 cells/well) were grown in a 96-well plate for
24 h. For each well, cells transfected with 3 nM of FTL siRNA and
12 nM of FTH siRNA reagent were mixed with 0.06 µl of
Lipofectamine 2000 in OptiMEM® medium. The cells were
kept in the transfection mixture for 4 h. Subsequently, 100
µl of antibiotic-free DMEM complete medium were added and
the cells were maintained for an additional 20 h. The cells were
treated with 1 µM doxorubicin for an addtional 24 h prior to
performing the cell viability assay as described above.
Intracellular reactive oxygen species
formation
Intra cellular reactive oxygen species (ROS)
generation was measured using the cell-permeable fluorescent probe,
5-(and-6)-chloro-methyl-2′,7′-dichlorodihydrofluorescein diacetate,
acetyl ester (CM-H2DCFDA). MCF-7 cells
(2.5×104 cells/well) were cultured in 96-well plates for
24 h. Treatment with human liver ferritin and doxorubicin was
performed, alone and in combination with 10 µM
CM-H2DCFDA for 90 min at 37°C in the dark.
DCF fluorescence was measured at 488 (excitation) and 520 nm
(emission) on a fluorescence microplate reader. The data were
presented as the percentage of ROS relative to the untreated
controls.
Reverse transcription-polymerase chain
reaction
The MCF-7 cells (1.5×106 cells/well) were
seeded in 6-well plates and allowed to grow for 24 h. Subsequently,
the cells were treated with the test compounds and RNA was isolated
using the RNeasy Mini kit according to the manufacturer's
instructions. Recovered RNA was quantified by its absorbance at 260
nm. cDNA was prepared by reverse transcription of isolated RNA
using the RT2 First Strand kit. The reverse
transcription products served as a template for RT-PCR. RT-PCR was
carried out according to the TaqMan® manufacturer's
instructions (code number for FTL: Hs00830226_gH, FTH:
Hs01060665_g1, and β-actin: Hs01694011_s1, internal control). The
expression of each gene was monitored using an ABI 7900 (Applied
Biosystems, Bedford, MA, USA). Gene expression difference levels
were calculated using the 2−ΔΔCt method for relative
quantification and expressed as the fold change relative to the
untreated control. Data were normalized to the expression of
messenger RNA for β-actin, included on the same polymerase chain
reaction array plate with targeted genes.
Western blot analysis
Western blot analysis was used to determine the
expression levels of FTL, p21 and the internal control β-actin.
MCF-7 cells were cultured in 6-well plates and treated with human
liver ferritin, doxorubicin or a combination of the two test
compounds at the indicated times. The cultured cells were washed
with PBS, lysed with RIPA buffer (Sigma Co.) and protease inhibitor
cocktail (1:100, M221; Amresco, Solon, OH, USA) at 4°C
for 30 min prior to being transferred to a microcentrifuge tube.
After vigorous vortex mixing, the suspension was centrifuged at
12,000 × g for 30 min and the supernatant was collected and stored
at −70°C until use. Total protein concentration was
determined using Pierce BCA Protein Assay kit (Thermo
Scientific).
The protein samples were mixed with SDS loading
buffer and subjected to separation by electrophoresis in 4–20%
Criterion polyacrylamide Tris-HCl gel (Bio-Rad, Hercules, CA, USA).
The bands were blotted onto a nitrocellulose membrane. The
membranes were blocked for 1 h at room temperature with 5% (w/v)
skimmed milk powder in Tris-buffered saline (TBS) containing 0.1%
Tween-20. The membranes were then incubated overnight at 4°C with
primary antibodies of rabbit polyclonal anti-human FTL (1:1,000,
ab69096), rabbit polyclonal anti-human p21Cip/WAF1 (1:500, ab7960),
and rabbit polyclonal anti-human β-actin (1:2,500, ab8227) (all
from Abcam, Cambridge, USA) in TBS. After washing with TBS, the
blots were incubated for 1 h at room temperature with the
HRP-conjugated secondary antibodies (anti-rabbit IgG-HRP, 1:5,000,
ab97051; Abcam). After removal of the secondary antibody and TBS
buffer washes, the blots were incubated in chemiluminescent (ECL)
system (Perkin Elmer, Waltham, MA, USA). The densities of the
specific protein bands were visualized and captured by Multi Gauge
software (V3.0; FujiFilm Systems Medical Systems, Stamford, CT,
USA).
Statistical analysis
Data are presnted as mean ± SEM of duplicate assays
from three independent experiments. An analysis of variance with
repeated measurement was used to determine significant differences
between each experimental group. The level of significance was set
at P<0.05.
Results
Ferritin and doxorubicin effects on MCF-7
cell viability
To assess the proliferative roles of ferritin with
or without doxorubicin in MCF-7 cells, the cells were exposed to
ferritin and doxorubicin and assessed for viability by MTS and MTT.
Doxorubicin reduced cell viability in a dose-dependent manner with
IC50 values of 3.43±0.13 µM at 24 h (Fig. 1A). Ferritin, by contrast, induced
cell proliferation with maximal induction observed after 24–48 h
(Fig. 1B). When MCF-7 cells were
treated with ferritin (25 and 50 nM) for 24 h the cytotoxic effect
of doxorubicin was significantly decreased (Fig. 1C).
Ferritin and doxorubicin effects on FTL
expression in MCF-7 cells
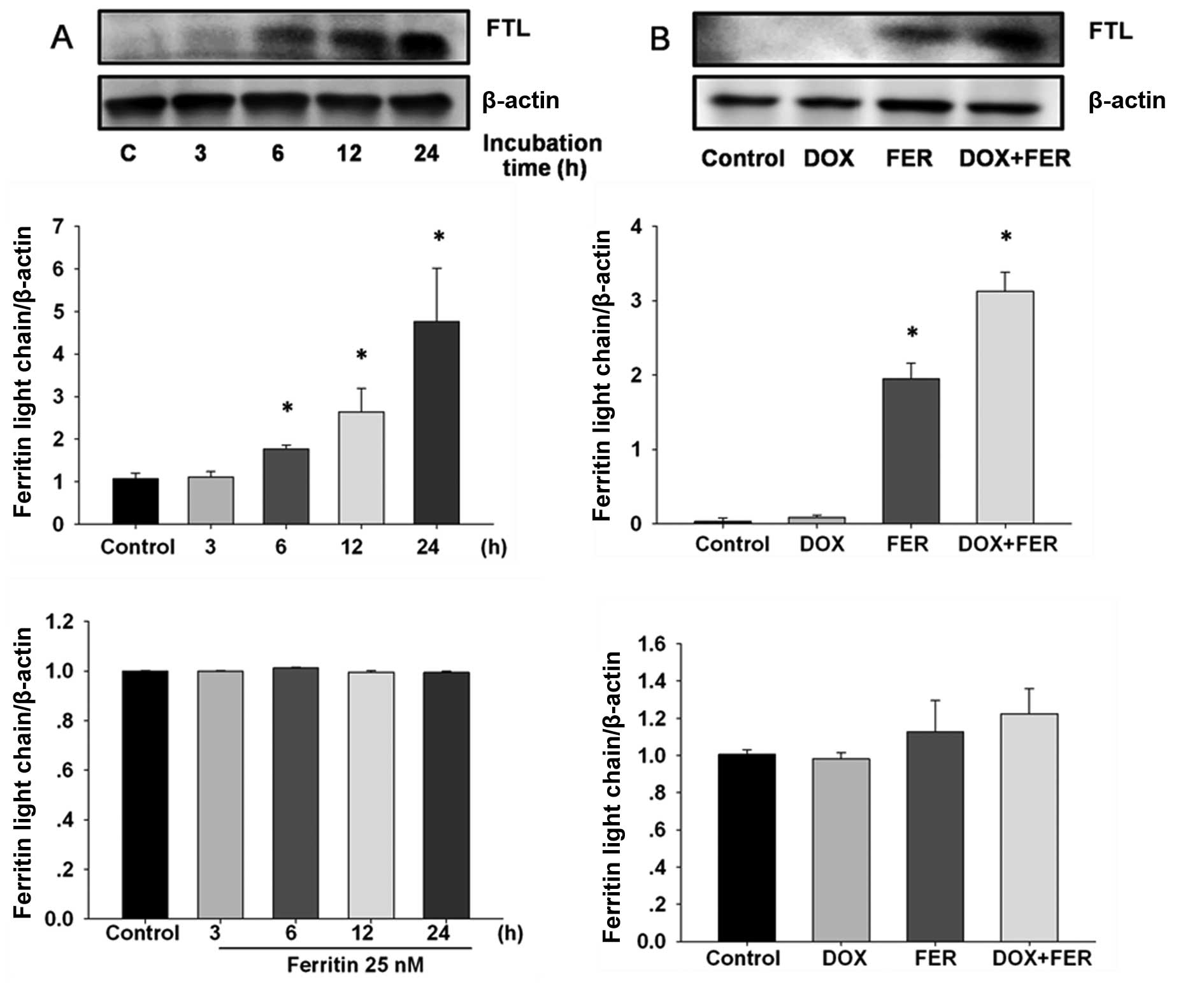
To establish whether ferritin and doxorubicin was
capable of inducing FTL expression in MCF-7, cells were incubated
with 25 nM ferritin and 1 µM doxorubicin and the time-course
of FTL expression was determined by western blotting and RT-PCR.
Ferritin-treated MCF-7 cells showed rapidly elevated FTL protein
within 6 h compared with the control, although FTL expression was
unaltered (Fig. 2A). The combined
treatment significantly increased levels of FTL protein compared to
the control and ferritin treatment alone (Fig. 2B). FTL mRNA expression was unaltered
by ferritin in the presence or absence of doxorubicin.
FTL and FTH gene silencing sensitizes
MCF-7 cells to doxorubicin
To confirm that FTL and FTH inhibition induced the
sensitization of MCF-7 cells to anticancer agents, we examined the
effects of FTL and FTH gene silencing on doxorubicin sensitivity.
Basal levels of FTL and FTH were determined by RT-PCR. FTL mRNA
expression was found to be higher than that of FTH (Fig. 3A). FTL and FTH mRNA significantly
decreased 24 h after transfection of siRNA, respectively (Fig. 3B and C). At 24 h after siRNA
treatment, MCF-7 cells were exposed to 1 µM doxorubicin for an
additional 24 h. The results showed that concurrent inhibition of
FTL with FTH increased cytotoxicity in the MCF-7 cells. Therefore,
inhibition of FTL and FTH sensitizes MCF-7 cells to
doxorubicin.
Ferritin and doxorubicin effects on ROS
formation in MCF-7 cells
To establish the mechanism of action by which
ferritin sensitizes MCF-7 cells to doxorubicin, we monitored the
intracellular accumulation of ROS using the
H2DCFDA-enhanced chemiluminescence method. Doxorubicin-
and ferritin-treated cells showed significantly increased ROS
production (Fig. 4A and B), which
were particularly high for the doxorubicin-treated groups.
Doxorubicin induced intracellular ROS production in MCF-7 cells in
a dose-dependent manner. For the combined treatment, ferritin
abolished the ROS formation that had been induced by doxorubicin
(Fig. 4C).
Ferritin and doxorubicin alter
p21Cip/WAF1 protein in MCF-7 cells
To establish whether combined doxorubicin and
ferritin treatment mediated the cell cycle pathway, p21Cip/WAF1 was
quantified. This is a protein related to the cyclin-dependent
kinase inhibitor 1 or CDK-interacting protein 1 for cell
proliferation inhibition. The combined drug treatment reduced the
levels of the p21Cip/WAF1 protein compared to the doxorubicin
treatment alone (Fig. 5). Treatment
with ferritin did not only induce p21Cip/WAF1 protein but slightly
reduced the protein expression compared to the control. The
combination of ferritin and doxorubicin caused a reduction in
p21Cip/WAF1 and was associated with a marked proliferative effect
in MCF-7 cells.
Discussion
Ferritin is a major iron storage protein essential
to iron homeostasis. Ferritin plays a key protective role against
oxidative stress due to its ability to sequester iron (18), and can protect normal and cancer
cells. In cancer patients, elevated levels of ferritin are detected
in the serum, and very high levels correlate with aggressive
disease and poor clinical outcome (18). In this study, we investigated how
ferritin affects cell proliferation and cell death in the
doxorubicin-treated cells of the MCF-7 breast cancer line. Our
results show that ferritin plays a critical role in breast cancer
cell proliferation and extracellular ferritin protects cancer cells
against doxorubicin. Additionally, inhibition of ferritin by FTL
and FTH siRNA sensitizes MCF-7 cells to anticancer agents.
Inhibition of doxorubicin-mediated cell death occurs probably due
to a reduction in intracellular ROS formation, leading to reduced
p21, a potent cyclin-dependent kinase inhibitor.
Ferritin is overexpressed in many malignancies
including hepatocellular carcinoma (19), Hodgkin's lymphoma (15), pancreatic cancer (3), and breast cancer (10,17).
Serum ferritin is elevated in breast cancer patients and surgical
resection of tumors lowers serum ferritin levels by ~50% (6). However, the source of serum ferritin
elevation has not been firmly established. Previous in vivo
studies have argued that serum ferritin is primarily derived from
macrophages and not hepatocytes (10,12,21).
Higher levels of serum ferritin also induce cancer cell
proliferation and resistance to anticancer drug treatment. Ferritin
significantly stimulated the proliferation of the T47D and MCF-7
breast cancer cell lines (10).
This proliferative effect was iron-independent of ferritin. In our
study, human liver ferritin activated the breast cancer cells in a
dose- and time-dependent manner. Ferritin also reduced the
sensitivity of breast cancer cells to doxorubicin in a
dose-dependent manner, consistent with findings showing that
increased serum ferritin was associated with poorer outcome in
breast cancer patients undergoing chemotherapy (10).
Mounting evidence has shown the proliferative
effects of ferritin in many types of cancer including breast cancer
(10) and HeLa cancer cells
(11). Furthermore, FTL was found
to be an independent predictor for breast cancer and present in
TAMs (16). In another study,
overexpression of L-ferritin, but not H-ferritin, increased
proliferation in HeLa cells without affecting intracellular iron
levels (11). Ferritin was absorbed
by breast cancer cells in a temperature-dependent manner indicating
a direct interaction. The proliferative effect of ferritin is
apparently independent of iron because apoferritin (iron-poor
ferritin) has similar effects to holoferritin (iron-rich ferritin)
(10). Our results show that human
liver ferritin is absorbed by MCF-7 cells in a time-dependent
manner. The results also show that doxorubicin significantly
induces human liver ferritin in MCF-7, but that ferritin and
doxorubicin do not alter FTL expression in breast cancer cells.
L-ferritin complexes can directly interact with
breast cancer cells and stimulate proliferation independently of
iron. The proliferative effects of ferritin remain to be
elucidated. One study has shown that addition of L-ferritin
complexes to primary rat hepatic stellate cells leads to an
increase in the phosphorylation of IKK α/β and subsequent
activation of NF-κB transcription factor (22). This may activate cell proliferation.
TAMs are rich in ferritin and this has led to the hypothesis
regarding the contribution of ferritin in cancer biology (16). An elevated expression of ferritin in
TAMs may protect them from iron-induced damage and stimulate
proliferation (11). For example,
L-ferritin levels are 6-fold higher in breast cancer compared to
benign breast tissue (10,16). This increase correlated with
proliferation, histopathological dedifferentiation and shorter
survival. This mechanism may promote cell proliferation and tumor
growth. The elevation in serum ferritin is partly due to localized
release from TAMs and directly activated cancer cell
proliferation.
In the present study, we investigated how ferritin
inhibition induces the sensitization of MCF-7 cells to doxorubicin.
MCF-7 cells were treated with FTL and FTH siRNA. Of note is that
ferritin plays an important role in cancer cell viability
especially under severe stress conditions. Due to its antioxidant
activity, ferritin has a protective effect against oxidative
stress. Our study reveals that FTL and FTH silencing significantly
sensitized breast cancer cells to doxorubicin. Inhibition of FTL
and FTH by siRNA may result in a significant increase in ROS
formation and directly lead to cancer cell death. Doxorubicin
treatment alone highly increases ROS levels in MCF-7 cells, while
ferritin alone weakly stimulates ROS formation. In the combined
treatment, ferritin reduced ROS production by doxorubicin
suggesting ferritin may sequester iron that is in released in a
particular manner by doxorubicin treatment.
It has been suggested that doxorubicin-induced
apoptosis occurs due to the formation of ROS derived from redox
activation of doxorubicin. As ferritin acts as an antioxidant, it
may decrease the efficacy of doxorubicin as well as other
chemotherapeutic drugs (alkylating agents and anthracyclines) whose
cytotoxicity is attributable to the production of ROS and the
induction of oxidative stress (22). Notably, ROS is capable of inducing
cell death via p53-dependent and -indepdent mechanisms (9,23). The
results of our study have shown that ferritin reduced the levels of
p21, a p53-dependent downstream gene product, and a potent
cyclin-dependent kinase inhibitor. Treatment with doxorubicin alone
significantly induced p21 expression, while ferritin weakly reduced
p21 protein. This is consistent with the strong protective effect
ferritin confers on MCF-7 cells to doxorubicin treatment. The
mechanism by which ferritin inhibition sensitizes cancer cells to
doxorubicin merits further investigation.
In conclusion, ferritin plays an important role in
cell proliferation in breast cancer cells. We have demonstrated
that ferritin exhibits proliferative activity in MCF-7 cells by
promoting cell growth, inhibiting doxorubicin-induced ROS
formation, and suppressing the p21Cip/WAF1 expression. A high level
ferritin expression has been previously reported in the serum of
breast cancer patients. Taken together, these findings suggest that
the targeted suppression of ferritin may be a useful strategy to
overcome drug resistance in breast cancer chemotherapy. Therefore,
ferritin is a potentially useful prognostic factor and candidate
drug target for the treatment of breast cancer patients in the
future.
Acknowledgments
This study was supported by the Mahasarakham
University Development Fund and the National Institutes of Health
grant (JC). The authors would like to thank Dr Tim Cushnie, Faculty
of Medicine, Mahasarakham University, for editing the
manuscript.
References
|
1
|
O'Driscoll L and Clynes M: Biomarkers and
multiple drug resistance in breast cancer. Curr Cancer Drug
Targets. 6:365–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coley HM: Mechanisms and strategies to
overcome chemotherapy resistance in metastatic breast cancer.
Cancer Treat Rev. 34:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arosio P, Adelman TG and Drysdale JW: On
ferritin heterogeneity. Further evidence for heteropolymers. J Biol
Chem. 253:4451–4458. 1978.PubMed/NCBI
|
|
4
|
Boyd D, Vecoli C, Belcher DM, Jain SK and
Drysdale JW: Structural and functional relationships of human
ferritin H and L chains deduced from cDNA clones. J Biol Chem.
260:11755–11761. 1985.PubMed/NCBI
|
|
5
|
Levi S, Yewdall SJ, Harrison PM,
Santambrogio P, Cozzi A, Rovida E, Albertini A and Arosio P:
Evidence of H- and L-chains have co-operative roles in the
iron-uptake mechanism of human ferritin. Biochem J. 288:591–596.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cozzi A, Corsi B, Levi S, Santambrogio P,
Albertini A and Arosio P: Overexpression of wild type and mutated
human ferritin H-chain in HeLa cells: In vivo role of ferritin
ferroxidase activity. J Biol Chem. 275:25122–25129. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macip S, Igarashi M, Berggren P, Yu J, Lee
SW and Aaronson SA: Influence of induced reactive oxygen species in
p53-mediated cell fate decisions. Mol Cell Biol. 23:8576–8585.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wade VJ, Levi S, Arosio P, Treffry A,
Harrison PM and Mann S: Influence of site-directed modifications on
the formation of iron cores in ferritin. J Mol Biol. 221:1443–1452.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lawson DM, Treffry A, Artymiuk PJ,
Harrison PM, Yewdall SJ, Luzzago A, Cesareni G, Levi S and Arosio
P: Identification of the ferroxidase centre in ferritin. FEBS Lett.
254:207–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alkhateeb AA, Han B and Connor JR:
Ferritin stimulates breast cancer cells through an iron-independent
mechanism and is localized within tumor-associated macrophages.
Breast Cancer Res Treat. 137:733–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cozzi A, Corsi B, Levi S, Santambrogio P,
Biasiotto G and Arosio P: Analysis of the biologic functions of H-
and L-ferritins in HeLa cells by transfection with siRNAs and
cDNAs: Evidence for a proliferative role of L-ferritin. Blood.
103:2377–2383. 2004. View Article : Google Scholar
|
|
12
|
Ferring-Appel D, Hentze MW and Galy B:
Cell-autonomous and systemic context-dependent functions of iron
regulatory protein 2 in mammalian iron metabolism. Blood.
113:679–687. 2009. View Article : Google Scholar
|
|
13
|
Jézéquel P, Campion L, Spyratos F,
Loussouarn D, Campone M, Guérin-Charbonnel C, Joalland MP, André J,
Descotes F, Grenot C, et al: Validation of tumor-associated
macrophage ferritin light chain as a prognostic biomarker in
node-negative breast cancer tumors: A multicentric 2004 national
PHRC study. Int J Cancer. 131:426–437. 2012. View Article : Google Scholar
|
|
14
|
Levi S, Luzzago A, Cesareni G, Cozzi A,
Franceschinelli F, Albertini A and Arosio P: Mechanism of ferritin
iron uptake: Activity of the H-chain and deletion mapping of the
ferro-oxidase site. A study of iron uptake and ferro-oxidase
activity of human liver, recombinant H-chain ferritins, and of two
H-chain deletion mutants. J Biol Chem. 263:18086–18092.
1988.PubMed/NCBI
|
|
15
|
Alkhateeb AA and Connor JR: The
significance of ferritin in cancer: Anti-oxidation, inflammation
and tumorigenesis. Biochim Biophys Acta. 1836:245–254.
2013.PubMed/NCBI
|
|
16
|
Fargion S, Fracanzani AL, Brando B, Arosio
P, Levi S and Fiorelli G: Specific binding sites for H-ferritin on
human lymphocytes: Modulation during cellular proliferation and
potential implication in cell growth control. Blood. 78:1056–1061.
1991.PubMed/NCBI
|
|
17
|
Cohen LA, Gutierrez L, Weiss A,
Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern
A, Galy B, Hentze MW, et al: Serum ferritin is derived primarily
from macrophages through a nonclassical secretory pathway. Blood.
116:1574–1584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li JY, Paragas N, Ned RM, Qiu A, Viltard
M, Leete T, Drexler IR, Chen X, Sanna-Cherchi S, Mohammed F, et al:
Scara5 is a ferritin receptor mediating non-transferrin iron
delivery. Dev Cell. 16:35–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Madhankumar AB, Slagle-Webb B,
Sheehan JM, Surguladze N and Connor JR: Heavy chain ferritin siRNA
delivered by cationic liposomes increases sensitivity of cancer
cells to chemotherapeutic agents. Cancer Res. 71:2240–2249. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shpyleva SI, Tryndyak VP, Kovalchuk O,
Starlard-Davenport A, Chekhun VF, Beland FA and Pogribny IP: Role
of ferritin alterations in human breast cancer cells. Breast Cancer
Res Treat. 126:63–71. 2011. View Article : Google Scholar
|
|
21
|
Eshhar Z, Order SE and Katz DH: Ferritin,
a Hodgkin's disease associated antigen. Proc Natl Acad Sci USA.
71:3956–3960. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keizer HG, Pinedo HM, Schuurhuis GJ and
Joenje H: Doxorubicin (adriamycin): A critical review of free
radical-dependent mechanisms of cytotoxicity. Pharmacol Ther.
47:219–231. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knovich MA, Storey JA, Coffman LG, Torti
SV and Torti FM: Ferritin for the clinician. Blood Rev. 23:95–104.
2009. View Article : Google Scholar :
|