Introduction
Cancers of the oral cavity are common tumors in male
and female patients with an estimated incidence of 264,000 and
128,000 mortalities worldwide in 2008 (1). Oral squamous cell carcinoma (OSCC)
constitutes ~90% of oral malignancies (2). Chronic alcohol abuse, tobacco use and
HPV infection are the most important risk factors for the
development of OSCC. Smoking and alcohol synergistically contribute
to the disease (1). Therefore, OSCC
occurs most frequently in middle-aged to elderly patients who smoke
and drink heavily. However, the incidence rates of OSCC in young
adults in US and some European countries are on the increase, which
is possibly caused by HPV infection, which may partly be attributed
to oral sexual behavior (3).
NIN1/RPN12 binding protein 1 homolog (NOB1) was
first identified in Saccharomyces cerevisiae by two-hybrid
screening. NOB1 encodes an essential protein Nin one binding
protein (NOB1p) in growing cells (4). As a nucleoprotein, NOB1p forms a
complex with the 19S regulatory particle in the nucleus by binding
at NIN1/RPN12, a subunit of the 19S regulatory particle of the
yeast 26S proteasome and facilitates the maturation of the 20S
proteasome and is then degraded by 26S proteasome to complete 26S
proteasome biogenesis (5). NOB1p
was also found to accompany the pre-40S ribosomes during nuclear
export and be cleaves at the D-site of 20S pre-rRNA to form mature
18S rRNA (6–8). The human NOB1 gene is localized
on chromosome 16q22.1 and expresses an ~50 kDa protein NOB1. NOB1
is expressed mainly in the liver, lung and spleen in human and is
mainly localized in the nucleus (9,10).
The function in ribosome assembly and proteasome
biogenesis of NOB1 suggest that NOB1 may be associated with protein
homeostasis and may play important roles in mediating certain
physiological and pathological functions. Specifically, ribosome
assembly and ubiquitin-proteasome pathway were involved in certain
types of cancer (11–13). Dysfunction of the NOB1 gene
was reported to contribute to certain human cancers. Upregulation
of NOB1 was first identified in esophageal squamous cell carcinoma
(14). The aberrant expression of
NOB1 was also found in breast-infiltrating ductal carcinoma and was
possibly involved in tumorigenesis and development (15). Expression of NOB1 mRNA and protein
in papillary thyroid carcinoma tissue was significantly higher than
in normal and benign thyroid tissue (16). There were also significant
associations between NOB1 expression and TNM stage, lymph node
metastasis and histopathological grade of non-small cell lung
cancer (NSCLC) and prostate carcinoma (17,18).
Knockdown of NOB1 decreased the proliferation of several types of
human cancer cells, including breast and ovarian cancer,
hepatocellular carcinoma, gliomas, osteosarcoma, NSCLC and colon
cancer cells (19–25). However, the roles of NOB1 in OSCC
have not been reported.
To determine the potential role of NOB1 in OSCC, an
immunohistochemical analysis of NOB1 protein in OSCC tumors was
performed. A loss-of-function analysis was then performed by
applying a NOB1 short hairpin RNA (shNOB1)-expressing lentivirus
(Lv-shNOB1) to two OSCC cell lines, CAL27 and TCA-8113. The effect
of NOB1 knockdown on OSCC cell proliferation, colony formation,
apoptosis and cell cycle progression was investigated.
Materials and methods
Cell lines, reagents and antibodies
Human OSCC CAL27 and TCA-8113 cell lines were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's
medium (DMEM) (CAL27) or RPMI-1640 medium (TCA-8113) containing 10%
fetal bovine serum, 100 µg/ml streptomycin and 100 IU/ml
penicillin at 37°C with 5% CO2 and 95% humidity. Rabbit
anti-NOB1 polyclonal antibody used for western blot analysis was
purchased from Novus Biologicals (Littleton, CO, USA). Anti-rabbit
IgG secondary antibody was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Immunohistochemical assay reagents were
purchased from Abcam (Boston, MA, USA) and Rockland
Immunochemicals, Inc. (Gilbertsville, PA, USA). Anti-GAPDH mAb was
purchased from Santa Cruz Biotechnology, Inc. The Annexin V-APC
apoptosis detection kit was purchased from eBioscience (San Diego,
CA, USA). Propidium iodide (PI) was purchased from Sigma-Aldrich
(St. Louis, MO, USA).
Immunohistochemical staining
Immunohistochemical analysis was first performed on
tissue chip. The chip used in the present study was no. OR601a,
which was purchased from US Biomax, Inc. (Rockville, MD, USA). The
patient information is available at the link: http://www.biomax.us/tissue-arrays/Oral_Cavity/OR601a
(Table I).
 | Table IPatient information of OSCC tissue
chip OR601a. |
Table I
Patient information of OSCC tissue
chip OR601a.
| No. | Gender | Age (years) | Organ | Pathology
diagnosis | Grade | Stage | TNM | Type |
|---|
| 1 | M | 78 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 2 | F | 51 | Tongue | Squamous cell
carcinoma | 1 | Iva | T4N0M0 | Malignant |
| 3 | F | 75 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 4 | M | 69 | Tongue | Squamous cell
carcinoma | 1 | III | T3N0M0 | Malignant |
| 5 | F | 56 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 6 | F | 35 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 7 | F | 39 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 8 | M | 64 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 9 | M | 63 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 10 | F | 77 | Tongue | Squamous cell
carcinoma | 2 | I | T1N0M0 | Malignant |
| 11 | F | 41 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 12 | M | 53 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 13 | M | 50 | Tongue | Squamous cell
carcinoma | 1 | III | T3N0M0 | Malignant |
| 14 | F | 36 | Tongue | Squamous cell
carcinoma | 2 | I | T1N0M0 | Malignant |
| 15 | M | 58 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 16 | F | 63 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 17 | F | 55 | Tongue | Squamous cell
carcinoma | 2 | II | T2N0M0 | Malignant |
| 18 | M | 76 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 19 | F | 50 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 20 | M | 44 | Tongue | Squamous cell
carcinoma | 1 | III | T2N1M0 | Malignant |
| 21 | F | 53 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 22 | F | 67 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 23 | M | 60 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 24 | M | 55 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 25 | M | 61 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 26 | M | 55 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 27 | M | 59 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 28 | F | 46 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 29 | F | 45 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 30 | M | 61 | Tongue | Squamous cell
carcinoma (fibrous tissue and blood vessel) | – | II | T2N0M0 | Malignant |
| 31 | F | 48 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 32 | F | 52 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 33 | M | 64 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 34 | F | 46 | Tongue | Squamous cell
carcinoma (sparse) | 1 | II | T2N0M0 | Malignant |
| 35 | F | 48 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 36 | M | 80 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 37 | M | 49 | Tongue | Squamous cell
carcinoma (fibrous tissue and skeletal muscle) | – | I | T1N0M0 | Malignant |
| 38 | M | 60 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 39 | M | 57 | Tongue | Squamous cell
carcinoma | 1 | I | T1N0M0 | Malignant |
| 40 | M | 45 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 41 | F | 47 | Tongue | Squamous cell
carcinoma | 1 | II | T2N0M0 | Malignant |
| 42 | M | 37 | Tongue | Squamous cell
carcinoma | 1 | III | T2N1M0 | Malignant |
| 43 | M | 60 | Tongue | Squamous cell
carcinoma | 2 | II | T2N0M0 | Malignant |
| 44 | F | 40 | Tongue | Squamous cell
carcinoma | 3 | II | T2N0M0 | Malignant |
| 45 | M | 49 | Tongue | Squamous cell
carcinoma | 1–2 | I | T1N0M0 | Malignant |
| 46 | M | 50 | Tongue | Squamous cell
carcinoma | 3 | II | T2N0M0 | Malignant |
| 47 | M | 60 | Tongue | Squamous cell
carcinoma | 3 | I | T1N0M0 | Malignant |
| 48 | F | 56 | Tongue | Squamous cell
carcinoma | 3 | II | T2N0M0 | Malignant |
| 49 | M | 77 | Tongue | Squamous cell
carcinoma | 3 | II | T2N0M0 | Malignant |
| 50 | M | 56 | Tongue | Squamous cell
carcinoma | 2 | III | T2N1M0 | Malignant |
| 51 | M | 76 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 51 | M | 76 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 52 | M | 38 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 53 | F | 51 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 54 | M | 30 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 55 | M | 50 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 56 | M | 2 months | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 57 | M | 49 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 58 | M | 40 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 59 | M | 25 | Tongue | Cancer adjacent
normal tongue tissue | – | – | – | NAT |
| 60 | M | 62 | Tongue | Cancer adjacent
normal mucous membrane tissue of pars palatalis | – | – | – | NAT |
| 61 | M | 42 | Adrenal gland | Pheochromocytoma
(tissue marker) | – | | | Malignant |
Freshly prepared immunohistochemical staining was
carried out. Briefly, tissue sections (5-µm) were dewaxed,
followed by quenching the endogenous peroxidase with 3%
H2O2 in methanol for 30 min. Prior to
staining, non-specific binding was blocked by incubation with 10%
BSA in phosphate-buffered saline (PBS) at 37°C for 1 h. Tissue
sections were incubated with specific antibodies in PBS containing
1% BSA at 4°C overnight, followed by incubation with a horseradish
peroxidase-conjugated anti-mouse or rabbit antibody. Color was then
developed by incubation with an ImmunoPure Metal Enhanced
Diaminobenzidine (DAB) Substrate kit (Pierce, Rockford, IL, USA).
After each incubation, the tissue sections were washed three times
with PBS for 10 min, and then counterstained with hematoxylin. For
determination of NOB1, cytosolic and nuclear staining of yellowish
or brownish granules was graded as: 0 for background, 1 for faint,
2 for moderate and 3 for strong staining. In addition, positive
staining areas in the entire tissue section were graded as: 0 for
<5%, 1 for 5–25%, 2 for 26–50%, 3 for 51–75% and 4 for 76–100%.
Combining these two parameters, 0–2 and ≥3 were considered negative
and positive stainings, respectively.
Lentivirus-mediated shRNA delivery
Sequences of NOB1 shRNA were inserted into the
pGCSIL-GFP lentivirus RNAi expression system (GeneChem, Shanghai,
China). The shRNA-containing vectors were co-transfected together
into 293T cells with pHelper1.0 and lentiviral helper plasmid
pHelper2.0 using Lipofectamine 2000 (Invitrogen-Life Technologies,
Carlsbad, CA, USA) to generate the respective lentiviruses. Viral
stocks collected from 293T cells were used to infect CAL27 and
TCA-8113 cells three days after infection. The target sequence of
NOB1 siRNA was: GGTTAAGGTGAGCTCATCG. The negative control scramble
sequence of siRNA was: TTCTCCGAACGTGTCACGT, which does not target
any genes in humans, mice or rats as determined by screening with
NCBI RefSeq. The stem-loop-stem oligos [short-hairpin RNAs
(shRNAs)] were produced, annealed and ligated into the
AgeI/EcoRI-linearized pGV112 vector (GeneChem). The
lentiviral-based shRNA-expressing vectors were confirmed by DNA
sequencing. The mRNA and protein levels were measured 72 h after
lentivirus infection.
RT-PCR analysis
Total RNA was isolated using TRIzol reagent
(Invitrogen-Life Technologies) and reverse transcribed using a
PrimeScript® RT reagent kit (Takara, Dalian, China).
cDNA was normalized using GAPDH. RT-PCR was performed by three-step
methods using a SYBR® Premix Ex Taq™ II kit
(Takara) with 55°C annealing temperature and 40 amplification
cycles. The individual test was carried out in triplicate. GAPDH
was used as an internal control. The relative amount of each cDNA
was analyzed by means of 2−ΔΔCt. Primers for qPCR for
NOB1 and GAPDH were as follows: NOB1-F, ATCTGCCCTACAAGCCTAAAC and
-R, TCCTCCTCCTCCTCCTCAC; and GAPDH-F, TGACTTCAACAGCGACACCCA and -R,
CACCCTGTTGCTGTAGCCAAA.
Western blotting
Protein samples prepared from CAL27 and TCA-8113
cells five days after NOB1 shRNA lentivirus or control shRNA
lentivirus infection were subjected to 10% SDS-PAGE (20 µg
protein each lane), transferred to PVDF membranes (Millipore,
Kankakee, IL, USA) and detected with rabbit anti-NOB1 or rabbit
anti-GAPDH antibodies followed by horseradish peroxidase-conjugated
goat anti-rabbit IgG. GAPDH was used as an internal control.
Proteins were then detected using an ECL kit (Amersham, Piscataway,
NJ, USA) and exposed to X-ray film. Bands on the X-ray film were
quantified with an ImageQuant densitometric scanner (Molecular
Dynamics, Sunnyvale, CA, USA).
Cell proliferation assay
Five days after lentivirus infection, CAL27 or
TCA-8113 cells were trypsinized, resuspended, seeded in a 96-well
plate with a density of 2×103 cells/well and incubated
at 37°C. The number of viable cells was measured at daily intervals
(days 1–5). At each time-point, 20 µl of 5 mg/ml MTT
(Dingguo Biotechnology, Beijing, China) was added and incubation
was continued for 4 h. At the end of the incubation period, the
medium was removed carefully and 150 µl of acidified
isopropanol (in 0.01 M HCl) was added. The plates were agitated and
the absorbance was measured at 490 nm on the spectrophotometer
Biotek EL×800 (Beijing, China). Each data point was collected from
five parallel wells.
Colony formation assay
The CAL27 and TCA-8113 cells were seeded in 6-well
plates (8×102 cells/well) (in three duplicate wells) and
cultured at 37°C in 5% CO2. After two weeks, the cells
were washed with PBS once and fixed with paraformaldehyde for 30
min and washed with PBS and stained with Giemsa for 20 min.
ddH2O was used to wash the cells three times to obtain a
clean background. The number of colonies and cell number in each
colony were counted and statistically analyzed.
Flow cytometric analysis
Apoptosis assay was carried out with Annexin V-APC
staining. Cells were harvested by centrifugation at 1,200 rpm for 5
min after six days of infection. The pellets were washed twice with
cold PBS, fixed with chilled 70% ethanol, centrifuged at 1,500 rpm
for 5 min to discard ethanol and resuspended with PBS sequentially.
Suspensions were filtrated through 400-mesh membrane and
centrifuged at 1,200 rpm for 5 min. The cells were resuspended with
1X Annexin V staining buffer and stained with Annexin V-APC at room
temperature for 15 min in the dark for the flow cytometric
analysis. Each experiment was carried out in triplicate.
The cell cycle distribution was analyzed with PI
staining. Briefly, 1.5×105 cells that were infected with
the lentivirus constructs for 4 days were seeded in 6-cm dishes and
cultured for 40 h at 37°C. The cells were harvested, washed with
PBS and fixed with 70% cold ethanol. The cells were then collected
by centrifugation, resuspended in PBS containing 100 µg/ml
of DNase-free RNase and 40 µg/ml PI, and incubated for 1 h
at 37°C. A total of 1.0×104 fixed cells were analyzed by
FACS (Becton-Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
One-way ANOVA and the Student's t-test were used for
raw data analysis. Statistical analysis was performed using SPSS
12.0 software package. Values in the text and figures are presented
as the mean ± SD. P<0.05 was considered to indicate a
statistically significant result.
Results
Expression of NOB1 in OSCC tissue
Immunohistochemical analysis was performed on tissue
chip with 50 OSCC cancer tissues and 10 cancer adjacent normal
tongue tissues from patients with medical records (no. OR601a; US
Biomax, Inc.). NOB1-positive stainings in different genders, ages,
TNM and pathological grades were calculated (Table II). NOB1 expression in OSCC cancers
had no statistical significance in patients with different genders
and ages. By contrast the NOB1-positive rate in TNM grades II, III
and IV (88.24%) was significantly higher than grade I (51.52%,
P=0.01). In addition, the NOB1-positive rate in pathological grades
II and III (84.21%) was significantly higher than grade I (51.61%,
P=0.026). The same result was observed in immunohistochemical
staining of OSCC tissue sections. As shown in Fig. 1, NOB1-positive staining increased
with the pathological grades of OSCC cancer. Thus, elevation of
NOB1 expression plays an important role in the pathogenesis of
human OSCC.
 | Table IIAssociation between NOB1 expression
and pathological parameters in tissue chip. |
Table II
Association between NOB1 expression
and pathological parameters in tissue chip.
| Parameters | N | NOB1
| χ2 | P-value |
|---|
| + | − | (%) |
|---|
| Gender | | | | | 0.148 | 0.7 |
| Male | 28 | 18 | 10 | 64.28 | | |
| Female | 22 | 14 | 8 | 63.63 | | |
| Age (years) | | | | | 2.002 | 0.157 |
| ≥50 | 34 | 24 | 10 | 70.59 | | |
| <50 | 16 | 8 | 8 | 50.00 | | |
| TNM | | | | | 6.566 | 0.01 |
| I | 33 | 17 | 16 | 51.52 | | |
| II, III, IV | 17 | 15 | 2 | 88.24 | | |
| Pathological
grades | | | | | 4.93 | 0.026 |
| I | 31 | 16 | 15 | 51.61 | | |
| II, III | 19 | 16 | 3 | 84.21 | | |
Lentivirus-mediated shRNA inhibited NOB1
mRNA and protein expression in CAL27 and TCA-8113 cells
Approximately half of intraoral squamous cell
carcinomas begin on the floor of the mouth or on the lateral and
ventral surfaces of the tongue (2).
Thus, to investigate the role of NOB1 in OSCC, a human OSCC cell
line, CAL27, and a human tongue squamous cell carcinoma cell line,
TCA-8113, were selected to study in vitro. A
loss-of-function study was carried out by lentivirus-mediated shRNA
knockdown of NOB1. The lentiviral vector system was constructed to
express a shRNA targeting NOB1 and GFP as a reporter gene. To
determine the infection efficiency of lentivirus of CAL27 and
TCA-8113 cells, at 30% cell density, the cells infected with
Lv-shNOB1 and Lv-shCon vectors were observed under a fluorescence
microscope after three days of infection. As shown in Fig. 2A, >90% of CAL27 and TCA-8113
expressed GFP, which indicated a high efficiency infection of the
lentivirus.
To verify the knockdown efficiency of Lv-shNOB1, the
mRNA and protein expression of NOB1 in CAL27 and TCA-8113 was
detected by qPCR and western blot analysis five days after
lentivirus infection. As shown in Fig.
2B, the mRNA expression levels of NOB1 in CAL27 and TCA-8113
infected by Lv-shNOB1 were downregulated by 80.8% (P<0.05) and
80.2% (P<0.05) compared to the Lv-shCon infection group,
respectively. As shown in Fig. 2C,
the protein expression of NOB1 in CAL27 and TCA-8113 cells infected
with Lv-shNOB1 was also significantly decreased compared to the
Lv-shCon infection group.
Knockdown of NOB1 significantly inhibited
OSCC cell proliferation
To investigate the effect of NOB1 knockdown on cell
proliferation, an MTT assay was performed in CAL27 and TCA-8113
cell lines five days after lentivirus infection. Data were
collected from five parallel wells in each group for 5 days
consecutively. As shown in Fig. 3,
lentivirus-mediated shRNA knockdown of NOB1 significantly inhibited
the proliferation of CAL27 (Fig.
3A) and TCA-8113 (Fig. 3B)
compared to cells infected with the control lentivirus. On day 5 of
the assay, Lv-shRNA infection inhibited the proliferation of CAL27
by 52.8% (P<0.01) and TCA-8113 by 67.9% (P<0.01). Compared to
the mock group (CAL27 and TCA-8113 cells without lentivirus
infection), the cell number in Lv-shCon was slightly decreased,
which indicated that lentivirus infection had a slight effect on
the proliferation of CAL27 and TCA-8113. The result indicated that
NOB1 played an important role in the proliferation of CAL27 and
TCA-8113 cells.
Knockdown of NOB1 significantly inhibited
the colony-forming ability of OSCC cells
To study the long-term effect of NOB1 shRNA
lentivirus on the cell growth, colony-forming experiments were
performed on CAL27 and TCA-8113 cell lines. After 72 h of
lentivirus infection, the cells were allowed to grow for 11 days
with media replacement every two days to form colonies. As shown in
Fig. 4A upper left, the number of
colonies in Lv-shNOB1-treated CAL27 were significantly less than
that in the Lv-shCon-treated group. As shown in Fig. 4A upper right, the colony number was
48±3 in Lv-shNOB1 compared to 144±7 in the Lv-shCon group
(P<0.01). The sizes of colonies in Lv-shNOB1 were significantly
smaller than that in the Lv-shCon group (as shown in Fig. 4A lower left). As shown in Fig. 4B upper left, the number of colonies
in Lv-shNOB1-treated TCA-8113 were significantly less than that in
the Lv-shCon-treated group. As shown in Fig. 4B upper right, the colony number was
5±2 in Lv-shNOB1 compared to 206±6 in the Lv-shCon group
(P<0.01). The sizes of the colonies in Lv-shNOB1 were
significantly smaller than that in the Lv-shCon group (as shown in
Fig. 4B lower left). Our results
indicated that the downregulation of NOB1 significantly decreased
the colony formation of OSCC cells in vitro.
Knockdown of NOB1 significantly increased
apoptosis of OSCC cells
To detect the effect of NOB1 knockdown in OSCC
cells, Annexin V staining and flow cytometric analysis were carried
out on CAL27 and TCA-8113 after six days of lentivirus infection
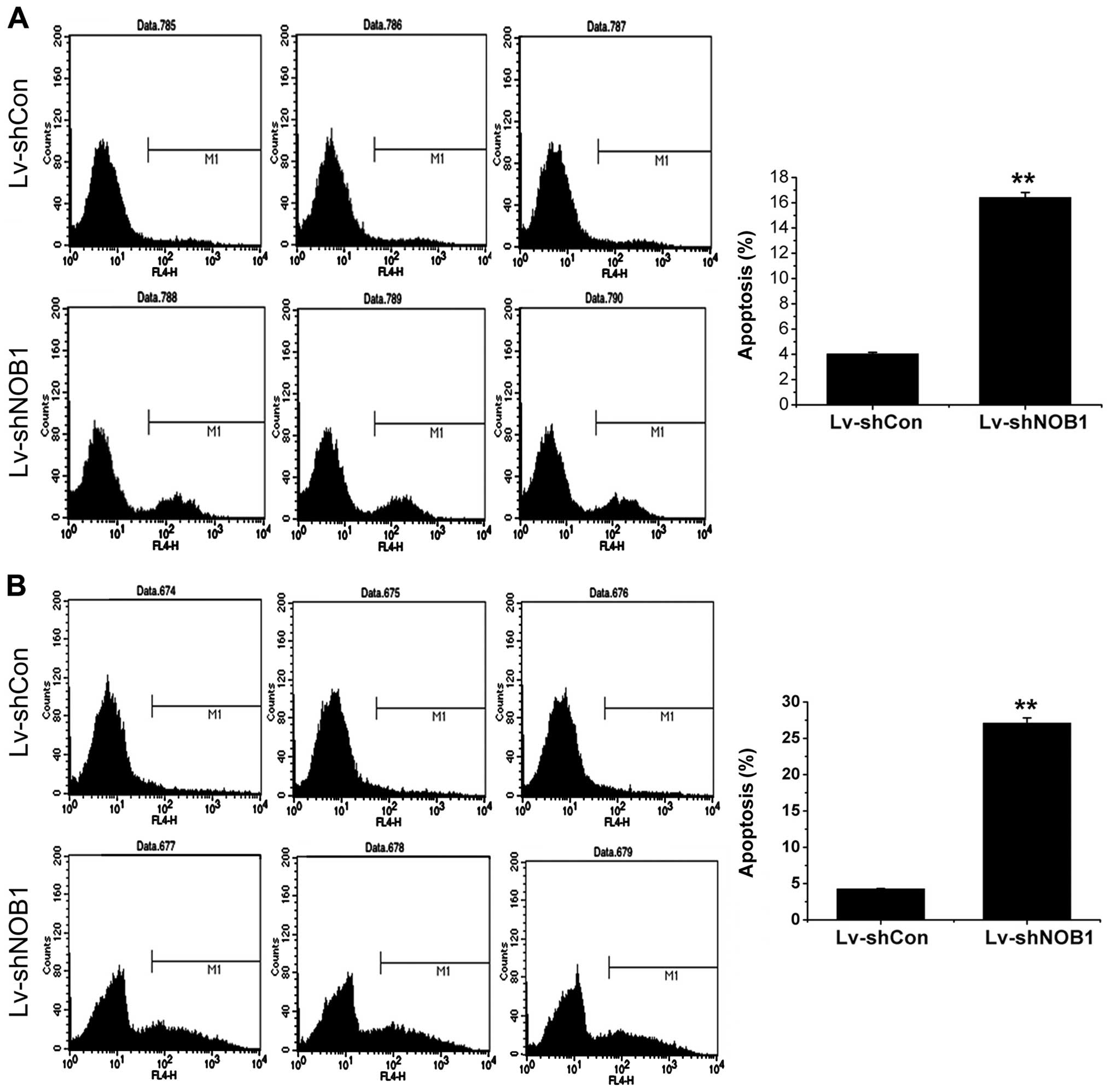
when cell confluency reached 90%. As shown in Fig. 5A, the apoptotic rate of CAL27 cells
in the Lv-shNOB1 group was ~16.40 compared to 4.04% in the Lv-shCon
group (P<0.05). As shown in Fig.
5B, the apoptotic rate of TCA-8113 cells in the Lv-shNOB1 group
was ~27.10 compared to 4.27% in the Lv-shCon group (P<0.05).
Effects of NOB1 knockdown on cell cycle
distribution
To elucidate the impact of Lv-shNOB1 knockdown of
NOB1 on the cell cycle progression of OSCC cells, CAL27 and
TCA-8113 cell lines were subjected to a PI staining flow cytometric
assay six days after lentivirus infection. The Lv-shNOB1-infected
CAL27 and TCA-8113 cells exhibited a decreasing portion of cells in
the G1 phase (P<0.01, Fig. 6A and
B) and an increasing portion of cells in the S phase (p<0.01
for CAL27 Fig. 6A, and p<0.05
for TCA-8113 Fig. 6B), compared to
the cells infected with Lv-shCon. Of note, the Lv-shNOB1 knockdown
of NOB1 also increased the portion of G2/M phase cells in TCA-8113
but not in CAL27, suggesting that NOB1 showed various function in
cell cycle in different cell lines. The results indicated that the
knockdown of NOB1 induced the S-phase arrest in OSCC cells and
induced G2/M-phase arrest in the OSCC cell lines.
Discussion
OSCC, one of the most common types of cancer usually
found in smoking and heavy-drinking middle-aged men, is on the
increase in non-smoking and non-heavily-drinking young individuals,
especially, young men. This phenomenon may be partially attributed
to HPV infection from oral sexual behavior (3,26).
OSCC is becoming an important cause of morbidity and mortality,
especially in developing countries and its prevalence may rise in
the foreseeable future. Therefore, the study on the mechanism of
OSCC genesis and development, especially, the mechanism of how the
virus involves, is of great importance.
In normal cells, NOB1 was found to be involved in
two key cell processes. Firstly, NOB1 facilitates the maturation of
the 20S proteasome and is then degraded by 26S proteasome to
complete 26S proteasome biogenesis, which means NOB1 is important
in ubiquitin-mediated protein degradation (5). Secondly, NOB1 accompanies the pre-40S
ribosomes during nuclear export and is cleaved at the D-site of 20S
pre-rRNA to form mature 18S rRNA, which means NOB1 is also involved
in protein synthesis (6–8). The two functions of NOB1 indicate that
NOB1 is important in protein homeostasis. Abnormal regulation of
the expression of NOB1 in cells may lead to dysfunction of the
protein synthesis and protein degradation and disturbs the protein
homeostasis. Rapidly growing cancer cells require more proteins for
DNA replication and cell division. Therefore, it is not surprising
to identify NOB1 upregulation in cancer. In addition, NOB1 was
found to be a target of microRNA-326 and may increase the
proliferation of glioma cells by activating the MAPK pathway by
increasing the phosphorylation of ERK1/2, JNK and p38 (27). Therefore, in cancer cells, the
protein homeostasis and signal transduction function of NOB1 were
modulated to facilitate the proliferation and development of
cancers.
In the present study, NOB1 was highly expressed in
OSCC cancers in clinical specimens. Expression of NOB1 increased
with the pathological grades of OSCC, which indicated that NOB1 may
be involved in the development of OSCC. The hypothesis was tested
with loss-of-function of NOB1 in two OSCC cell lines. In the cell
assay, NOB1 knockdown in OSCC cells decreased cell proliferation
and colony formation, increased cell apoptosis and induced cell
cycle arrest in S phase. The results were confirmed by the
knockdown of NOB1 in normal cells HEK293 (data not shown).
Knockdown of NOB1 in HEK293 did not show any significant difference
in cell proliferation, colony formation and apoptosis, which means
NOB1 is not a housekeeping gene in cells. Expression of NOB1 was
specifically increased in OSCC cancers but not in normal cells.
It was reported that NOB1 is also involved in the
MAPK pathway (27). The MAPK
pathway plays a central role in tumorigenesis and tumor development
(28). The MAPK pathway is also
reported to mediate the insulin-like growth factor (IGF) signaling
to promote the proliferation of OSCC. In view of the importance of
MAPK in cell proliferation and differentiation, many viruses hijack
the MAPK pathway in the progress of virus induced tumor genesis.
MAPK was reported to participate in human papillomavirus type
(HPV)-induced human cervical squamous carcinoma (29,30).
It is hypothesized that HPV infection is one of the histopathologic
risk factors in young OSCC patients (1,31).
However, the mechanism of HPV infection on the tumorigenesis and
tumor development of OSCC remains to be elucidated. Based on the
above information, we hypothesize that NOB1 is involved in the
malignant transformation of oral squamous cell after HPV
infection.
In conclusion, we have shown that the expression
level of NOB1 may be an indicator of the aggressiveness of OSCC. A
lentivirus-mediated siRNA study in CAL27 and TCA-8113 revealed that
NOB1 plays an important role in proliferation and anti-apoptosis in
OSCC cells. NOB1 may be involved in the malignant transformation of
oral squamous cells after HPV infection. Notably, the mechanism of
how NOB1 exerts its effect in OSCC cells remains to be
determined.
Acknowledgments
The present study was supported by grants from the
Nature Science Foundation of China (no. 31340014), and the Youth
Development Project of Medical Technology in Army (no.
13QNP166).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Zyl A and Bunn BK: Clinical features
of oral cancer. SADJ. 67:566–569. 2012.
|
|
3
|
Kansy K, Thiele O and Freier K: The role
of human papillomavirus in oral squamous cell carcinoma: Myth and
reality. Oral Maxillofac Surg. 18:165–172. 2014. View Article : Google Scholar
|
|
4
|
Tone Y, Tanahashi N, Tanaka K, Fujimuro M,
Yokosawa H and Toh-e A: Nob1p, a new essential protein, associates
with the 26S proteasome of growing Saccharomyces cerevisiae cells.
Gene. 243:37–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tone Y and Toh-E A: Nob1p is required for
biogenesis of the 26S proteasome and degraded upon its maturation
in Saccharomyces cerevisiae. Genes Dev. 16:3142–3157. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fatica A, Oeffinger M, Dlakić M and
Tollervey D: Nob1p is required for cleavage of the 3′ end of 18S
rRNA. Mol Cell Biol. 23:1798–1807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fatica A, Tollervey D and Dlakić M: PIN
domain of Nob1p is required for D-site cleavage in 20S pre-rRNA.
RNA. 10:1698–1701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamanna AC and Karbstein K: Nob1 binds the
single-stranded cleavage site D at the 3′-end of 18S rRNA with its
PIN domain. Proc Natl Acad Sci USA. 106:14259–14264. 2009.
View Article : Google Scholar
|
|
9
|
Zhou GJ, Zhang Y, Wang J, Guo JH, Ni J,
Zhong ZM, Wang LQ, Dang YJ, Dai JF and Yu L: Cloning and
characterization of a novel human RNA binding protein gene PNO1.
DNA Seq. 15:219–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Ni J, Zhou G, Yuan J, Ren W, Shan
Y, Tang W, Yu L and Zhao S: Cloning, expression and
characterization of the human NOB1 gene. Mol Biol Rep. 32:185–189.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mani A and Gelmann EP: The
ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol.
23:4776–4789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montanaro L, Treré D and Derenzini M:
Changes in ribosome biogenesis may induce cancer by downregulating
the cell tumor suppressor potential. Biochim Biophys Acta.
1825:101–110. 2012.
|
|
13
|
Belin S, Beghin A, Solano-Gonzàlez E,
Bezin L, Brunet-Manquat S, Textoris J, Prats AC, Mertani HC,
Dumontet C and Diaz JJ: Dysregulation of ribosome biogenesis and
translational capacity is associated with tumor progression of
human breast cancer cells. PLoS One. 4:e71472009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deschamps C: Retraction. Up-regulation of
NOB1 in esophageal squamous cell carcinoma (p). Dis Esophagus.
21:5742008. View Article : Google Scholar
|
|
15
|
Li XY, Luo QF, Li J, Wei CK, Kong XJ,
Zhang JF and Fang L: Clinical significance of NOB1 expression in
breast infiltrating ductal carcinoma. Int J Clin Exp Pathol.
6:2137–2144. 2013.PubMed/NCBI
|
|
16
|
Lin S, Meng W, Zhang W, Liu J, Wang P, Xue
S and Chen G: Expression of the NOB1 gene and its clinical
significance in papillary thyroid carcinoma. J Int Med Res.
41:568–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu K, Gu MM, Chen HL and You QS: NOB1 in
non-small-cell lung cancer: Expression profile and clinical
significance. Pathol Oncol Res. 20:461–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu G, Shen D, Jiao L and Sun Y: Nin one
binding protein expression as a prognostic marker in prostate
carcinoma. Clin Transl Oncol. 16:843–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang WY, Chen DH, Ning L and Wang LW:
siRNA mediated silencing of NIN1/RPN12 binding protein 1 homolog
inhibits proliferation and growth of breast cancer cells. Asian Pac
J Cancer Prev. 13:1823–1827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin Y, Peng S, Yu H, Teng H and Cui M:
RNAi-mediated downregulation of NOB1 suppresses the growth and
colony-formation ability of human ovarian cancer cells. Med Oncol.
29:311–317. 2012. View Article : Google Scholar
|
|
21
|
Lu Z, Guo Q, Shi A, Xie F and Lu Q:
Downregulation of NIN/RPN12 binding protein inhibit the growth of
human hepatocellular carcinoma cells. Mol Biol Rep. 39:501–507.
2012. View Article : Google Scholar
|
|
22
|
Wang H, Li P and Zhao B: Knockdown of NOB1
expression by RNAi inhibits cellular proliferation and migration in
human gliomas. Gene. 528:146–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen B, Liu J, Wu D, Qin Y, Peng C, Li C
and Wang J: Gene silencing of NOB1 by lentivirus suppresses growth
and migration of human osteosarcoma cells. Mol Med Rep.
9:2173–2179. 2014.PubMed/NCBI
|
|
24
|
Li Y, Ma C, Qian M, Wen Z, Jing H and Qian
D: Downregulation of NOB1 suppresses the proliferation and tumor
growth of non-small cell lung cancer in vitro and in vivo. Oncol
Rep. 31:1271–1276. 2014.PubMed/NCBI
|
|
25
|
Liu Y, Huang H, Yuan B, Zhuang LY, Luo TP
and Zhang Q: Lentivirus-mediated knockdown of NOB1 suppresses the
prolife ration of colon cancer cells. Z Gastroenterol. 52:429–435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Majchrzak E, Szybiak B, Wegner A,
Pienkowski P, Pazdrowski J, Luczewski L, Sowka M, Golusinski P,
Malicki J and Golusinski W: Oral cavity and oropharyngeal squamous
cell carcinoma in young adults: A review of the literature. Radiol
Oncol. 48:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Xu T, Yan Y, Qin R, Wang H, Zhang
X, Huang Y, Wang Y, Lu Y, Fu D, et al: MicroRNA-326 functions as a
tumor suppressor in glioma by targeting the Nin one binding protein
(NOB1). PLoS One. 8:e684692013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar
|
|
29
|
Kim JE, Lee JI, Jin DH, Lee WJ, Park GB,
Kim S, Kim YS, Wu TC, Hur DY and Kim D: Sequential treatment of HPV
E6 and E7-expressing TC-1 cells with bortezomib and celecoxib
promotes apoptosis through p-p38 MAPK-mediated downregulation of
cyclin D1 and CDK2. Oncol Rep. 31:2429–2437. 2014.PubMed/NCBI
|
|
30
|
Gao LJ, Gu PQ, Zhao W, Ding WY, Zhao XQ,
Guo SY and Zhong TY: The role of globular heads of the C1q receptor
in HPV 16 E2-induced human cervical squamous carcinoma cell
apoptosis is associated with p38 MAPK/JNK activation. J Transl Med.
11:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
El-Mofty SK: Histopathologic risk factors
in oral and oropharyngeal squamous cell carcinoma variants: An
update with special reference to HPV-related carcinomas. Med Oral
Patol Oral Cir Bucal. 19:e377–e385. 2014. View Article : Google Scholar : PubMed/NCBI
|