Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of
the most common head and neck cancers worldwide. In China, its
incidence has been increasing gradually, particularly in the
northeastern region. While surgery or radiotherapy can often cure
early-stage LSCC, the outcome has not improved greatly for most
patients with advanced disease in the last two decades despite the
advances in therapy (1). The poor
prognosis of laryngeal carcinoma is attributed to postoperative
recurrence and distant metastasis (2). Therefore, it is crucial to develop a
better understanding of the molecular mechanisms involved in the
progression of laryngeal carcinoma to improve treatment
efficacy.
The Notch signaling pathway is highly conserved
evolu-tionally, playing central roles in embryonic development and
adult life. In mammals, the Notch receptors (Notch1-4) are
transmembrane proteins. Notch receptor activation takes place when
the receptor binds with a ligand from the Jagged (Jagged1 and 2) or
Delta family (DLL-1, -3 and -4), which are themselves attached to
their respective cell membranes (3,4).
Therefore, Notch receptor-ligand interaction occurs between two
adjacent cells. Notch proteins are synthesized as precursors in the
endoplasmic reticulum, and then the Notch intracellular domain
(NICD) is released, translocating to the nucleus, driving the
expression of HEY1, HES1, Myc, CCND1, Bcl-2 and other cellular
process-regulating genes involved in proliferation,
differentiation, stem cell maintenance and apoptosis (5).
The Notch pathway is genetically altered in many
hematopoietic and solid tumors; Notch signaling can be oncogenic or
tumor-suppressive in tumorigenesis. A recent review (4) demonstrated that the Notch pathway
plays an oncogenic role in T cell acute lymphoblastic and chronic
lymphocytic leukemias, breast, pancreatic and colorectal cancer,
and lung adenocarcinoma; a tumor-suppressive role was observed in B
cell acute lymphoblastic and acute myeloid leukemias, ovarian
carcinoma and small cell lung cancer. Moreover, Notch signaling has
opposite effects in the same type of cancer (6–9). For
example, Notch exhibits both tumor-promoting and inhibitory
functions in lung carcinoma, depending on cell type (10,11).
Clearly, Notch activity regulates tumor biology context-dependently
and in a complex manner.
The roles of the Notch pathway in human LSCC remain
unclear. Early studies suggested a pro-tumorigenic role in head and
neck squamous cell carcinoma (HNSCC), mainly based on the increased
Notch1 and Notch2 expression levels in HNSCC tissues (12–14).
Recent exome sequencing data revealed that NOTCH1 is the second
most frequently mutated gene in HNSCC, unexpectedly raising
questions regarding the accepted role of Notch in HNSCC (15). Therefore, detailed clarification of
the role of Notch1 in LSCC is urgent. In the present study, we
analyzed Notch1 expression in clinical LSCC samples using quantum
dot immunohistochemistry (QD-IHC) and conventional IHC. Using short
hairpin RNA (shRNA), we silenced Notch1 expression in a human
laryngeal carcinoma HEp-2 cell line to elucidate the effects of
Notch1 on LSCC cell proliferation, apoptosis, migration and
invasion.
Materials and methods
Laryngeal carcinoma specimens and cell
lines
Formalin-fixed, paraffin-embedded tissue samples
were collected from Renmin Hospital of Wuhan University, China,
from January 2009 to December 2010, from patients aged 39–81 years
(median, 58 years). There were 31 normal vocal polyp tissues and 55
LSCC samples (18 cases with metastasis, 37 cases without
metastasis). A tissue microarray of 40 LSCC cases (5 cases with
metastasis and 35 cases without metastasis) was purchased from
AiLiNa Technologies (Xian, China). We obtained written informed
consent from the patients. The Ethics Committee of Renmin Hospital
of Wuhan University approved the study protocol.
The HEp-2 cell line was obtained from the China
Center for Type Culture Collection (Wuhan, China). HEp-2 cells were
cultured in RPMI-1640 culture medium (HyClone, Logan, UT, USA) and
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
invitrogen, Carlsbad, CA, USA), 100 u/ml penicillin and 20
µg/ml streptomycin in a fully humidified incubator at 37°C
and in an atmosphere of 5% Co2 in air.
shRNA transfection
The enhanced green fluorescent protein
(EGFP)-V-RS-Notch1 shRNA plasmid was purchased from Wuhan XiMa
Technologies Co. Ltd. (Wuhan, China). The shRNA plasmid structure
is as follows: stop-mir30 flank ing-shRNA1-mir30
flanking-EGFP-CMV-u6 shRNA2-stop; the shRNA sequences are as
follows: Notch1 shRNA1, 5′-CCGGGACATCACGGATCATAT-3′; Notch1 shRNA2,
5′-CGCTGCCTGGACAAGATCAAT-3′. Three treatments were designed for the
present study. Untreated HEp-2 cells were considered as the blank
control, and termed the non-transfected group. The plasmid
EGFP-V-RS-Notch1 shRNA, containing Notch1-specific shRNA, and the
plasmid EGFP-V-RS-negative shRNA, containing non-specific shRNA
(Wuhan XiMa Technologies Co., Ltd.), were the Notch1 shRNA and
negative shRNA groups, respectively. These plasmids were
transfected into HEp-2 cells using Lipofectamine RNAiMAX
(invitrogen) according to the manufacturer's instructions.
QD-IHC
The collected tissue samples and tissue microarray
were used for QD-based immunofluorescence histochemical detection.
For antibody binding, slides were incubated with rabbit anti-human
Notch1 (1:200; Cell Signaling Technology, Danvers, MA, USA)
overnight at 4°C. For QD conjugation, the slides were incubated
with zinc sulfate-capped cadmium selenide QDs conjugated to
anti-rabbit immunoglobulin G probes with an emission wavelength of
605 nm (1:50 in 2% bovine serum albumin; Jiayuan Quantum Dot Co.,
Ltd., Wuhan, China) for 30 min at 37°C. Following incubation, the
slides were vigorously washed with phosphate-buffered saline (PBS),
mounted with neutral glycerol and stored at 4°C for observation.
The QDs were excited by blue light (excitation wavelength of
450–480 nm under u-MWB filters) and present red light under
excitation. IHC staining was observed under light microscopy, and
positive cells appeared brown-yellow and granular, primarily to
prevent drying of tissues. The staining results were scored
according to existing standards: negative, no staining; 1+, weak
staining; 2+, strong staining. The IHC results were graded as
negative, positive or strongly positive as follows: negative, no
staining or 1+ staining in ≤30% of cells; positive, 1+ staining in
>30% of cells or 2+ staining in ≤50% of cells; strongly
positive, 2+ staining in >50% of cells.
IHC
The collected tissue samples were used for IHC
detection. Rabbit anti-human Notch1 (1:200; Cell Signaling
Technology) was used to detect Notch1 expression. The
immunohisto-chemistry protocols have been described elsewhere
(16). Immunostaining intensity was
estimated semi-quantitatively according to signal intensity and
distribution. The staining results were assessed on a three-tier
scale: negative, no staining; 1+, weak staining, 2+, strong
staining. The IHC results were graded as negative, positive, or
strongly positive as follows: negative, no staining or 1+ staining
in ≤30% of cells; positive, 1+ staining in >30% of cells or 2+
staining in ≤50% of cells; strongly positive, 2+ staining in
>50% of cells.
Quantitative reverse transcription-PCR
(qRT-PCR)
Total RNA was extracted from cells using TRizol
reagent (Invitrogen) and reverse-transcribed into complementary DNA
(cDNA) using a PrimeScript RT reagent kit (Takara, Kyoto, Japan).
The primer sequences used were as follows: Notch1 forward and
reverse, 5′-ATGACCAGTGGCTACGTGTG-3′ and 5′-CGGCAACGTCGTCAATACAC-3′,
respectively; glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
forward and reverse, 5′-GAAAGCCTGCCGGTGACTAA-3′ and
5′-AGGAAAAGCATCACCCGGAG-3′, respectively. The cDNA was used as the
template for qRT-PCR detection of the expression of the genes of
interest with SYBR Premix Ex Taq™ (Takara). Data were analyzed
according to the comparative threshold cycle value
(2−ΔΔCt) method. Relative mRNA expression was normalized
to GAPDH expression.
Cell proliferation assay
Cells were plated into 96-well culture plates at
3,000 cells/well and transfected with shRNA mentioned above. At the
same time each day for 24, 48 and 72 h, the original culture medium
was removed and 10 µl Cell Counting Kit-8 (CCK-8) solution
(Dojindo, Kumamoto, Japan) and 90 µl fresh RPMI-1640 medium
were added to each well. The cells were incubated at 37°C for 1 h,
and the absorbance of the medium at 450 nm was measured.
Apoptosis assay
Cells were plated into 6-well culture plates and
transfected with shRNA. After 72 h, apoptosis was analyzed using a
FACScan instrument (Becton-Dickinson, San Jose, CA, USA). The cells
were harvested and washed in PBS, and then stained with adenomatous
polyposis coli (APC) and 7-amino actinomycin D (7-AAD) (Lianke,
Hangzhou, China). The stained cells were analyzed with a FACSCanto™
ii spectrometer (Becton-Dickinson). Data were analyzed using FlowJo
7.6.5 software.
Scratch wound-healing motility assay
Cells were transfected with shRNA for 24 h, and then
plated into 6-well culture plates. Then, a scratch was made by
running a pipette tip through the dish; the cells were then
cultured under standard conditions for 24 h. The plates were washed
twice with fresh medium to remove non-adherent cells and then
photographed. The number of cells that had migrated from the edge
of the wound was counted.
In vitro cell invasion and migration
assays
For the cell invasion assays, Transwell membranes
(Corning Inc., New York, NY, USA) were coated with Matrigel (2.5
mg/ml). Twenty-four hours after transfection, cells were
serum-starved for 8 h, and were then collected in RPMi-1640 medium
containing 3% FBS. Cells were seeded onto the top chambers of the
precoated Transwells in the same medium alone at 1.0×105
cells/well. The bottom chambers of the Transwells contained 600
µl RPMI-1640 medium and 10% FBS. After 48 h, the Matrigel
and cells in the top chamber were swabbed with a Q-tip, and the
membranes were stained with crystal violet for 10 min. Cells in at
least five random fields were counted and photographed under
microscopy (×200).
Cell migration assays were also performed using
Transwell membranes (Corning). The procedure used was similar to
that of the cell invasion assay, except the Transwell membranes
were not coated with Matrigel.
Western blotting
Total protein from the plasmid-transfected HEp-2
cells was extracted using radioimmunoprecipitation assay buffer [1
mM MgCl2, 10 mM Tris-HCl (pH 7.4), 1% Triton X-100, 0.1%
sodium dodecyl sulfate (SDS), 1% NP-40]; and protein expression was
analyzed by western blotting. GAPDH was used as the loading
control. Total protein extracts were separated by
SDS-polyacrylamide gel electrophoresis on 12% gels and transferred
to polyvinylidene difluoride membranes. The proteins were
visualized and were quantified using Odyssey Western Blotting
Detection Reagents (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD); the data were analyzed with SPSS 16.0 (SPSS, inc.,
Chicago, iL, USA). The QD- IHC and IHC results were analyzed using
the rank-sum test. The results from the PCR, western blotting,
apoptosis assay and in vitro cell migration/invasion assays
were analyzed using the Student's t-test. P<0.05 was considered
statistically significant (Table
I).
 | Table IP-values for the western blot analysis
(Student's t-test). |
Table I
P-values for the western blot analysis
(Student's t-test).
| P-value (Notch1-shRNA
vs. non-transfection) | P-value (Notch1-shRNA
vs. negative-shRNA) |
|---|
| p-ERK | 0.0100 | 0.0219 |
| t-ERK | 0.5230 | 0.5968 |
| c-Myc | 0.0012 | 0.0016 |
| Bcl-2 | 0.0048 | 0.0089 |
| Bax | 0.0467 | 0.0439 |
| CDK4 | 0.0483 | 0.0307 |
| Cyclin E | 0.0183 | 0.0272 |
| p-Akt | 0.0304 | 0.0231 |
| t-Akt | 0.8955 | 0.6798 |
| p21 | 0.0263 | 0.0313 |
| Cyclin D1 | 0.0389 | 0.0443 |
Results
Notch1 is upregulated in LSCC
Compared with normal samples, Notch1 is both
overexpressed and downregulated in human cancers. In the present
study, we investigated Notch1 expression in LSCC and normal vocal
polyp tissues using QD-IHC and conventional IHC. QD-IHC has better
image quality and sensitivity than conventional staining methods,
and detected Notch1 expression in a total of 95 LSCC and 31 normal
vocal polyp specimens. Fig. 1 and
Table II show no or very low
Notch1 expression in the normal vocal polyp tissues, but higher
expression in the LSCC tissues. There was gradual Notch1
upregulation in the normal vocal polyp tissues and in LSCC without
and with metastasis. We determined that Notch1 was localized to the
cytoplasm and nucleus. To confirm our results, we characterized
Notch1 expression levels using IHC. Among the 55 LSCC tissues, 48
samples (85.4%) had high Notch1 expression; among the normal vocal
polyp tissues, Notch1 expression was high in only 12 samples
(38.7%). Notch1 expression levels were higher in LSCC with
metastasis than in LSCC without metastasis (Fig. 1 and Table III). These results indicate that
Notch1 is upregulated in LSCC tissues and plays an important role
in LSCC tumorigenesis and metastasis.
 | Table IIQD-IHC detection of Notch1 expression
in LSCC and normal vocal polyp tissues. |
Table II
QD-IHC detection of Notch1 expression
in LSCC and normal vocal polyp tissues.
| Sample | Case | Negative | Positive | Strong
positive | P-value |
|---|
| Normal vocal
polyps | 31 | 24 | 5 | 2 | |
| LSCC | 95 | 11 | 44 | 41 | <0.01a |
| Without
metastasis | 72 | 11 | 39 | 22 | <0.01a |
| With
metastasis | 23 | 0 | 4 | 19 | <0.01b |
 | Table IIIIHC detection of Notch1 expression in
LSCC and normal vocal polyp tissues. |
Table III
IHC detection of Notch1 expression in
LSCC and normal vocal polyp tissues.
| Sample | Case | Negative | Positive | Strong
positive | P-value |
|---|
| Normal vocal
polyps | 31 | 19 | 7 | 5 | |
| LSCC | 55 | 7 | 21 | 27 | <0.01a |
| Without
metastasis | 37 | 5 | 19 | 13 | <0.01a |
| With
metastasis | 18 | 2 | 2 | 14 | <0.01b |
Notch1 shRNA downregulates Notch1 mRNA
and protein expression effectively in HEp-2 cells
The efficacy of Notch1 shRNA in HEp-2 cells was
analyzed using RT-PCR and western blotting. Compared with the
negative shRNA group, Notch1 mRNA (Fig.
2A) and protein (Fig. 2B and C)
expression in the Notch1 shRNA group was decreased by 72.6 and
76.8%, respectively. There were no significant changes in the
Notch2 mRNA or protein levels in the non-transfected and negative
shRNA groups (P>0.05).
Notch1 knockdown induces morphological
changes, inhibits proliferation and induces apoptosis in HEp-2
cells
To investigate the effect of Notch1 on cancer cell
morphology, proliferation and apoptosis, we used shRNA to knock
down Notch1 in HEp-2 cells. Following a 72-h Notch1 shRNA
transfection in HEp-2 cells, the cells were examined and
photographed under phase contrast microscopy at ×400 to observe the
cell morphological changes. Fig. 3A
shows that the cell morphological changes were consistent with the
reduced cell numbers in culture. Notch1-specific shRNA
significantly inhibited HEp-2 cell growth at 24, 48 and 72 h
post-transfection (Fig. 3B, 24 h,
P<0.05; 48 and 72 h, P<0.01).
To validate the effect of Notch1 on apoptosis, we
detected apoptotic cells using APC and 7-AAD staining and flow
cytometry following Notch1 shRNA transfection in HEp-2 cells. At 72
h after transfection, there were higher percentages of both early
apoptotic cells (APC-positive, 7-AAD-negative) and late apoptotic
cells (APC-positive, 7-AAD-positive) in the Notch1
shRNA-transfected HEp-2 cells as compared with the non-transfected
and negative shRNA HEp-2 cells (Fig. 3C
and D, P<0.05). These results confirmed that Notch1 is an
oncogene in LSCC and may contribute to LSCC cell proliferation and
apoptosis.
Notch1 knockdown inhibits HEp-2 cell
migration and invasion abilities
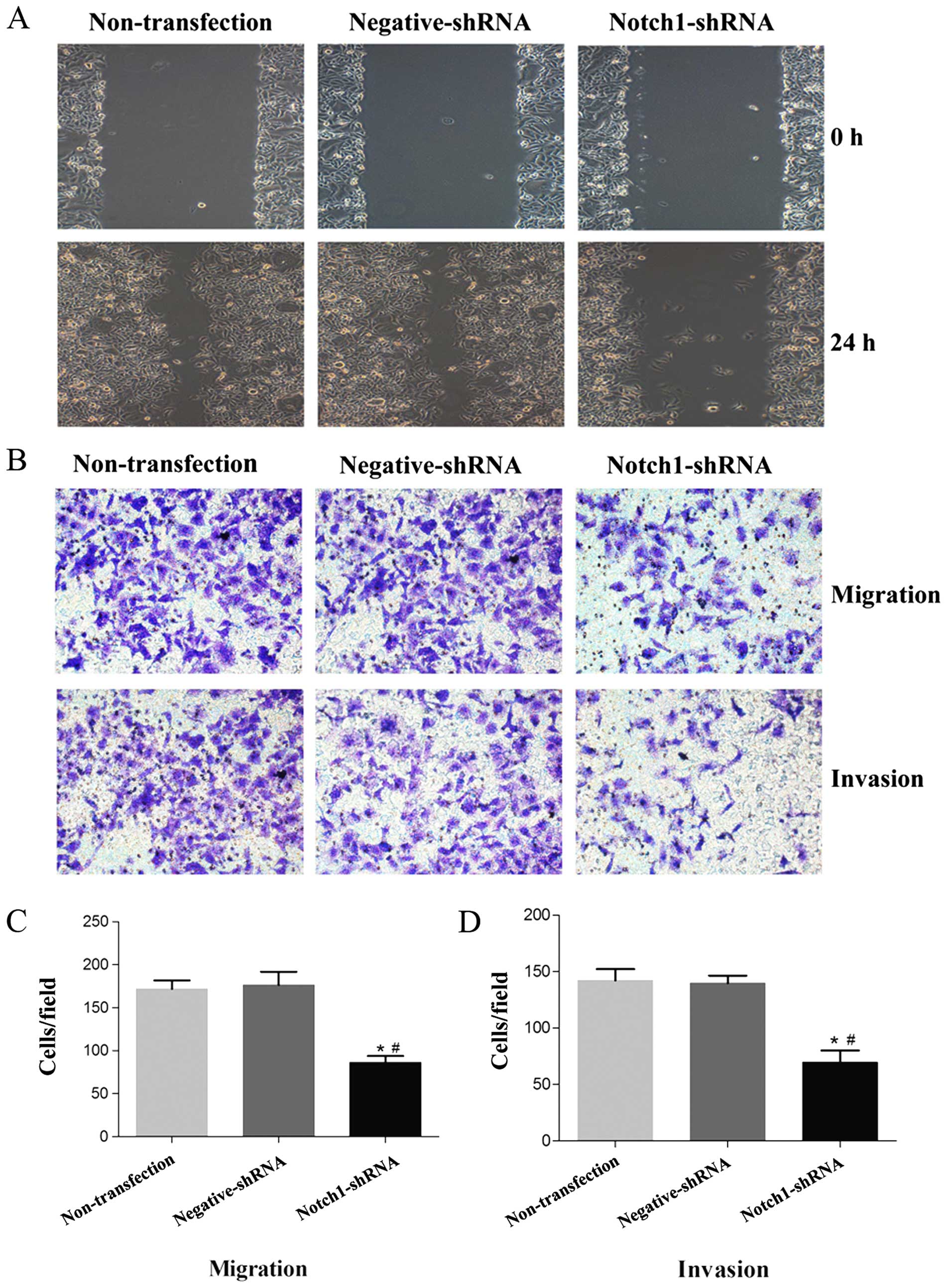
We examined whether Notch1 plays a role in LSCC cell
migration and invasion. The wound healing assay determined that
Notch1 knockdown significantly decreased HEp-2 cell motility and
migration (Fig. 4A). To confirm
this, we performed in vitro Transwell cell migration and
invasion assays following a 48-h Notch1 shRNA transfection. There
were significantly decreased numbers of migrating and invasive
Notch1 shRNA-transfected HEp-2 cells compared with the
non-transfected and negative shRNA cells (Fig. 4B-D, P<0.05). These results
indicate that Notch1 is crucial for inhibiting laryngeal cancer
cell migration and invasion in vitro.
Notch1 knockdown inhibits the expression
of proteins related to cell proliferation and apoptosis
We measured the expression of Notch pathway-related
proteins using western blotting to investigate the underlying
molecular mechanisms. Notch1 knockdown in HEp-2 cells inhibited
phosphorylated extracellular signal-related kinase (p-ERK),
phosphorylated protein kinase B (p-AKT), c-Myc, Bcl-2, p21, cyclin
D1, cyclin-dependent kinase 4 (CDK4) and cyclin E expression
(Fig. 5, P<0.05) and increased
Bax expression (Fig. 5, P<0.05).
There was no obvious change to t-ERK and t-Akt protein expression
(Fig. 5, P>0.05).
Discussion
Many aspects of cancer biology, including cell
renewal, proliferation, tumor angiogenesis and metastasis are
regulated by the Notch signaling pathway (17). Notch signaling is implicated in the
tumorigenesis of both solid and hematological malignancies,
regulating tumor biology context-dependently and in a complex
manner. Notch signaling has oncogenic effects in T cell acute
lymphoblastic leukemia, breast and colorectal cancer, where Notch
upregulation has been observed in these tumor types as compared
with normal samples. Notch signaling also has tumor-suppressive
effects, such as in B cell acute lymphoblastic leukemia, ovarian
carcinoma and small cell lung cancer, in which Notch is
downregulated in these cancer tissues or cells (3,18–20).
However, Notch signaling pathway molecular alterations in LSCC are
less well defined. A recent report found that QD- IHC has good
correlation and consistency with conventional IHC and in
comparison, has better image quality and sensitivity (21). Therefore, we used both QD- IHC and
IHC to comprehensively confirm Notch1 expression levels in LSCC
tissues. Notch1 was upregulated in LSCC tissues as compared with
normal vocal polyp tissues; the upregulation was even higher in
LSCC tissues with metastasis when compared with LSCC tissues
without metastasis, indicating Notch1 may be oncogenic in LSCC
tumorigenesis and metastasis.
The role of Notch1 in cellular proliferation and
apoptosis has been described in many cell types, and the results
have been controversial (5). In the
present study, we used shRNA interference to silence the NOTCH1
gene in human laryn-gocarcinoma HEp-2 cells. RT-PCR and western
blotting detected successful NOTCH1 knockdown in the HEp-2 cells,
where the shRNA inhibited Notch1 expression effectively at the mRNA
and protein levels. We then examined the effect of Notch1 silencing
on HEp-2 cell proliferation. Notch1 shRNA suppressed the
proliferative potential of HEp-2 cells compared with that of the
non-transfected and negative shRNA-trans-fected HEp-2 cells.
Furthermore, Notch1 knockdown induced apoptosis in the HEp-2 cells
at a rate of 37%, which was significantly higher than the rate of
apoptosis in the non-transfected and negative shRNA-transfected
groups.
Notch1 promotes or suppresses tumorigenesis by
regulating different target genes in specific tissue environments
and in cancer microenvironments. Abdel Aziz et al (22) found that downregulating NOTCH1 and
its target gene HES1 signifi-cantly decreased the rate of
hepatocellular carcinoma (HCC) HepG2 cell proliferation. Moriyama
et al (23) confirmed that
Notch signaling acts through HES1 to play an important role in
immature melanoblast survival by preventing apoptosis. Li et
al (24) found that Notch1
mediates the proliferation of smooth muscle cells via HEY2. We
examined the conventional Notch target genes in HEp-2 cells, and
found that silencing of Notch1 downregulated p-ERK, p-Akt, c-Myc,
Bcl-2, p21, cyclin D1, CDK4 and cyclin E and upregulated Bax; t-ERK
and t-Akt expression levels were unchanged.
Among the validated genes, Bcl-2 is a well-known
anti-apoptosis gene (25), and Bax
is a pro-apoptosis protein (26);
these findings were consistent with the increased number of
apoptotic cells following Notch1 knockdown. Furthermore, we found
downregulation of p-Akt after Notch1 knockdown, confirming that
deactivation of the Pi3K/Akt pathway attenuated Notch1-induced
apoptosis. Knockdown of Notch1 deactivated Akt phosphorylation,
decreased Bcl-2 and increased Bax expression, and induced cell
apoptosis. ERK is a major effect target of the mitogen-activated
protein kinase (MAPK) pathway, and p-ERK was downregulated
following Notch1 knockdown, which also inhibited cell proliferation
and promoted apoptosis. At the same time, c-Myc was down-regulated.
c-Myc activates Bax, which induces cytochrome c release from
the mitochondria into the cytoplasm, resulting in caspase
cascade-induced apoptosis. c-Myc also activates transcription
factor p53, ARF and apoptosis regulation indirectly, inhibiting
MAPK and nuclear factor κB activity, which is sensitive to tumor
necrosis factor-mediated signaling, promoting apoptosis (27). In the present study, it was also
found that p21, cyclin D1, CDK4 and cyclin E protein expression was
downregulated, indicating that Notch1 induces cell cycle arrest and
thereby inhibits cell proliferation in HEp-2 cells. This is
consistent with previous studies in different carcinoma cells. In
small cell lung cancer cells, Notch signaling can increase p21 and
p27 expression and hence induce cell cycle arrest (28). In human glioma cells, Notch
signaling induces cell cycle arrest by downregulating MCM2 and p21
protein expression (29). In the
present study, downregulation of Notch1 inhibited HEp-2 cell
proliferation by altering cell cycle-related protein expression.
Metastasis and invasion are two important factors affecting LSCC
prognosis and recurrence. We found that Notch1 controls HEp-2 cell
migration and invasion, although the underlying molecular mechanism
remains unclear. Luo et al found that inhibition of NICD
decreased tumor invasion in gastric cancer (30). Zhou et al reported that
downregulation of Notch1 decreased HCC cell migration and invasion
by regulating E-cadherin and CD44v6 (31). These results all demonstrate that
Notch1 contributes to the migration and invasion of cancer cells.
Nevertheless, further studies are warranted to more precisely
determine the molecular mechanisms of Notch1 in LSCC invasion and
metastasis.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81172569 and
81372880).
References
|
1
|
Liu M, Wu H, Liu T, Li Y, Wang F, Wan H,
Li X and Tang H: Regulation of the cell cycle gene, BTG2, by miR-21
in human laryngeal carcinoma. Cell Res. 19:828–837. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu M, Yin F, Yang L, Chen S, Chen R, Zhou
X, Jing W, Fan X, Jia R, Wang H, et al: Contribution of TIP30 to
chemoresistance in laryngeal carcinoma. Cell Death Dis.
5:e14682014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capaccione KM and Pine SR: The Notch
signaling pathway as a mediator of tumor survival. Carcinogenesis.
34:1420–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ntziachristos P, Lim JS, Sage J and
Aifantis I: From fly wings to targeted cancer therapies: A
centennial for notch signaling. Cancer Cell. 25:318–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su BH, Qu J, Song M, Huang XY, Hu XM, Xie
J, Zhao Y, Ding LC, She L, Chen J, et al: NOTCH1 signaling
contributes to cell growth, anti-apoptosis and metastasis in
salivary adenoid cystic carcinoma. Oncotarget. 5:6885–6895. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu H, Zhao X, Huang S, Jian L, Qian G and
Ge S: Blocking Notch1 signaling by RNA interference can induce
growth inhibition in HeLa cells. Int J Gynecol Cancer. 17:511–516.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Qin H, Chen B, Xin X, Li J and Han
H: Overexpressed active Notch1 induces cell growth arrest of HeLa
cervical carcinoma cells. Int J Gynecol Cancer. 17:1283–1292. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng Q, Qin H, Zhang H, Li J, Hou L, Wang
H, Zhang X, Zhang S, Feng L, Liang Y, et al: Notch signaling
inhibits growth of the human lung adenocarcinoma cell line A549.
Oncol Rep. 17:847–852. 2007.PubMed/NCBI
|
|
9
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito T, Udaka N, Okudela K, Yazawa T and
Kitamura H: Mechanisms of neuroendocrine differentiation in
pulmonary neuroendocrine cells and small cell carcinoma. Endocr
Pathol. 14:133–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunnimalaiyaan M and Chen H: Tumor
suppressor role of Notch-1 signaling in neuroendocrine tumors.
Oncologist. 12:535–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng Q, Li S, Chepeha DB, Giordano TJ, Li
J, Zhang H, Polverini PJ, Nor J, Kitajewski J and Wang CY:
Crosstalk between tumor and endothelial cells promotes tumor
angiogenesis by MAPK activation of Notch signaling. Cancer Cell.
8:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leethanakul C, Patel V, Gillespie J,
Pallente M, Ensley JF, Koontongkaew S, Liotta LA, Emmert-Buck M and
Gutkind JS: Distinct pattern of expression of differentiation and
growth-related genes in squamous cell carcinomas of the head and
neck revealed by the use of laser capture microdissection and cDNA
arrays. Oncogene. 19:3220–3224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hijioka H, Setoguchi T, Miyawaki A, Gao H,
Ishida T, Komiya S and Nakamura N: Upregulation of Notch pathway
molecules in oral squamous cell carcinoma. Int J Oncol. 36:817–822.
2010.PubMed/NCBI
|
|
15
|
Agrawal N, Frederick MJ, Pickering CR,
Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, et
al: Exome sequencing of head and neck squamous cell carcinoma
reveals inactivating mutations in NOTCH1. Science. 333:1154–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schacht V and Kern JS: Basics of
immunohistochemistry. J Invest Dermatol. 135:e302015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yap L, Lee D, Khairuddin A, Pairan M,
Puspita B, Siar C and Paterson I: The opposing roles of NOTCH
signalling in head and neck cancer: A mini review. Oral Dis. Jan
7–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun W, Gaykalova DA, Ochs MF, Mambo E,
Arnaoutakis D, Liu Y, Loyo M, Agrawal N, Howard J, Li R, et al:
Activation of the NOTCH pathway in head and neck cancer. Cancer
Res. 74:1091–1104. 2014. View Article : Google Scholar :
|
|
19
|
Dang TP, Gazdar AF, Virmani AK, Sepetavec
T, Hande KR, Minna JD, Roberts JR and Carbone DP: Chromosome 19
trans-location, overexpression of Notch3, and human lung cancer. J
Natl Cancer Inst. 92:1355–1357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bedogni B, Warneke JA, Nickoloff BJ,
Giaccia AJ and Powell MB: Notch1 is an effector of Akt and hypoxia
in melanoma development. J Clin Invest. 118:3660–3670. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun JZ, Chen C, Jiang G, Tian WQ, Li Y and
Sun SR: Quantum dot-based immunofluorescent imaging of Ki67 and
identification of prognostic value in HER2-positive (non-luminal)
breast cancer. Int J Nanomed. 9:1339–1346. 2014. View Article : Google Scholar
|
|
22
|
Abdel Aziz MT, Khaled HM, El Hindawi A,
Roshdy NK, Rashed LA, Sabry D, Hassouna AA, Taha F and Ali WI:
Effect of mesenchymal stem cells and a novel curcumin derivative on
Notch1 signaling in hepatoma cell line. Biomed Res Int.
2013:1296292013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moriyama M, Osawa M, Mak SS, Ohtsuka T,
Yamamoto N, Han H, Delmas V, Kageyama R, Beermann F, Larue L, et
al: Notch signaling via Hes1 transcription factor maintains
survival of melanoblasts and melanocyte stem cells. J Cell Biol.
173:333–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Takeshita K, Liu PY, Satoh M, Oyama
N, Mukai Y, Chin MT, Krebs L, Kotlikoff MI, Radtke F, et al: Smooth
muscle Notch1 mediates neointimal formation after vascular injury.
Circulation. 119:2686–2692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar
|
|
26
|
Cory S and Adams JM: Killing cancer cells
by flipping the Bcl-2/Bax switch. Cancer Cell. 8:5–6. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pelengaris S and Khan M: The many faces of
c-MYC. Arch Biochem Biophys. 416:129–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sriuranpong V, Borges MW, Ravi RK, Arnold
DR, Nelkin BD, Baylin SB and Ball DW: Notch signaling induces cell
cycle arrest in small cell lung cancer cells. Cancer Res.
61:3200–3205. 2001.PubMed/NCBI
|
|
29
|
Li X, He X, Tian W and Wang J: Short
hairpin RNA targeting Notch2 inhibits U87 human glioma cell
proliferation by inducing cell cycle arrest and apoptosis in vitro
and in vivo. Mol Med Rep. 10:2843–2850. 2014.PubMed/NCBI
|
|
30
|
Luo DH, Zhou Q, Hu SK, Xia YQ, Xu CC, Lin
TS, Pan YT, Wu JS and Jin R: Differential expression of Notch1
intracellular domain and p21 proteins, and their clinical
significance in gastric cancer. Oncol Lett. 7:471–478.
2014.PubMed/NCBI
|
|
31
|
Zhou L, Zhang N, Song W, You N, Li Q, Sun
W, Zhang Y, Wang D and Dou K: The significance of Notch1 compared
with Notch3 in high metastasis and poor overall survival in
hepatocel-lular carcinoma. PLoS One. 8:e573822013. View Article : Google Scholar
|