Introduction
Ovarian cancer is one of the most common gynecologic
malignancies worldwide, and is a leading cause of cancer-related
death among women (1). The overall
5-year survival of early-stage ovarian cancer patients is higher
than 65%. However, advanced ovarian cancer develops resistance to
chemotherapy and radiotherapy, and eventually leads to death
(2). Therefore, the search for
novel biomarkers for the early diagnosis of ovarian cancer is
urgently needed.
Extracellular proteolytic enzymes, including matrix
metalloproteinases (MMPs) and serine proteases, have been found to
contribute to tumor cell invasion and metastasis (3,4).
Recently, type II transmembrane serine proteases (TTSPs) have been
recognized as a new subfamily of the serine proteases (5,6).
Numerous TTSPs are highly expressed in cancer, and have been
implicated in tumor development and progression (4). Belonging to the TTSP family, the
TMPRSS subfamily consists of four members, TMPRSS2-5. As one member
of the TMPRSS family, transmembrane protease, serine 3 (TMPRSS3)
expression has been detected in a number of human tissues,
including the heart, kidney, liver, lung and ovary (7). TMPRSS3 has been found to be
overexpressed in numerous types of cancers including pancreatic,
gastric and colon cancer (8).
Microarray of pancreatic cancer has identified that TMPRSS3 is one
of the most differentially expressed genes, and acts as a tumor
marker for screening pancreatic cancer (9). It has been reported that the TMPRSS3
expression level in ovarian carcinomas is elevated, and may be
useful as a molecular target for diagnosis and therapeutic
intervention in ovarian cancer. However, the molecular mechanisms
of TMPRSS3 in ovarian cancer remain unclear.
In the present study, we investigated the expression
of TMPRSS3 in ovarian cancer cell lines. Moreover, we explored the
effects of TMPRSS3 expression on the proliferation, invasion and
metastasis of ovarian cancer cells. We also aimed to elucidate the
molecular mechanisms of TMPRSS3 in ovarian cancer. These data may
provide information for the diagnosis of ovarian cancer.
Materials and methods
Reagents
Antibodies against TMPRSS3, E-cadherin, vimentin,
Twist and β-actin were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Antibodies against ERK1/2 and phosho-ERK1/2
were obtained from Cell Signaling Technology (Danvers, MA, USA).
ERK1/2 special inhibitor U0126 was purchased from Sigma (St. Louis,
MO, USA).
Cell lines and culture condition
Human ovarian surface epithelial cell line IOSE144
and human ovarian cancer cell lines A2780, OVCAR3, SKOV3 and HO8910
were all purchased from the Cell Bank of the Chinese Academy of
Science (Shanghai, China). The IOSE144 cell line was maintained in
MCDB105 medium containing 10% fetal bovine serum (FBS). A2780 and
SKOV3 cell lines were maintained in Dulbecco's modified Eagle's
medium (DMEM) containing 10% FBS, while OVCAR3 and HO8910 cell
lines were maintained in RPMI-1640 medium containing 10% FBS. All
cell lines were incubated in a CO2 incubator at
37°C.
Cell transfection
To upregulate the expression of TMPRSS3 in A2780
cells, the human TMPRSS3 full length cDNA was amplified and
inserted into the pcDNA3.1 vector (GenePharma Co., Ltd., Shanghai,
China) to obtain pcDNA3.1-TMPRSS3, and a scramble sequence was
inserted into the pcDNA3.1 vector as the control vector. To knock
down the expression of TMPRSS3 in HO8910 cells, a TMPRSS3 shRNA was
designed and obtained from GenePharma Co., Ltd. A scrambled shRNA
was used as the control shRNA. For transfection, the cells were
seeded into 6-well plates. When cell confluency reached 50%,
TMPRSS3 shRNA or pcDNA3.1-TMPRSS3 was transfected into the cells
using Lipofectamine 2000 transfection reagent (Invitrogen)
according to the manufacturer's instructions. Culture medium was
replaced after 6 h of incubation. Finally, the stable transfected
cells were selected by G418 selection and the efficiency was
determined by western blotting.
Western blotting
Cells were lysed in RIPA buffer with protease and
phosphatase inhibitors, and then the concentration of protein was
determined by the BCA assay. Equal amount of protein samples was
loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and was then transferred to PVDF
membranes. After blocking with 5% BSA for 1 h at room temperature,
the membranes were incubated with primary antibodies specific to
TMPRSS3, E-cadherin, vimentin, Twist, β-actin, ERK1/2 or
phosho-ERK1/2 at 4°C overnight. Then, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibody
(Zhongshan Biotech Co., Ltd, Beijing, China) for 1 h at room
temperature. The protein bands were visualized by enhanced
chemiluminescence (Applygen Technologies Inc., Beijing, China) and
were densitometrically analyzed by Quantity One software (Bio-Rad,
Hercules, CA, USA).
Proliferation assay
Cell proliferation was determined by the CCK-8 assay
(Jingmei Biotech, Shanghai, China). In brief, the cells were seeded
at 1,000 cells/well in a 96-well plate. After incubation with U0126
or dimethylsulfoxide (DMSO) for the indicated time, 10 µl of
CCK-8 was added into the plate and incubated for 2 h. The optical
density (OD) was measured by a microplate reader (Bio-Rad Model
680) at 490 nm wavelength. The OD value was measured every day for
seven days.
In vitro invasion assay
A 24-well Transwell chamber (Corning Costar, New
York, NY, USA), which was covered with 30 µl of Matrigel (BD
Biosciences, USA) to create an artificial basement membrane, was
used to examine the invasive ability of the ovarian cancer cells.
Cells (5×105) were suspended in 200 µl serum-free
1640 medium and were added into the upper Transwell chamber. The
lower Transwell chamber was filled with 600 µl of 1640
medium supplemented with 20% FBS. After incubation of 16 h at 37°C,
the non-invading cells were removed with a sterile cotton swab, and
the invaded cells were stained with 0.1% crystal violet for 20 min
at room temperature. The numbers of cells were calculated under a
light microscope in five random fields.
In vitro migration assay
Migratory ability was determined using a 24-well
Transwell chamber (Corning Costar) not covered with Matrigel. Cells
(1×105 cells/200 µl serum-free 1640 medium) were
added into the upper Transwell chamber, and 600 µl of 1640
medium supplemented with 20% FBS was added into the lower Transwell
chamber. After incubation of 16 h at 37°C, the non-migrating cells
were removed with a sterile cotton swab, and the migrated cells
were stained with 0.1% crystal violet for 20 min at room
temperature. The numbers of cells were calculated under a light
microscope in five random fields.
In vivo growth and metastasis
experiments
Female BABL/c nude mice, four weeks old, were
maintained in pathogen-free conditions, and in vivo growth
and metastasis experiments were performed after approval by the
Animal Care and Use Committee of Harbin Medical University. Mice
were subcutaneously injected with 1.0×105 cells at the
back (n=8 for each group). Tumors were observed after one week.
Then the length (L) and width (W) of the tumors in mice were
measured every week, and the tumor volumes were estimated with the
formula: 0.52 × L × W2. Seven weeks later, the mice were
sacrificed and the livers were fixed in 4% paraformaldehyde,
sectioned into slices and were then stained with hematoxylin and
eosin (H&E). Finally, every slice was observed under a
microscope, and the number of micrometastases was counted.
Statistical analysis
Each experiment was performed at least three times.
Data are expressed as the mean ± standard deviation (SD). SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA) was used to perform
statistical analysis. Differences between groups were evaluated by
the Student's t-test or one-way ANOVA. p<0.05 was considered to
indicate a statistically significant result.
Results
Expression of TMPRSS3 in ovarian cancer
cells
We first detected the protein level of TMPRSS3 in
human ovarian surface epithelial (IOSE144) and ovarian cancer cells
(A2780, OVCAR3, SKOV3 and HO8910) by western blotting. The results
showed that TMPRSS3 was highly expressed in all ovarian cancer cell
lines. A higher protein level of TMPRSS3 was observed in the HO8910
cells, whereas the expression of TMPRSS3 expression was weak in the
A2780 cells (Fig. 1).
TMPRSS3 participates in the proliferation
of ovarian cancer cells in vitro
To investigate the biological functions of TMPRSS3
in ovarian cancer cells, A2780 cells were transfected with
pcDNA3.1-TMPRSS3 to increase TMPRSS3 expression (Fig. 2A), and HO8910 cells were transfected
with TMPRSS3 shRNA to deplete endogenous TMPRSS3 expression
(Fig. 2B). Cells transfected with
the scrambled pcDNA3.1 or shRNA were used as a negative control.
The proliferation assay was then performed to explore the effect of
TMPRSS3 expression on ovarian cancer cell proliferation. The
results showed that overexpression of TMPRSS3 promoted the
proliferation of A2780 cells, whereas knockdown of TMPRSS3 led to a
decrease in the growth of HO8910 cells (Fig. 2C and D), indicating the involvement
of TMPRSS3 in regulating ovarian cancer cell proliferation.
TMPRSS3 is involved in the invasion and
migration of ovarian cancer cells in vitro
We further examined the effect of TMPRSS3 expression
on ovarian cancer cell invasion and migration. Using invasion and
migration assays, we found that the invasive and migratory
abilities of the TMPRSS3-expressing A2780 cells were significantly
higher when compared with the vector-transfected cells (Fig. 3A and B). In contrast, knockdown of
TMPRSS3 attenuated the invasion and migration of the HO8910 cells
(Fig. 3C and D). These data suggest
that TMPRSS3 plays a pivotal role in the regulation of ovarian
cancer cell invasion and migration.
TMPRSS3 affects invasion-related genes in
ovarian cancer cells
Numerous studies have revealed that the expression
levels of various genes, including E-cadherin, vimentin and Twist
are associated with tumor invasion and metastasis (10). In the present study, we found that
overexpression of TMPRSS3 resulted in decreased E-cadherin
expression and elevated vimentin and Twist expression in the A2780
cells. In contrast, knockdown of TMPRSS3 led to increased
expression of E-cadherin and decreased expression of vimentin and
Twist in the HO8910 cells (Fig.
4A-C).
TMPRSS3 induces activation of the ERK1/2
pathway
ERK1/2, a key player in the MAPK signaling pathway,
is essential for tumor progression (11). In the present study, we detected the
effect of TMPRSS3 expression on the activation of ERK1/2 by western
blotting. As shown in Fig. 5,
overexpression of TMPRSS3 stimulated the phosphorylation of ERK1/2
in the A2780 cells, whereas knockdown of TMPRSS3 suppressed the
phosphorylation of ERK1/2 in the HO8910 cells.
TMPRSS3 contributes to ovarian cancer
cell proliferation and invasion via the ERK1/2 pathway
To determine the role of the ERK1/2 pathway in the
TMPRSS3-mediated proliferation and invasion of ovarian cancer
cells, a special ERK1/2 inhibitor U0126 (20 µM) was used to
block the activation of ERK1/2. The results showed that
overexpression of TMPRSS3 promoted the proliferation, invasion and
migration of A2780 cells. However, after inhibition of the ERK1/2
pathway, the proliferation, invasion and migration mediated by
TMPRSS3 expression were markedly suppressed (Fig. 6A–C). These results indicate that the
ERK1/2 pathway is involved in the TMPRSS3-mediated proliferation,
invasion and migration of ovarian cancer cells.
The ERK1/2 pathway is required for
TMPRSS3-mediated expression of E-cadherin, vimentin and Twist
We further detected the role of the ERK1/2 pathway
in the TMPRSS3-mediated expression of E-cadherin, vimentin and
Twist. Notably, we found that U0126 treatment attenuated the
TMPRSS3-mediated expression changes in E-cadherin, vimentin and
Twist in the A2780 cells (Fig.
7A–C). These data suggest that TMPRSS3 regulates the expression
of E-cadherin, vimentin and Twist via the ERK1/2 pathway in ovarian
cancer cells.
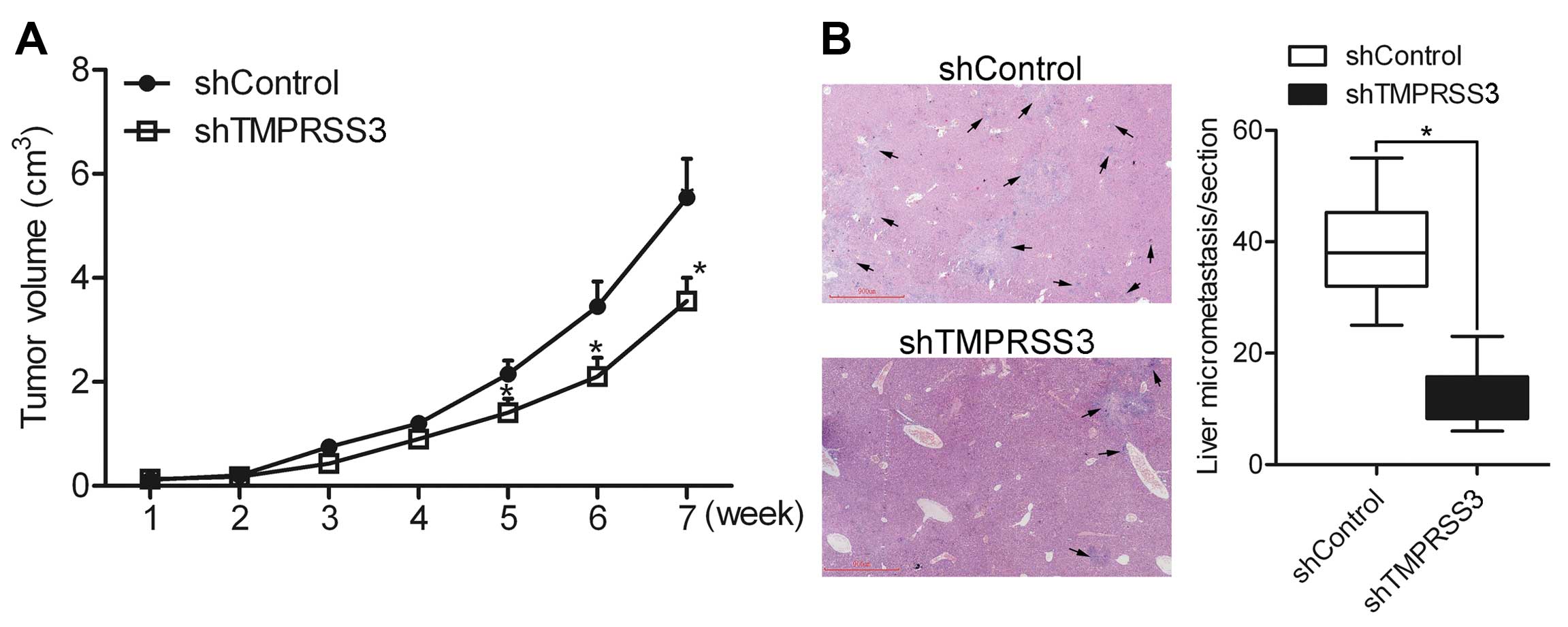
Knockdown of TMPRSS3 suppresses ovarian
cancer cell growth and metastasis in vivo
In vivo growth and metastasis experiments
were performed to detect the effect of TMPRSS3 on cell growth and
metastasis in vivo. shControl and shTMPRSS3 cells were
subcutaneously injected in the back of the mice, respectively.
Growth analysis showed that knockdown of TMPRSS3 suppressed ovarian
cancer HO8910 cell growth (Fig.
8A), suggesting that TMPRSS3 regulates the growth of ovarian
cancer cells in vivo. In addition, liver micrometastasis in
the section was counted under a microscope. The results showed that
the number of micrometastases was greatly decreased in the
shTMPRSS3 group as compared to the shControl group (Fig. 8B), indicating that TMPRSS3
contributes to the metastasis of ovarian cancer cells in
vivo.
Discussion
In the present study, we found that TMPRSS3 was
significantly expressed in ovarian cancer cells. To investigate the
effect of TMPRSS3 expression on ovarian cancer cells, gain-or-loss
of TMPRSS3 expression was introduced through ectopic
over-expression or RNAi-mediated knockdown. The data showed that
TMPRSS3 was able to promote the proliferation, invasion and
metastasis of ovarian cancer cells. We further found that TMPRSS3
stimulated activation of ERK1/2, and the ERK1/2 pathway was
involved in the biological functions of TMPRSS3. These findings
suggest that TMPRSS3 promotes ovarian cancer cell proliferation,
invasion and metastasis via activation of the ERK1/2 pathway.
The TMPRSS family has been reported in many tumor
types (12,13). Studies have found that TMPRSS3 is
highly expressed in ovarian cancer (14,15).
In the present study, using western blotting we found that TMPRSS3
was significantly expressed in ovarian cancer cells, indicating a
positive role of TMPRSS3 in ovarian cancer. It has been reported
that TMPRSS4 promotes thyroid cancer proliferation via CREB
phosphorylation (16), and TMPRSS4
knockdown in non-small cell lung cancer cells resulted in a
significant reduction in proliferation and clonogenic capacity
(17). However, the role of TMPRSS3
in cancer growth remains unknown. In the present study, we found
that overexpression of TMPRSS3 enhanced the growth of ovarian
cancer A2780 cells, whereas knockdown of TMPRSS3 suppressed the
proliferation of ovarian cancer HO8910 cells in vitro.
Moreover, knockdown of TMPRSS3 inhibited the proliferation of
ovarian cancer HO8910 cells in vivo. These data suggest that
TMPRSS3 contributes to the cell proliferation of ovarian cancer
cells. It has been reported that TMPRSS3 expression is correlated
with the metastatic potential of the clonal SUIT-2 pancreatic
cancer cell line, implying that TMPRSS3 is involved in metastasis
formation and tumor invasion (8).
In the present study, our findings confirmed that TMPRSS3
contributed to the invasion and migration of ovarian cancer cells
in vitro, and affected the metastasis of ovarian cancer cell
in vivo.
Epithelial-mesenchymal transition (EMT) plays an
important role in the invasion and metastasis of cancer. During the
process of EMT, numerous invasion-related genes are altered.
E-cadherin is a well-characterized single-pass transmembrane
protein that mediates cell-cell adhesion. E-cadherin expression is
usually decreased during cancer progression, and down-regulation of
E-cadherin is associated with tumor invasion and metastasis.
Studies have found that TMPRSS4 negatively regulates E-cadherin
expression in colorectal cancer and hepatocellular cancer cells
(18,19), and TMPRSS2 downregu-lates E-cadherin
expression in prostate cancer cells (20). In the present study, we found that
overexpression of TMPRSS3 decreased the expression of E-cadherin in
A2780 cells, whereas knockdown of TMPRSS3 increased the level of
E-cadherin in the HO8910 cells. Twist is a basic helix-loop-helix
protein that plays a role both in human development and in cancer
biogenesis. Overexpression of Twist downregulates E-cadherin, which
is the hallmark of EMT (21).
Vimentin is the major subunit protein of the intermediate filaments
of mesen-chymal cells, and acts as an important hallmark of EMT
(22). However, no study has
reported the effect of TMPRSS3 on the expression of Twist and
vimentin. In the present study, we found that TMPRSS3 positively
affected the expression of Twist and vimentin in ovarian cancer
cells.
It is well known that the ERK1/2 pathway plays a
pivotal role in regulating tumor progression, including cell
proliferation, invasion and metastasis (23,24).
In the present study, we found that TMPRSS3 expression induced
activation of ERK1/2, suggesting that TMPRSS3 expression affects
the ERK1/2 signaling pathway in ovarian cancer cells. We further
found that the ERK1/2 pathway was required for TMPRSS3-mediated
proliferation, invasion and migration, and participated in
regulating TMPRSS3-mediated expression of E-cadherin, vimentin and
Twist.
In summary, the present study demonstrated that
TMPRSS3 contributes to ovarian cancer cell proliferation, invasion
and metastasis, probably via activation of the ERK1/2 signaling
pathway. Therefore, TMPRSS3 acts as an attractive diagnostic marker
and a new target for ovarian cancer treatment.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodríguez Villalba S1, Díaz-Caneja Planell
C and Cervera Grau JM: Current opinion in cervix carcinoma. Clin
Transl Oncol. 13:378–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roy DM and Walsh LA: Candidate prognostic
markers in breast cancer: Focus on extracellular proteases and
their inhibitors. Breast Cancer. 6:81–91. 2014.PubMed/NCBI
|
|
4
|
Netzel-Arnett S, Hooper JD, Szabo R,
Madison EL, Quigley JP, Bugge TH and Antalis TM: Membrane anchored
serine proteases: A rapidly expanding group of cell surface
proteolytic enzymes with potential roles in cancer. Cancer
Metastasis Rev. 22:237–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bugge TH, Antalis TM and Wu Q: Type II
transmembrane serine proteases. J Biol Chem. 284:23177–23181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hooper JD, Clements JA, Quigley JP and
Antalis TM: Type II transmembrane serine proteases. Insights into
an emerging class of cell surface proteolytic enzymes. J Biol Chem.
276:857–860. 2001. View Article : Google Scholar
|
|
7
|
Scott HS, Kudoh J, Wattenhofer M, Shibuya
K, Berry A, Chrast R, Guipponi M, Wang J, Kawasaki K, Asakawa S, et
al: Insertion of beta-satellite repeats identifies a transmembrane
protease causing both congenital and childhood onset autosomal
recessive deafness. Nat Genet. 27:59–63. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wallrapp C, Hähnel S, Müller-Pillasch F,
Burghardt B, Iwamura T, Ruthenbürger M, Lerch MM, Adler G and Gress
TM: A novel transmembrane serine protease (TMPRSS3) overexpressed
in pancreatic cancer. Cancer Res. 60:2602–2606. 2000.PubMed/NCBI
|
|
9
|
Iacobuzio-Donahue CA, Ashfaq R, Maitra A,
Adsay NV, Shen-Ong GL, Berg K, Hollingsworth MA, Cameron JL, Yeo
CJ, Kern SE, et al: Highly expressed genes in pancreatic ductal
adenocarcinomas: A comprehensive characterization and comparison of
the transcription profiles obtained from three major technologies.
Cancer Res. 63:8614–8622. 2003.PubMed/NCBI
|
|
10
|
Al Saleh S, Sharaf LH and Luqmani YA:
Signalling pathways involved in endocrine resistance in breast
cancer and associations with epithelial to mesenchymal transition
(Review). Int J Oncol. 38:1197–1217. 2011.PubMed/NCBI
|
|
11
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kebebew E, Peng M, Reiff E, Duh QY, Clark
OH and McMillan A: ECM1 and TMPRSS4 are diagnostic markers of
malignant thyroid neoplasms and improve the accuracy of fine needle
aspiration biopsy. Ann Surg. 242:353–361. 2005.PubMed/NCBI
|
|
13
|
Liang B, Wu M, Bu Y, Zhao A and Xie F:
Prognostic value of TMPRSS4 expression in patients with breast
cancer. Med Oncol. 30:4972013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guerrero K, Wang Z, Bachvarova M, Gregoire
J, Renaud MC, Plante M and Bachvarov D: A novel genome-based
approach correlates TMPRSS3 overexpression in ovarian cancer with
DNA hypomethylation. Gynecol Oncol. 125:720–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Guo H, Mi Z, Gao C, Bhattacharya
S, Li J and Kuo PC: EF1A1-actin interactions alter mRNA stability
to determine differential osteopontin expression in HepG2 and Hep3B
cells. Exp Cell Res. 315:304–312. 2009. View Article : Google Scholar
|
|
16
|
Guan H, Liang W, Liu J, Wei G, Li H, Xiu
L, Xiao H and Li Y: Transmembrane protease serine 4 promotes
thyroid cancer proliferation via CREB phosphorylation. Thyroid.
25:85–94. 2015. View Article : Google Scholar
|
|
17
|
Larzabal L, Nguewa PA, Pio R, Blanco D,
Sanchez B, Rodríguez MJ, Pajares MJ, Catena R, Montuenga LM and
Calvo A: Overexpression of TMPRSS4 in non-small cell lung cancer is
associated with poor prognosis in patients with squamous histology.
Br J Cancer. 105:1608–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim S, Kang HY, Nam EH, Choi MS, Zhao XF,
Hong CS, Lee JW, Lee JH and Park YK: TMPRSS4 induces invasion and
epithelial-mesenchymal transition through upregulation of integrin
alpha5 and its signaling pathways. Carcinogenesis. 31:597–606.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Zeng ZC, Wang L, Qiu SJ, Zhou JW,
Zhi XT, Yu HH and Tang ZY: Radiation enhances long-term metastasis
potential of residual hepatocellular carcinoma in nude mice through
TMPRSS4-induced epithelial-mesenchymal transition. Cancer Gene
Ther. 18:617–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leshem O, Madar S, Kogan-Sakin I, Kamer I,
Goldstein I, Brosh R, Cohen Y, Jacob-Hirsch J, Ehrlich M,
Ben-Sasson S, et al: TMPRSS2/ERG promotes epithelial to mesenchymal
transition through the ZEB1/ZEB2 axis in a prostate cancer model.
PLoS One. 6:e216502011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neuzillet C, Tijeras-Raballand A, de
Mestier L, Cros J, Faivre S and Raymond E: MEK in cancer and cancer
therapy. Pharmacol Ther. 141:160–171. 2014. View Article : Google Scholar
|
|
24
|
Schmitt JM, Abell E, Wagner A and Davare
MA: ERK activation and cell growth require CaM kinases in MCF-7
breast cancer cells. Mol Cell Biochem. 335:155–171. 2010.
View Article : Google Scholar
|