Introduction
Hepatocellular carcinoma (HCC) is a common liver
malignancy and its prognosis is poor despite advancements in
treatment (1). The treatment
options for HCC include local ablation, surgery, transcatheter
arterial chemoembolization, and systemic chemotherapy (2,3).
Molecular therapy has been under investigation for the development
of a novel treatment for HCC (4).
Glycolysis is more pronounced in cancer cells than
in normal cells. Cancer cells require more glucose under conditions
of a sufficient oxygen supply (Warburg effect) (5). The Warburg effect is strong in cancer
cells, but weak in normal cells. If glycolysis is inhibited, it is
expected that cancer cells die while normal cells survive (6). Thus, inhibition of glycolysis is a new
treatment strategy for HCC (7).
Pyruvate is the final product of glycolysis and
enters the citric acid cycle (8).
3-Bromopyruvate (3BP) is an analogue of pyruvate (9) and is used as an inhibitor of
glycolysis. 3BP inhibits the activity of glyceraldehyde-3-phosphate
dehydrogenase (10), and unlike
other chemotherapeutic agents, it is less toxic to cells as it
mimics pyruvate (9). Therefore, 3BP
can be used for the treatment of cancer (11).
In the present study, we investigated the
suppressive effects of 3BP on the proliferation and motility of HCC
cells.
Materials and methods
Cell culture
HCC cell lines, HLF and PLC/PRF/5, were purchased
from the Riken Cell Bank (Tsukuba, Japan) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis,
MO, USA) supplemented with 10% fetal bovine serum (FBS; Life
Technologies, Grand Island, NY, USA). The cells were cultured in
10-cm dishes (Asahi Techno Glass, Funabashi, Japan) with 5% carbon
dioxide at 37°C in a humidified chamber.
Cell proliferation assay
The cells were trypsinized, harvested, and spread on
96-well plates (Asahi Techno Glass) at a density of 1,000
cells/well. The cells were cultured in DMEM supplemented with 10%
FBS. 3BP was added at 0, 1, 3, 10, 30, or 100 µM to the
culture medium. The cells were cultured for 72 h and subjected to
3-(4,5-dimethylthiazol-2-yl)-5-(3-carb
oxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
(MTS) assay, according to the manufacturer's instructions (Promega
Corporation, Madison, WI, USA). MTS is reduced by cells to a
colored formazan product with an absorbance maximum at 490 nm. The
absorbance was measured using an iMark microplate absorbance reader
(Bio-Rad, Hercules, CA, USA).
Real-time quantitative polymerase chain
reaction
Total RNA (5 µg), which was isolated using
Isogen (Nippon Gene, Tokyo, Japan), was used for the first-strand
cDNA synthesis with SuperScript III and oligo(dT) according to the
manufacturer's instructions (Life Technologies). Real-time
quantitative PCR was performed using Fast SYBR Green Master Mix
(Life Technologies) with Mini Opticon (Bio-Rad). The results were
analyzed using the Mini Opticon system (Bio-Rad). Real-time
quantitative PCR was performed for 40 cycles, with 5 sec of
denaturation and 5 sec of annealing-extension. Table I shows primer sequences used. RPL19
was used as an internal control since it is a housekeeping gene
that is constitutively expressed (12).
 | Table IPrimer sequences used for real-time
quantitative PCR. |
Table I
Primer sequences used for real-time
quantitative PCR.
| Primer name | Sequence | Description | Product size
(bp) | Annealing temperature
(°C) | Cycle | GenBank |
|---|
| OMC355 |
5′-AGAGGCGGAGGAGAACAAACAG-3′ | Cyclin D1,
forward | 180 | 60 | 40 | NM_053056 |
| OMC356 |
5′-AGGCGGTAGTAGGACAGGAAGTTG-3′ | Cyclin D1,
reverse | | | | |
| OMC749 |
5′-CCTGGGCAGATTCCAAACCT-3′ | MMP9, forward | 89 | 60 | 40 | NM_004994 |
| OMC750 |
5′-GCAAGTCTTCCGAGTAGTTTTGGAT-3′ | MMP9, reverse | | | | |
| OMC321 |
5′-CGAATGCCAGAGAAGGTCAC-3′ | RPL19, forward | 157 | 60 | 40 | BC095445 |
| OMC322 |
5′-CCATGAGAATCCGCTTGTTT-3′ | RPL19, reverse | | | | |
Scratch assay and hematoxylin and eosin
staining
The cells were plated on 4-well chamber slides
(Becton Dickinson, Franklin Lakes, NJ, USA). When the cells reached
confluency, they were scratched with a sterile razor. The cells
were incubated with 3BP (0 or 100 µM) for 48 h and stained
with hematoxylin and eosin. The cells were plated in 4-well chamber
slides (Becton Dickinson) for the analysis of apoptosis. The cells
were incubated with 3BP (0 or 100 µM) for 48 h and stained
with hematoxylin and eosin. The stained slides were observed under
an AX80 microscope (Olympus, Tokyo, Japan) for the analysis of
apoptosis and scratch assay. For the scratch assay, the distance
between the scratched line and the growing edges of the cells was
measured at five different points.
Statistical analysis
Data were analyzed by one-way analysis of variance
(ANOVA) and statistical analysis was carried out with JMP 10.0.2
software (SAS Institute, Cary, NC, USA). P-values <0.05 were
determined to be statistically significant.
Results
To address the possibility that 3BP suppresses cell
proliferation, HLF (Fig. 1A) and
PLC/PRF/5 cells (Fig. 1B) were
incubated with 3BP. After 72 h of incubation, the cells were
subjected to MTS assay. Proliferation of both cell lines was
significantly suppressed (P<0.05).
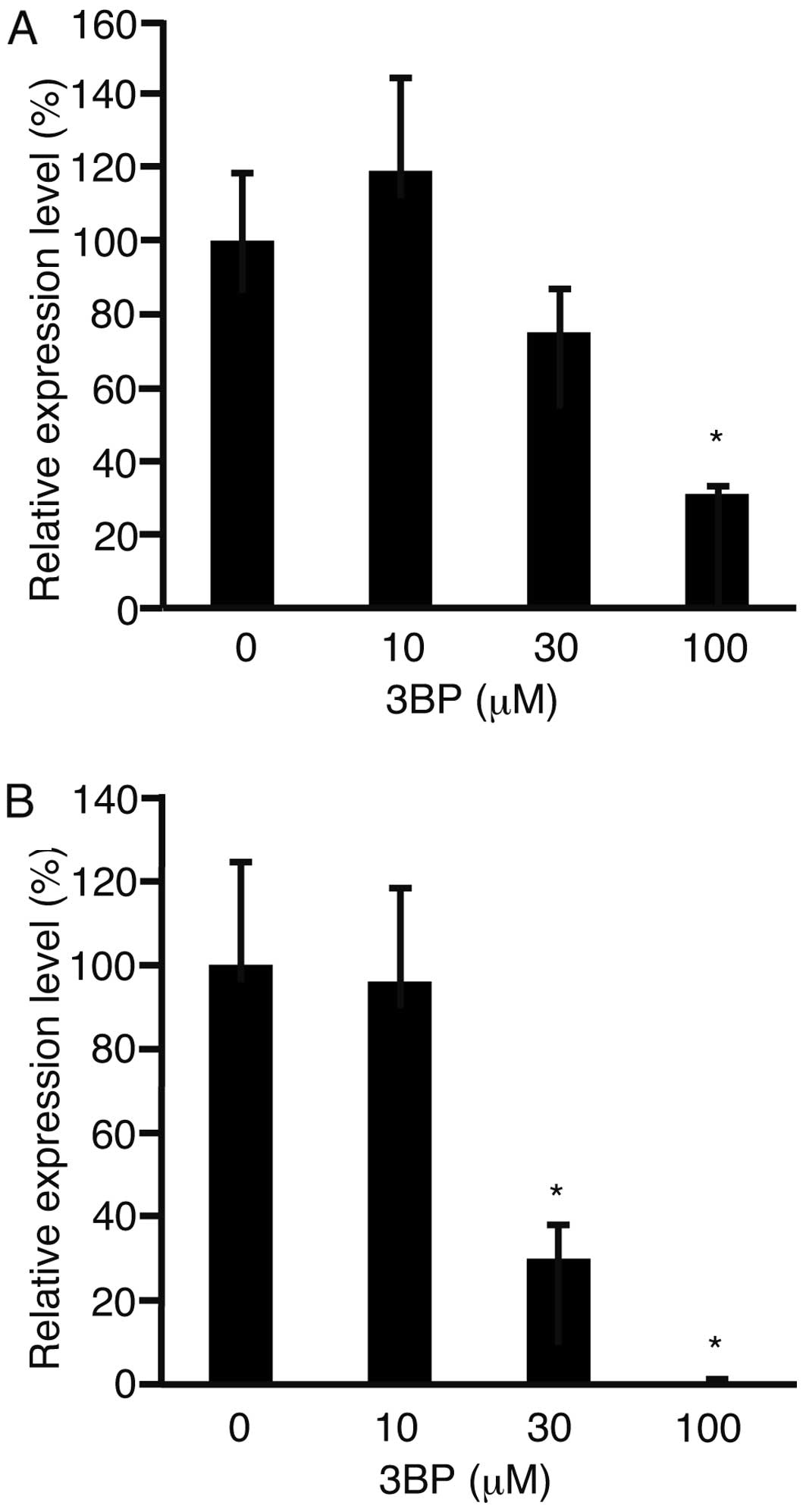
The expression levels of cyclin D1 were analyzed
with real-time quantitative PCR, as this gene plays a role in cell
proliferation (Fig. 2) (13). The expression levels of cyclin D1
were decreased in both cell lines (P<0.05).
We hypothesized that apoptosis may be involved in
the suppression of cell proliferation. To clarify the role of
apop-tosis, hematoxylin and eosin staining was performed in the HLF
(Fig. 3A and B) and PLC/PRF/5 cells
(Fig. 3C and D) after a 48-h
incubation with 3BP at 0 µM (Fig. 3A and C) or 100 µM (Fig. 3B and D). Pyknotic nuclei were
observed in the cells cultured with 3BP at 100 µM
(arrows).
In order to investigate cell motility, a scratch
assay was performed in the HLF (Fig. 4A
and B) and PLC/PRF/5 cells (Fig. 4C
and D) after a 48-h incubation with 3BP at 0 µM
(Fig. 4A and C) or 100 µM
(Fig. 4B and D). The distance
between the growing edges of the cells and the scratched line was
measured (Fig. 4E). Cell motility
was significantly suppressed in both cell lines (P<0.05).
MMP9 is involved in cell motility (14). RNA was isolated from the HLF
(Fig. 5A) and PLC/PRF/5 cells
(Fig. 5B) and subjected to
real-time quantitative PCR. The expression levels of MMP9 were
significantly decreased in both cell lines (P<0.05).
Discussion
3BP reduces HCC cell activity and induces apoptosis
(15). Ganapathy-Kanniappan et
al found that 3BP at 150 µM suppressed the proliferation
of Hep3B cells (16). In our study,
3BP at 100 µM induced the apoptosis of HLF and PLC/PRF/5
cells. Although the cells were different, the concentration of 3BP
was almost the same between the previous report and our study.
These reports and our data suggest that 3BP induces apoptosis in
HCC cells at 100–150 µM.
Our results clearly showed that proliferation of HCC
cells was suppressed by 3BP, and 3BP also inhibited the cell cycle
(17). No reports exist on the
effects of 3BP on cyclin D1 expression. In the present study, the
expression of cyclin D1 decreased after 3BP treatment. This result
indicated that cyclin D1 was involved in the suppression of cell
proliferation by 3BP. However, the detailed mechanism underlying
the decrease in the expression of cyclin D1 is yet to be
elucidated.
3BP in combination with 2-deoxyglucose, which is
another inhibitor of glycolysis, was found to suppress the motility
of breast cancer cells (18). In
the present study, 3BP alone successfully suppressed the motility
of HLF and PLC/PRF/5 cells. Moreover, the expression levels of MMP9
decreased. The previous report and our results clearly showed that
3BP suppressed the motility of cancer cells by decreasing the
expression of matrix metalloproteinase with or without the
combination of other agents.
One major limitation of 3BP is that it exerts
limited antitumor effects in in vivo animal models (19). To overcome this limitation, a
combination of 3BP and other agents could be employed (20). Furthermore, it is proposed that 3BP
should be administered to patients in combination with other
chemotherapeutic agents during transcatheter arterial
chemoembolization (21). No harmful
effects were reported in an animal model (22).
In conclusion, 3BP suppressed the proliferation and
motility of HCC cells by decreasing the expression of cyclin D1 and
matrix metalloproteinase.
References
|
1
|
Tejeda-Maldonado J, García-Juárez I,
Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M,
Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF and
Carrillo-Pérez DL: Diagnosis and treatment of hepatocellular
carcinoma: An update. World J Hepatol. 7:362–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar :
|
|
3
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: Past, present, and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen C and Wang G: Mechanisms of
hepatocellular carcinoma and challenges and opportunities for
molecular targeted therapy. World J Hepatol. 7:1964–1970. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaitheesvaran B, Xu J, Yee J, Q-Y L, Go
VL, Xiao GG and Lee WN: The Warburg effect: A balance of flux
analysis. Metabolomics. 11:787–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang M, Kim SS and Lee J: Cancer cell
metabolism: Implications for therapeutic targets. Exp Mol Med.
45:e452013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Granja S, Pinheiro C, Reis RM, Martinho O
and Baltazar F: Glucose addiction in cancer therapy: Advances and
drawbacks. Curr Drug Metab. 16:221–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ngo DC, Ververis K, Tortorella SM and
Karagiannis TC: Introduction to the molecular basis of cancer
metabolism and the Warburg effect. Mol Biol rep. 42:819–823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shoshan MC: 3-Bromopyruvate: Targets and
outcomes. J Bioenerg Biomembr. 44:7–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganapathy-Kanniappan S, Geschwind JF,
Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, Cole RN, Syed
LH, Rao PP, Ota S, et al: Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell
death. Anticancer Res. 29:4909–4918. 2009.
|
|
11
|
Cardaci S, Desideri E and Ciriolo MR:
Targeting aerobic glycolysis: 3-bromopyruvate as a promising
anticancer drug. J Bioenerg Biomembr. 44:17–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metallopro-teinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ganapathy-Kanniappan S, Geschwind JF,
Kunjithapatham R, Buijs M, Syed LH, Rao PP, Ota S, Kwak BK, Loffroy
R and Vali M: 3-Bromopyruvate induces endoplasmic reticulum stress,
overcomes autophagy and causes apoptosis in human HCC cell lines.
Anticancer Res. 30:923–935. 2010.PubMed/NCBI
|
|
16
|
Ganapathy-Kanniappan S, Kunjithapatham R,
Torbenson MS, Rao PP, Carson KA, Buijs M, Vali M and Geschwind JF:
Human hepatocellular carcinoma in a mouse model: Assessment of
tumor response to percutaneous ablation by using
glyceral-dehyde-3-phosphate dehydrogenase antagonists. Radiology.
262:834–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XH, Zheng XF and Wang YL: Inhibitive
effect of 3-bromo-pyruvic acid on human breast cancer MCF-7 cells
involves cell cycle arrest and apoptotic induction. Chin Med J
(Engl). 122:1681–1685. 2009.
|
|
18
|
Feng X, Wang P, Liu Q, Zhang T, Mai B and
Wang X: Glycolytic inhibitors 2-deoxyglucose and 3-bromopyruvate
synergize with photodynamic therapy respectively to inhibit cell
migration. J Bioenerg Biomembr. 47:189–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jae HJ, Chung JW, Park HS, Lee MJ, Lee KC,
Kim HC, Yoon JH, Chung H and Park JH: The antitumor effect and
hepatotoxicity of a hexokinase II inhibitor 3-bromopyruvate: In
vivo investigation of intraarterial administration in a rabbit VX2
hepatoma model. Korean J Radiol. 10:596–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu SJ, Yoon JH, Yang JI, Cho EJ, Kwak MS,
Jang ES, Lee JH, Kim YJ, Lee HS and Kim CY: Enhancement of
hexokinase II inhibitor-induced apoptosis in hepatocellular
carcinoma cells via augmenting Er stress and anti-angiogenesis by
protein disulfide isomerase inhibition. J Bioenerg Biomembr.
44:101–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liapi E and Geschwind JF: Interventional
oncology: new options for interstitial treatments and intravascular
approaches: targeting tumor metabolism via a loco-regional
approach: a new therapy against liver cancer. J Hepatobiliary
Pancreat Sci. 17:405–406. 2010. View Article : Google Scholar
|
|
22
|
Geschwind JF, Ko YH, Torbenson MS, Magee C
and Pedersen PL: Novel therapy for liver cancer: Direct
intraarterial injection of a potent inhibitor of ATP production.
Cancer Res. 62:3909–3913. 2002.PubMed/NCBI
|