Introduction
Ovarian cancer is a common lethal gynecological
malignancy worldwide, causing over 140,000 deaths every year
(1,2). Although improved debulking surgery and
the introduction of platinum-taxane regimens have been applied in
its treatment, the 5-year survival rate is ~40% (3). This poor prognosis is mostly related
to late diagnosis; approximately 70% of patients are diagnosed at
the advanced stages of ovarian cancer [International Federation of
Gynecology and Obstetrics (FIGO) III/IV] (2,4). It is
difficult to diagnose ovarian cancer at its early stages (FIGO
I/II) before it spreads and advances to later stages (FIGO III/IV),
as the majority of symptoms are non-specific and thus of little use
in the diagnosis at the early stage (4). Serum molecular tests such as cancer
antigen (CA) 125 test are useful in differential diagnosis, but
have not been demonstrated as effective methods for screening for
early-stage ovarian cancer due to the unacceptable low sensitivity
and specificity (5). It has been
considered that numerous other genetic changes are involved in the
development and progression of ovarian cancer (6,7).
However, little is known concerning the exact molecular events
leading to its development and progression. Therefore, further
understanding of the molecular mechanism and discovering valuable
diagnostic markers as well as novel therapeutic strategies are
major challenges in ovarian cancer.
Prostate and breast cancer overexpressed 1
(PBOV1, or UROC28 or UC28), a human
protein-coding gene with a 135-amino acid open reading frame, is
overexpressed in prostate, breast and bladder cancer, but has not
been demonstrated as being overexpressed in lung and colon cancer
tissues (8). PBOV1 mRNA and protein
were found to be upregulated in glandular epithelial cells of
prostate cancer, and were regulated by androgen treatment (9). Compared with normal individuals,
patients with prostate cancer had higher serum PBOV1 protein
levels. In breast cancer cells, frequently overexpressed PBOV1 was
downregulated by estradiol in a dose-dependent manner (9). Fluorescence in situ
hybridization showed that there were increased copy numbers of the
gene loci encoding PBOV1 in 67% of prostate tumor foci (10). Another group suggested that the
PBOV1 rs6927706 polymorphism may be a risk factor for breast cancer
(11). Using comparative genomics
analysis, Samusik et al showed that the PBOV1 protein-coding
sequence is 80% unique to humans and originated de novo
during primate evolution through a series of frame-shift and stop
codon mutations. They found that PBOV1 is expressed in
multiple tumor types and higher levels of PBOV1 expresion
positively correlate with relapse-free survival in breast cancer
(12). As with breast and prostate
cancer, ovarian cancer is closely linked to sex hormones.
Therefore, fully elucidating the status of PBOV1 expression and its
clinical/prognostic relevance in ovarian cancer is warranted.
In the present study, we report, for the first time,
the characterization of PBOV1 expression in human ovarian cancer
tissues and its correlation with clinicopathological features.
PBOV1 expression was negatively correlated with ovarian cancer FIGO
stage, T/N/M classification, and growth. The effectiveness of PBOV1
as an independent prognostic factor was assessed using multivariate
analysis. Our results strongly suggest that PBOV1 could be a
potential promising biomarker for predicting the prognosis of
patients with ovarian cancer, serve as a tumor-suppressor gene, and
may be a potential target for ovarian cancer therapy.
Materials and methods
Patients and tissue specimens
A total of 209 paraffin-embedded epithelial ovarian
specimens, comprising 17 normal ovarian tissues [also scratched and
used as control in real-time reverse transcription (RT)-PCR and
western blotting], 13 cystadenoma tissues, 14 borderline tumor
tissues, and 165 invasive carcinoma tissues, were obtained from
paraffin-embedded tissues archived between 1996 and 2007 and
histopathologically diagnosed at the Department of Pathology,
Cancer Center, Sun Yat-Sen University (Guangzhou, China). The 17
normal ovary specimens were obtained from excise for non-ovarian
diseases. Four non-cancerous ovarian tissues (N1–N4) and five
ovarian cancer tissues (T1–T5) were obtained by resection from 9
different patients with ovarian cancer at the Cancer Center of Sun
Yat-Sen University. All tissues were pathologically characterized,
and Table I summarizes the clinical
information concerning the ovarian cancer samples. None of the
cancer patients in this study had received preoperative radiation
or chemotherapy. The use of the clinical specimens was approved by
the Local Institutional Review Board and the ethics Committee of
the Sun Yat-Sen University Cancer Center (Guangzhou, Guangdong,
China), and conformed to the ethical guidelines of the Helsinki
Declaration.
 | Table IAssociation of PBOV1 expression with
clinicopathological features of the ovarian carcinoma cases. |
Table I
Association of PBOV1 expression with
clinicopathological features of the ovarian carcinoma cases.
| All cases | PBOV1 protein
| P-value |
|---|
| Low expression | High
expression |
|---|
| Age at surgery
(years) |
| ≤47.6 | 74 | 44 | 30 | 0.25 |
| >47.6 | 91 | 62 | 29 | |
| Histological
type |
| Serous | 95 | 65 | 30 | 0.333 |
| Mucinous | 36 | 20 | 16 | |
| Others | 34 | 21 | 13 | |
| Histological grade
(Silverberg) |
| G1 | 15 | 7 | 8 | 0.01 |
| G2 | 94 | 56 | 38 | |
| G3 | 56 | 43 | 13 | |
| pT status |
| pT1 | 23 | 9 | 14 | <0.001 |
| pT2 | 25 | 10 | 15 | |
| pT3 | 117 | 87 | 30 | |
| pN status |
| pN0 | 53 | 27 | 26 | 0.014 |
| pN1 | 111 | 79 | 32 | |
| pM status |
| pMX | 129 | 77 | 52 | 0.021 |
| pM1 | 36 | 29 | 7 | |
| FIGO stage |
| I | 23 | 9 | 14 | <0.001 |
| II | 14 | 4 | 10 | |
| III | 92 | 64 | 28 | |
| IV | 36 | 29 | 7 | |
Cell culture
Primary normal breast epithelial cells (NBECs) were
purification and cultured from the excised tissue from a
30-year-old woman with breast plastic surgery at the First
Affiliated Hospital of Sun Yat-Sen University (China). Primary
NBECs and the ovarian cancer cell lines were maintained according
to our previous study (13).
RNA extraction and real-time quantitative
PCR
Total RNA from cultured cells was extracted using
TRIzol reagent (Invitrogen) according to the manufacturer's
instructions. Complementary DNA (cDNA) was amplified and quantified
using an ABI Prism 7500 Sequence Detection system (Applied
Biosystems, Foster City, CA, USA) and SYBR Green I (Molecular
Probes, Invitrogen). The primers used were as follows: PBOV1
forward, 5′-TGAGTCCCCTCTCGGTAATG-3′ and reverse,
5′-GCCCCGAGTTAAGAACATCA-3′. Expression data were normalized to the
geometric mean of the housekeeping gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) to control variability in expression
levels (forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′), and calculated as 2− [(CT
of PBOV1) − (CT of
GAPDH)], where CT represents the
threshold cycle for each transcript.
Plasmid and transfection
The full-length sequence of PBOV1 is 408-bp
long and was cloned into a pSin plasmid (Promega, Madison, WI,
USA). Retroviral production and infection were performed as
previously described (14).
Western blotting
Western blotting was performed according to standard
methods as previously described, using anti-PBOV1 and anti-Ki67
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) (13). Each sample was detected and analyzed
three times.
Immunohistochemistry (IHC)
The IHC procedure and PBOV1 expression scoring were
performed as previously described (15). The proportion of positively stained
tumor cells was graded as follows: 0 (no positive tumor cells), 1
(<10% positive tumor cells), 2 (10–50% positive tumor cells),
and 3 (>50% positive tumor cells). Each staining intensity was
scored on a scale of 0 (no staining), 1 (weak staining, light
yellow), 2 (moderate staining, yellowish brown), and 3 (strong
staining, brown). The staining index was calculated as in our
previous study (13). Cut-off
values for defining high and low PBOV1 expression were selected
based on a measure of heterogeneity with log-rank test statistics
with respect to overall survival, and an optimal cut-off value was
identified. The staining index scores >6 and <4 were used to
define tumors with high and low PBOV1 expression, respectively. The
AxioVision Rel. 4.6 computerized image analysis system assisted by
an automated measurement program (Carl Zeiss, Oberkochen, Germany)
was used for quantitative analysis of the IHC staining.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay
Cells were cultured in 96-well plates
(1×104/well). At various time-points, 0.5 mg/ml MTT was
used to treat cells for 4 h at 37° C. The medium was removed, and
150 µl dimethyl sulphoxide (DMSO; Sigma-Aldrich) was added
and then absorbance values were measured. All experiments were
performed in triplicate as previously reported (16).
Colony formation assay
Cells (1,000/plate) were incubated in 6-well plates
for 10 days. The colonies were stained with 1.0% crystal violet for
30 sec, followed by 5-min fixation in 10% formaldehyde.
Anchorage-independent growth assay
Cells [500 in 2 ml complete medium plus 0.3% agar
(Sigma-Aldrich)] were seeded in 6-well plates. All experiments were
performed in triplicate for each cell line according to our
previous study (16).
Xenograft tumor model
BALB/c-nu mice (4–5 weeks old, 18–20 g) were
purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha,
Hunan, China). The Institutional Animal Care and Use Committee of
Sun Yat-Sen University approved all experimental procedures. Each
mouse was subcutaneously injected in situ with SKOV3-vector
cells (5×106) on the left side and with SKOV3-PBOV1
cells (5×106) on the right side. Tumors were examined
every five days; length (L) and width (W) were measured using
calipers, and tumor volumes were calculated using the equation (L ×
W2)/2. On day 40, the animals were euthanized, and the
tumors were excised and weighed.
Statistical analysis
All statistical analyses were carried out using the
SPSS v. 13.0 statistical software packages. The relationship
between PBOV1 expression and clinicopathological characteristics
was analyzed using the Chi-square test. Bivariate correlations
between study variables were calculated by Spearman's rank
correlation coefficients. Survival curves were plotted using the
Kaplan-Meier method and compared using the log-rank test. Survival
data were evaluated using Univariate and multivariate Cox
regression analyses. A p-value <0.05 was considered to indicate
a statistically significant result in all cases.
Results
PBOV1 upregulation in ovarian cancer cell
lines
Representative overexpression of PBOV1 has been
reported in breast and prostate cancer (12). Its expression status in ovarian
cancer, however, remains unclear. To determine PBOV1 protein
expression, western blotting was performed using protein samples
from human normal ovarian tissues, NBECs, and ovarian cancer cell
lines (OVCAR4, SKOV3 COV644, OV56, TOV-21G, OV90). To investigate
whether PBOV1 was upregulated at the transcription level,
PBOV1 mRNA was quantified using real-time PCR. Both PBOV1
protein and mRNA expression were markedly upregulated in the
ovarian cancer cell lines (Fig. 1A and
B).
PBOV1 upregulation in ovarian cancer
tissues
To determine whether PBOV1 upregulation in ovarian
cancer cell lines is clinically correlated with ovarian cancer
progression, real-time PCR analysis and western blotting were
performed using non-cancerous ovarian tissues from four patients
with other ovarian diseases and ovarian cancer tissues from 5
patients with ovarian cancer. Both PBOV1 mRNA and protein levels
were differentially overexpressed in the primary ovarian cancer
samples (Fig. 1C and D).
Quantification determined that all five tumors had >3-fold
increased PBOV1 mRNA compared with the normal tissues (Fig. 1D).
IHC staining of PBOV1 protein in human
ovarian tissues
To investigate PBOV1 upregulation in ovarian cancer,
we further examined PBOV1 expression in 165 paraffin-embedded,
archived ovarian tissues, and found that PBOV1 protein was located
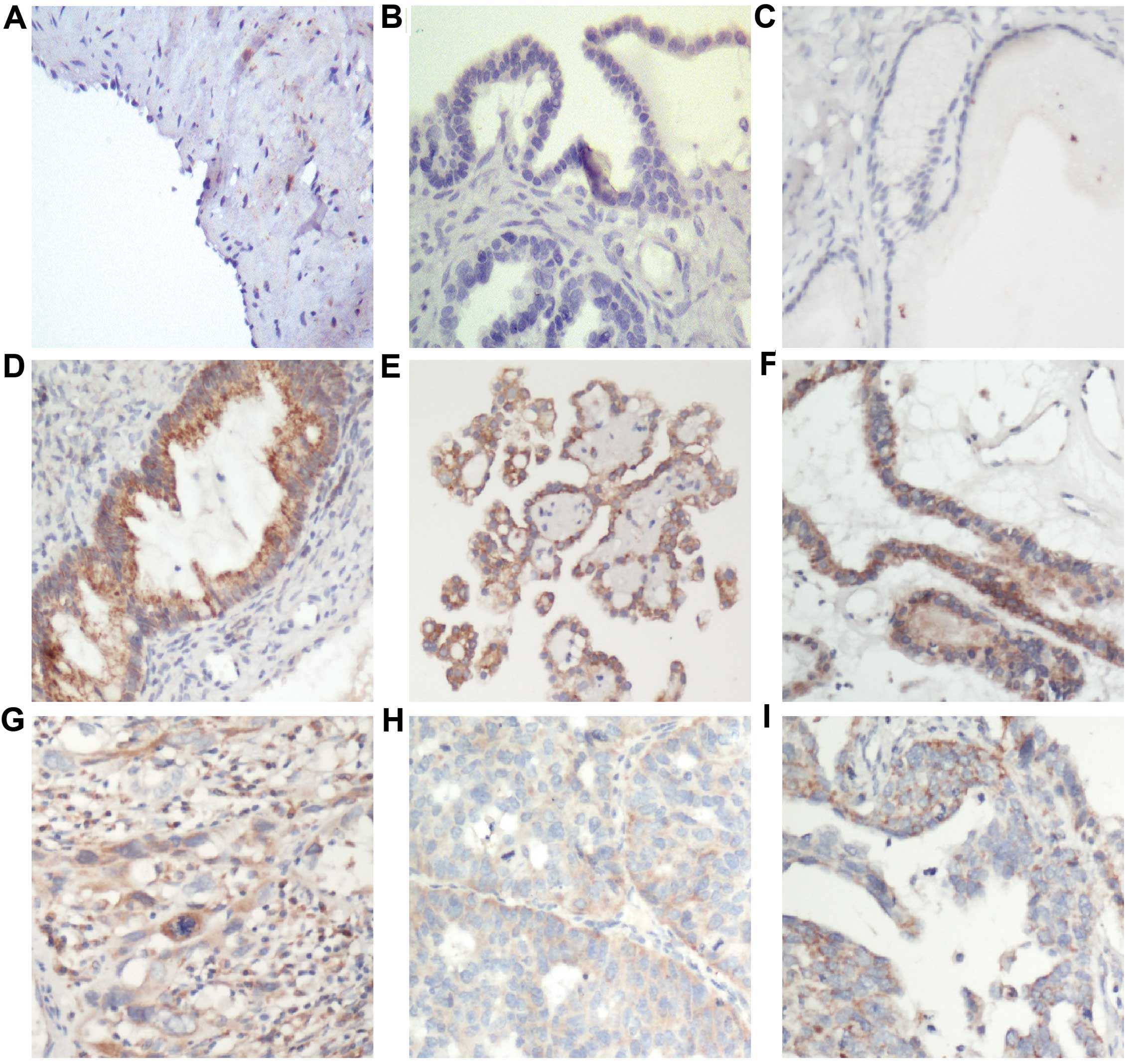
in the cytoplasm and cell membrane. As shown in Fig. 2, there was negative PBOV1 expression
in normal ovarian surface epithelium (Fig. 2A; detected in none of the 17
samples), cystadenoma (Fig. 2B;
detected in 1 of the 13 samples), and borderline tumors (Fig. 2C; detected in 2 of the 14 samples;
Table II). Although PBOV1 was
upregulated in the tumor tissues, the expression levels differed.
In the 165 patients with ovarian cancer, there was high PBOV1
expression in 35.8% of the samples (Fig. 2D–F) and low expression in 64.2% of
the samples (Fig. 2G–I). There was
differential expression of PBOV1 protein between the types of
ovarian cancer, such as serous ovarian cancer (Fig. 2e and H) and mucinous ovarian cancer
(Fig. 2F and I). Taken together,
these observations suggest that PBOV1 expression is a common
feature of ovarian cancer.
 | Table IIPBOV1 expression in normal ovaries
and in benign and malignant epithelial ovarian tumors. |
Table II
PBOV1 expression in normal ovaries
and in benign and malignant epithelial ovarian tumors.
| Samples | All cases | PBOV1 protein
|
|---|
| High expression
(%) | Low expression
(%) | Negative expression
(%) |
|---|
| Normal ovaries | 17 | 0 (0.0) | 0 (0.0) | 17 (100.0) |
| Cystadenomas | 13 | 0 (0.0) | 1 (7.7) | 12 (92.3) |
| Borderline
tumors | 14 | 0 (0.0) | 2 (16.7) | 12 (83.3) |
| Invasive
carcinomas | 165 | 59 (35.8) | 106 (64.2) | 0 (0) |
Association of PBOV1 expression with
ovarian cancer clinicopathological features
We studied the association between PBOV1 expression
in ovarian cancer and several known clinicopathological features.
PBOV1 expression was negatively correlated with histological grade
(P=0.01), pT status (P<0.001), pN status (P=0.014), pM status
(P<0.021), and FIGO stage (P<0.001). There was no significant
correlation between PBOV1 expression and histological type or
patient age at surgery (P>0.05, Table I). Taken together, these
observations support the notion that ovarian cancer progression is
associated with decreased PBOV1 expression.
Association between PBOV1 expression and
survival
The statistical analysis findings in Table IV revealed a positive correlation
between the PBOV1 level and patient survival (P<0.001). In
conjunction with PBOV1 protein expression, established prognostic
predictors of patient survival, including histological grade,
pT/pN/pM status, and FIGO stage, were evaluated with Kaplan-Meier
analysis and the log-rank test. As shown in Fig. 3, survival was significantly
different between patients with low and high PBOV1 expression
(P<0.001): patients with high PBOV1 expression had a longer
overall survival. The cumulative 5-year survival rate in the
high-PBOV1 expression group was 86%; in the low-PBOV1 expression
group, it was 63% (Fig. 3A).
Univariate and multivariate analyses were used to determine whether
PBOV1 expression level is an independent prognostic factor of
patient outcome. Tables III and
IV showed that PBOV1 expression,
as well as FIGO stage and histological grade, were independent
prognostic factors. Furthermore, the prognostic value of PBOV1
expression in specific patient subgroups was evaluated according to
clinical staging. Despite the difference in overall survival length
between the low- and high-PBOV1 expression groups, overall survival
did not differ in the early clinical subgroups (stages I and II,
n=37; log-rank, P<0.001; Fig.
3B). In the advanced disease group (stages III and IV, n=128),
patients with high PBOV1 expression had significantly higher
overall survival rates compared with those with low PBOV1
expression (P<0.001; Fig. 3C).
Taken together, our data suggest that PBOV1 may represent a novel
and potentially useful independent prognostic biomarker for
patients with ovarian cancer.
 | Table IVMultivariate analysis of overall
survival (Cox regression model). |
Table IV
Multivariate analysis of overall
survival (Cox regression model).
| Variable | Relative risk | 95% confidence
interval | P-value |
|---|
| PBOV1 | 3.017 | 1.771–5.141 | <0.001 |
| FIGO stage | 0.65 | 0.401–1.055 | 0.036 |
| Histological
grade | 0.519 | 0.335–0.804 | 0.005 |
 | Table IIIUnivariate survival analysis
(log-rank test) of the clinicopathological parameters and PBOV1
expression in the prognosis of 164 patients with ovarian
carcinoma. |
Table III
Univariate survival analysis
(log-rank test) of the clinicopathological parameters and PBOV1
expression in the prognosis of 164 patients with ovarian
carcinoma.
| Variable | All cases | Mean survival
(months) | Median survival
(months) | P-value |
|---|
| Age at surgery
(years) |
| ≤47.6 | 74 | 76.7 | 67.4 | 0.323 |
| >47.6 | 91 | 63.4 | 61.9 | |
| Histological
type |
| Serous | 95 | 67.3 | 58.6 | 0.157 |
| Mucinous | 36 | 82.6 | 91.7 | |
| Others | 34 | 69.3 | 84.0 | |
| Histological grade
(Silverberg) |
| G1 | 15 | 97.8 | 108.4 | <0.001 |
| G2 | 94 | 71.4 | 74.1 | |
| G3 | 56 | 51.6 | 48.6 | |
| pT status |
| pT1 | 23 | 82.5 | 108.4 | 0.003 |
| pT2 | 25 | 87.1 | – | |
| pT3 | 117 | 65.2 | 59.7 | |
| pN status |
| pN0 | 53 | 84.7 | 108.4 | 0.006 |
| pN1 | 112 | 65.4 | 59.7 | |
| pM status |
| pMX | 129 | 75.8 | 74.1 | 0.005 |
| pM1 | 36 | 55.9 | 58.6 | |
| FIGO stage |
| I | 23 | 82.4 | 108.4 | <0.001 |
| II | 14 | 102.6 | – | |
| III | 92 | 67.9 | 68.5 | |
| IV | 36 | 55.9 | 58.6 | |
| PBOV1
expression |
| Low | 106 | 57.6 | 55.3 | <0.001 |
| High | 58 | 99.2 | 121.6 | |
Overexpression of PBOV1 suppresses
ovarian cancer cell proliferation
To investigate the biological role of PBOV1 in
ovarian cancer progression, SKOV3 and OVCAR4 ovarian cancer cells
were transduced to stably overexpress PBOV1 (Fig. 4A). The expression of Ki67, an
acknowledged marker of proliferation, was found to be downregulated
in the PBOV1-overexpressing cells (Fig.
4A). MTT and colony formation assays showed that PBOV1
overexpression markedly reduced the growth rate of the SKOV3 and
OVCAR4 cells compared with that of the vector-transduced cells
(Fig. 4B and C). Moreover, ectopic
expression of PBOV1 significantly reduced SKOV3 and OVCAR4 cell
anchorage-independent growth, as indicated by the decreased colony
number and size (Fig. 4D).
In vivo assay reveals the suppressive
effect of PBOV1 on tumorigenicity
To validate the in vitro cell proliferation
assay results, we performed in vivo assays to evaluate the
tumorigenic effect of PBOV1 in non-obese diabetic/severe combined
immunodeficiency (NOD/SCID) mice using the SKOV3 cell line.
PBOV1-transfected cells exhibited an anti-proliferative tendency in
the nude mice (Fig. 5A and B). The
PBOV1 expression of the excised tumors was verified by western
blotting (Fig. 5C). Our results
demonstrated that PBOV1 plays an important role in the
tumorigenicity of ovarian cancer in vivo.
Discussion
The key finding of our study is that PBOV1
upregulation, a common molecular change in human ovarian cancer,
and PBOV1 overexpression inhibit ovarian cancer cell proliferation
and tumorigenicity. Our study identifies a close correlation
between PBOV1 downregulation and disease progression as well as
poor patient survival. The in vitro and in vivo
assays both demonstrated the suppressive role of PBOV1 on ovarian
cancer cells. Our findings suggest that PBOV1 can potentially be
targeted as a therapeutic strategy for ovarian cancer.
Ovarian, breast, endometrial and prostate cancer are
hormone-dependent cancers regulated by hormones such as estrogen,
progesterone, or androgen (17–20).
Based on hormonal responsiveness, the development of effective
prevention strategies for breast, endometrial, ovarian and prostate
cancer is of paramount importance in the care of patients at risk
for these malignancies (21–23).
Estrogen regulates proteases and anti-proteases in both ovarian and
breast cancer cells (24,25). These studies indicate that the study
direction of ovarian cancer can be traced from breast and prostate
cancer. It was previously reported that PBOV1 expression is
upregulated in breast and prostate cancer cells and is positively
regulated by estrogen and dihydrotestosterone, respectively
(9,10). A previous study stated that PBOV1 is
expressed in human ovarian serous cystadenocarcinoma, and the
expression level and function of PBOV1 protein in ovarian cancer
are unknown (12). We found that,
in comparison with that in normal ovarian cells and tissues, both
PBOV1 mRNA and protein were upregulated in ovarian cancer cell
lines and clinical tumors. The conclusion obtained from the above
research that PBOV1 emerged de novo as a
protein-coding gene, as reported by Samusik et al supported
our current data that PBOV1 is upregulated in ovarian cancer
(12). However, how PBOV1 is
upregulated in ovarian cancer requires further exploration.
The low PBOV1 protein expression in advanced FIGO
stages is of great interest, and PBOV1 correlates inversely with
clinical advancement and shorter survival in breast cancer. We
noted that expression was intense in samples obtained from low FIGO
stage patients and was lower in samples from high FIGO stage
patients. PBOV1 protein expression levels were significantly
correlated with the prognosis of ovarian cancer, where low PBOV1
protein expression in ovarian cancer lesions was closely associated
with advanced FIGO staging; higher T, N, and M classification;
histological grade (Silverberg); and shorter survival. It was
previously reported that higher levels of PBOV1 significantly
correlated with relapse-free survival, which could only be observed
in patients with lymph node metastasis (8). Analysis of a gene expression dataset
of clinical glioma samples showed that tumor samples from patients
with proneural glioma who survived for more than 209 weeks had
higher PBOV1 expression levels (12). Herein, we believe that PBOV1 protein
may act as a tumor suppressor. Its higher expression in stage I/II
disease indicates that PBOV1 may be involved in the origin of the
tumor and render it helpful for early diagnosis.
To evaluate the biological function of PBOV1 in
ovarian cancer, we constructed PBOV1-overexpressing cell models.
The proliferation inhibitory activity of PBOV1 was demonstrated in
the in vitro and in vivo assays. This is the first
suggestion that PBOV1 may be involved in suppressing ovarian cancer
cell proliferation and tumorigenicity.
Why does PBOV1 overexpression decrease as the degree
of malignancy increases? A transcription- or translation-associated
molecule may play a major role in regulating PBOV1 in ovarian
cancer. MicroRNAs (miRNAs), a class of small non-coding RNAs,
inhibit gene translation or facilitate mRNA degradation, resulting
in the repression of target gene expression (26,27).
Several miRNAs are involved in regulating ovarian cancer, such as
miR-22 (28), miR-200 (29), miR-210 (30) and miR-16 (31). By analyzing the 3′ untranslated
region of PBOV1 in TargetScan Human, we found 10 potential
conserved sites targeted by miR-431, miR-132, miR-212 and miR-1299
which require verification in our future research.
PBOV1 protein can be detected in the serum, and
higher serum levels of PBOV1 protein were detected in patients with
prostate cancer compared with that in normal individuals (9). It would be of great interest to
investigate whether such an important marker is also detectable in
other patient samples such as blood and ovarian fluid in addition
to biopsy or surgical tissues. Confirmation of this would require
future large-scale studies. Nevertheless, our study provides a
basis for developing a novel diagnostic and prognostic biomarker of
ovarian cancer. In summary, our study suggests that PBOV1
overexpression is a common feature in ovarian cancer and may
represent a novel predictive marker for the clinical outcome of the
disease.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81201568).
References
|
1
|
Whitmore SE, Rosenshein NB and Provost TT:
Ovarian cancer in patients with dermatomyositis. Medicine
(Baltimore). 73:153–160. 1994. View Article : Google Scholar
|
|
2
|
Markman M, Webster K, Zanotti K, Peterson
G, Kulp B and Belinson J: Survival following the documentation of
platinum and taxane resistance in ovarian cancer: A single
institution experience involving multiple phase 2 clinical trials.
Gynecol Oncol. 93:699–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed. Debulking surgery in
ovarian cancer. J Clin Oncol. 4:1716–1717. 1986.PubMed/NCBI
|
|
4
|
Rossing MA, Wicklund KG, Cushing-Haugen KL
and Weiss NS: Predictive value of symptoms for early detection of
ovarian cancer. J Natl Cancer Inst. 102:222–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chudecka-Głaz AM, Cymbaluk-Płoska AA,
Menkiszak JL, Sompolska-Rzechuła AM, Tołoczko-Grabarek AI and
Rzepka-Górska IA: Serum He4, CA125, YKL-40, bcl-2, cathepsin-L and
prediction optimal debulking surgery, response to chemotherapy in
ovarian cancer. J Ovarian Res. 7:622014. View Article : Google Scholar
|
|
6
|
DiSaia PJ and Bloss JD: Treatment of
ovarian cancer: New strategies. Gynecol Oncol. 90:S24–S32. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mantha S, Sarasohn D, Ma W, Devlin SM, Chi
DS, Roche KL, Suidan RS, Woo K and Soff GA: Ovarian vein thrombosis
after debulking surgery for ovarian cancer: Epidemiology and
clinical significance. Am J Obstet Gynecol. 213:208.e1–e4. 2015.
View Article : Google Scholar
|
|
8
|
Krukovskaia LL, Samusik ND, Shilov ES,
Polev DE and Kozlov AP: Tumor-specific expression of PBOV1, a new
gene in evolution. Vopr Onkol. 56:327–332. 2010.In Russian.
|
|
9
|
Kamagata C, Tsuji N, Kondoh K, Sasaki M,
Kobayashi D, Yagihashi A and Watanabe N: Enhanced expression of the
uROC28 gene in human breast cancer: Relationship to ERBB2 gene
expression. Anticancer Res. 22:4087–4091. 2002.
|
|
10
|
Doak SH, Jenkins SA, Hurle RA, Varma M,
Hawizy A, Kynaston HG and Parry JM: Bone morphogenic factor gene
dosage abnormalities in prostatic intraepithelial neoplasia and
prostate cancer. Cancer Genet Cytogenet. 176:161–165. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Loizidou MA, Cariolou MA, Neuhausen SL,
Newbold RF, Bashiardes E, Marcou Y, Michael T, Daniel M, Kakouri E,
Papadopoulos P, et al: Genetic variation in genes interacting with
BRCA1/2 and risk of breast cancer in the Cypriot population. Breast
Cancer Res Treat. 121:147–156. 2010. View Article : Google Scholar
|
|
12
|
Samusik N, Krukovskaya L, Meln I, Shilov E
and Kozlov AP: PBOV1 is a human de novo gene with tumor-specific
expression that is associated with a positive clinical outcome of
cancer. PLoS One. 8:e561622013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Zhang N, Song LB, Liao WT, Jiang LL,
Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS, et al: Astrocyte elevated
gene-1 is a novel prognostic marker for breast cancer progression
and overall patient survival. Clin Cancer Res. 14:3319–3326. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hahn WC, Dessain SK, Brooks MW, King JE,
Elenbaas B, Sabatini DM, DeCaprio JA and Weinberg RA: Enumeration
of the simian virus 40 early region elements necessary for human
cell transformation. Mol Cell Biol. 22:2111–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Li J, Zheng H, Yu C, Chen J, Liu
Z, Li M, Zeng M, Zhou F and Song L: Expression and cytoplasmic
localization of SAM68 is a significant and independent prognostic
marker for renal cell carcinoma. Cancer Epidemiol Biomarkers Prev.
18:2685–2693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song L, Wang L, Li Y, Xiong H, Wu J, Li J
and Li M: Sam68 up-regulation correlates with, and its
down-regulation inhibits, proliferation and tumourigenicity of
breast cancer cells. J Pathol. 222:227–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verkooijen HM, Koot VC, Fioretta G, van
der Heiden M, Schipper ME, Rapiti E, Peeters PH, Peterse JL and
Bouchardy C: Hormone replacement therapy, mammography screening and
changing age-specific incidence rates of breast cancer: An
ecological study comparing two european populations. Breast Cancer
Res Treat. 107:389–395. 2008. View Article : Google Scholar
|
|
18
|
Moran-Santa Maria MM, Flanagan J and Brady
K: Ovarian hormones and drug abuse. Curr Psychiatry Rep.
16:5112014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCullough ML, Patel AV, Patel R,
Rodriguez C, Feigelson HS, Bandera EV, Gansler T, Thun MJ and Calle
EE: Body mass and endometrial cancer risk by hormone replacement
therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev.
17:73–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ansari J, Hussain SA, Zarkar A, Tanguay
JS, Bliss J and Glaholm J: Docetaxel chemotherapy for metastatic
hormone refractory prostate cancer as first-line palliative
chemotherapy and subsequent re-treatment: Birmingham experience.
Oncol Rep. 20:891–896. 2008.PubMed/NCBI
|
|
21
|
Markman M, Glass T, Smith HO, Hatch KD,
Weiss GR, Taylor SA, Goodwin JW and Alberts DS: Phase II trial of
single agent carboplatin followed by dose-intense paclitaxel,
followed by maintenance paclitaxel therapy in stage IV ovarian,
fallopian tube, and peritoneal cancers: A Southwest Oncology Group
trial. Gynecol Oncol. 88:282–288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coleman RE: Current and future status of
adjuvant therapy for breast cancer. Cancer. 97(Suppl 3): 880–886.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Izadi-Mood N, Sarmadi S and Sanii S:
Strategies in the histologic diagnosis of low-grade glandular
endometrial neoplasm. Curr Opin Obstet Gynecol. 22:43–50. 2010.
View Article : Google Scholar
|
|
24
|
Rochefort H, Chalbos D, Cunat S, Lucas A,
Platet N and Garcia M: Estrogen regulated proteases and
antiproteases in ovarian and breast cancer cells. J Steroid Biochem
Mol Biol. 76:119–124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elguero S, Patel B and Liu JH:
Misperception of estrogen activity in patients treated with an
estrogen receptor antagonist. Am J Obstet Gynecol. 211:e1–e2. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krichevsky AM, King KS, Donahue CP,
Khrapko K and Kosik KS: A microRNA array reveals extensive
regulation of microRNAs during brain development. RNA. 9:1274–1281.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawasaki H and Taira K: Hes1 is a target
of microRNA-23 during retinoic-acid-induced neuronal
differentiation of NT2 cells. Nature. 423:838–842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Liang S, Yu H, Zhang J, Ma D and Lu
X: An inhibitory effect of miR-22 on cell migration and invasion in
ovarian cancer. Gynecol Oncol. 119:543–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu X, Macdonald DM, Huettner PC, Feng Z,
El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN and Wang X:
A miR-200 microRNA cluster as prognostic marker in advanced ovarian
cancer. Gynecol Oncol. 114:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giannakakis A, Sandaltzopoulos R, Greshock
J, Liang S, Huang J, Hasegawa K, Li C, O'Brien-Jenkins A, Katsaros
D, Weber BL, et al: miR-210 links hypoxia with cell cycle
regulation and is deleted in human epithelial ovarian cancer.
Cancer Biol Ther. 7:255–264. 2008. View Article : Google Scholar
|
|
31
|
Bhattacharya R, Nicoloso M, Arvizo R, Wang
E, Cortez A, Rossi S, Calin GA and Mukherjee P: miR-15a and miR-16
control Bmi-1 expression in ovarian cancer. Cancer Res.
69:9090–9095. 2009. View Article : Google Scholar : PubMed/NCBI
|