Introduction
Malignant glioma, especially glioblastoma multiforme
(GBM), is one of the most aggressive forms of all human cancers,
with a median survival time of 12–15 months for GBM and 2–5 years
for anaplastic gliomas (1). Despite
recent advances in therapeutic modalities, including surgery,
chemotherapy and radiotherapy, the overall survival of GBM patients
was not significantly improved over the past 20 years. Malignant
gliomas display extensive infiltration of cells into the normal
brain parenchyma, which makes complete surgical removal almost
impossible. What makes the situation worse is that malignant glioma
cells are highly resistant to radiotherapy and chemotherapy, thus,
leading to recurrence (2).
Furthermore, anti-angiogenic therapy using bevacizumab increased
survival in patients with recurrent glioblastoma, it also increased
tumor invasiveness (3). As is
known, the invasiveness of glioma cells is the key problem in the
management of malignant gliomas. To the best of our knowledge,
there are no established anti-invasive therapies available.
Membrane type 1-matrix metalloproteinase (MT1-MMP,
MMP-14), a key metalloproteinase with a C-terminal sequence that
functions as membrane-anchoring domain, plays an important role
during tumor invasion (4). Once
transported to cell surface, MT1-MMP will be cleaved and activated.
Activated MT1-MMP cleaves copious substrates, including another
critical MMP, namely proMMP-2. Malignant gliomas over-express
MT1-MMP and upregulation of MT1-MMP in glioma cells correlates with
their invasiveness (5,6). Additionally, the expression of MT1-MMP
negatively correlates with prognosis of glioma patients (7). The above results indicate that MT1-MMP
is a key metalloproteinase in the process of glioma cell
invasion.
KIFs are a family of molecular motors which drive
the transport of certain cargoes such as protein complexes along
microtubular tracks. They are involved in cellular functions,
including cell division and intracellular transport. Accumulating
evidence supports the important role of KIFs in tumor development
and progression (8). The expression
of kinesin family member 2A (KIF2A) negatively correlated with
prognosis of squamous cell carcinoma of the oral tongue (SCCOT) and
breast cancer and knocking down KIF2A inhibited invasion (9,10).
Additionally, kinesin family member 3A (KIF3A) correlated with
clinicopathological factors of prostate cancer, while silencing
KIF3A decreased proliferation and invasion (11). First discovered by Nangaku et
al (12) in 1994, kinesin
family member 1B (KIF1B) participated in not only axonal transport
of synaptic vesicles and mitochondria, but also axon myelination
and outgrowth (13,14). We have reported that leptin
stimulates MT1-MMP expression as well as its cell surface
localization, which is dependent on KIF1B and consequently
promoting invasion of gastric cancer cells (15). Neither the expression, nor the
function of KIF1B in glioma has been reported, and its molecular
mechanisms in tumorigenesis and progression require further
investigation.
According to previous findings, we examined the
protein and mRNA expression of KIF1B in glioma using tumor
specimens as well as databases, and analyzed the correlation of
KIF1B expression with pathological grades, Karnofsky performance
status (KPS) and patient survival in this study. Furthermore, we
identified that KIF1B promoted glioma cell migration and invasion
and was involved in cell surface localization of MT1-MMP. These
results suggest that increased KIF1B expression may facilitate the
localization of MT1-MMP, thus promoting glioma cell invasion.
Materials and methods
Patients and specimens
The research was approved by the Ethics Committee of
the Qilu Hospital. Archived paraffin-embedded glioma tissues were
collected from 67 patients who underwent surgery in the Department
of Neurosurgery, Qilu Hospital of Shandong University (Shandong,
China). The diagnosis of each case was confirmed by two
pathologists according to the 2007 World Health Organization (WHO)
classification (16). The tissue
specimens included WHO I (n=5) and WHO II (n=13), WHO III (n=13)
and WHO IV (n=28). We obtained written informed consent from all
patients or their guardians.
Reagents and antibodies
The membrane protein extraction kit was purchased
from Thermo Fisher Scientific (Rockford, IL, USA; cat no. 89826).
KIF1B rabbit anti-human mAb for immunohistochemical analysis was
from Santa Cruz Biotechnology (Santa Cruz, CA, USA; cat no.
sc-28540). KIF1B and MT1-MMP rabbit anti-human mAb for western blot
analysis were from Abcam (Cambridge, UK; cat no. ab69614 for KIF1B
and ab51074 for MT1-MMP). β-actin rabbit anti-human mAb was from
Cell Signaling Technology (Beverly, MA, USA; cat no. 4967).
Cell culture
The human glioma U87MG (referred to as U87
hereinafter for ease of presentation) cell line from the Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) was
grown in Dulbecco's modified Eagle's medium (DMEM)/F12 medium
containing 10% fetal bovine serum (FBS) obtained from Thermo Fisher
Scientific, 100 units/ml penicillin, and 100 µg/ml
streptomycin in humidified air with 5% CO2 at 37°C.
Immunohistochemical analysis
Paraffin-embedded tissues were deparaffinized and
rehydrated. After antigen retrieval, all tissues were exposed to
primary antibody (KIF1B, 1:200) overnight at 4°C, and then
incubated with poly-HRP (horseradish peroxidase) secondary
antibodies (ZSGB-BIO, Beijing, China; cat no. SP-9001) for 30 min
at 37°C. Staining was observed with 3,3N-diaminobenzidine
tertrahydrochloride and evaluated independently by two pathologists
without prior knowledge of the clinicopathological information of
the specimens. An immunohistochemical (IHC) score was generated as
previously described, with slight modifications (17). Briefly, the IHC score was calculated
by multiplying: i) the staining intensity (scored as: 0, no
staining; 1, weak staining; 2, moderate staining; and 3, strong
staining); and ii) the percentage of KIF1B-positive glioma cells
(scored as: 1, 1–10%; 2, 11–50%; 3, 51–80%; or 4, 81–100%). An IHC
score of 9–12 was considered strong immunoreactivity (+++), 5–8 as
moderate (++), 1–4 as weak (+), and 0 was considered negative
(−).
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Sangon
Biotech Co., Ltd., Shanghai, China), treated with DNase I (Thermo
Fisher Scientific) then reverse transcribed to cDNA using M-MuLV
reverse transcriptase (Thermo Fisher Scientific), following the
manufacturer's protocol. Quantitative polymerase chain reaction
(PCR) was performed as previously described (18). PCR was performed using the following
primers: GADPH forward, GGTGGTCTCCTCTGACTTC AACAG and reverse,
GTTGCTGTAGCCAAATTCGTTGT; KIF1B forward, TGGCAGTTACTTCCTACACAGA and
reverse, GGGAACGGCTACTTGTTTCAT.
Western blot analysis
Total protein from cells or tissues was extracted in
RIPA buffer [50 mM Tris-HCl, pH 7.5, 1% IGEPAL CA-630 (v/v), 150 mM
NaCl, and 0.5% sodium deoxycholate] and quantified using the BCA
method. Equal amounts of proteins from each sample were separated
on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred onto nitrocellulose membranes. The membranes were
blocked for 1 h at room temperature with TBST (50 mM Tris-HCl, 150
mM NaCl, 0.05% Tween-20, pH 7.0) containing 5% non-fat dry milk and
then incubated with the primary antibody [KIF1B (1:200), MT1-MMP
(1:1,000), calnexin (1:1,000) and β-actin (1:1,000)] overnight at
4°C. The membranes were exposed to the horseradish
peroxidase-labeled secondary antibodies (1:2,000) for 1 h at room
temperature and then were detected by enhanced chemiluminescence
detection system (Amersham Life Science Inc., Pittsburgh, PA,
USA).
RNA interference
Small interference RNA (siRNA) constructs targeting
KIF1B, and stable negative control were designed and purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). For transient
silencing, 3×105 cells/well were seeded onto 6-well
plates and transfected with relevant siRNA (100 nmol/well) using
Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA; cat
no. 13778150), following the manufacturer's protocol.
Cell invasion and migration assay
For Transwell Matrigel invasion assay,
5×104 cells in 100 µl of serum-free medium were
plated onto the upper chamber of 24-well Transwell inserts (8
µm pores; BD Biosciences, Bedford, MA, USA) coated with
Matrigel, as previously described with slight modifications
(19). The lower chamber was filled
with 600 µl medium containing 20% FBS. After 36–48 h, the
non-invaded cells were gently scraped off by cotton swab. The
migrated cells were fixed by 10% formalin, stained with crystal
violet and counted. Five random fields of each well were
photographed and cell numbers were determined by Kodak MI
software.
As for Transwell migration assay, 2×104
cells in 100 µl of serum-free medium were plated onto the
upper chamber of the same inserts and migrated for 10 h. The other
steps were the same as described above.
Immunofluorescence staining
After KIF1B siRNA transfection, U87 cells were
harvested and then plated on glass slides in 24-well culture plates
at a concentration of 1×105 cells/well for 12 h.
Thereafter, the cells were fixed with a 4% formaldehyde solution in
PBS, permeabilized with 0.5% Triton X-100 in PBS, stained for
filamentous actin using Alexa-Fluor 488-labeled phalloidin dyes
(Cell Signaling Technology; cat no. 8878) according to the
manufacturer's instructions. The cells were counterstained with
4′,6-diamidino-2-phenylindole (DAPI) and examined under an Olympus
BX61 fluorescence microscope.
Cell viability assay
The cell viability assay was previously described
(20), with slight modifications.
Briefly, 2.5×103 cells in 200 µl medium per well
were seeded into a 96-well plate. At the time indicated, 20
µl MTT (5 mg/ml) was added into each well. The medium was
incubated for another 4 h in the dark. Consequently, the medium was
expirated. A total of 200 µl DMSO was used to dissolve the
formazan grain. The absorbance at 570 nm was measured using an
Infinite 200 PRO microplate reader (Tecan Schweiz AG, Männedorf,
Switzerland).
In silico analysis
The Oncomine database and Repository of Molecular
Brain Neoplasia Data (REMBRANDT) of the National Cancer Institute
(http://www.betastasis.com/) were used to
analyze mRNA expression of KIF1B and its prognostic value in
glioma.
Statistical analysis
The SPSS software package (version 17.0; SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analysis. Chi-square
test and Fisher's exact test were applied to analyze the
correlation of clinicopathologcial parameters and KIF1B expression.
Other data from experiments were analyzed by paired Student's
t-test or one-way ANOVA analysis of variance wherever
appropriate.
Results
The expression of KIF1B correlates with
the clinicopathologcial information of glioma
First, we investigated the expression of KIF1B mRNA
in 39 glioma tissue samples using qRT-PCR. The levels of KIF1B mRNA
increased with the ascending pathological classification of glioma
(P<0.05; Fig. 1A). The aberrant
expression of KIF1B was further validated by immunohistochemical
analysis of 67 glioma tissue samples (Fig. 1B). The glioma tissuse samples were
grouped as KIF1BLow (IHC score: − to +) and as
KIF1BHigh (IHC score: ++ to +++). As shown in Table I, KIF1B expression was significantly
associated with glioma grade according to the WHO classification
and KPS.
 | Table IThe relationship between KIF1B
immunohistochemical expression and clinicopathologcial parameters
of patients. |
Table I
The relationship between KIF1B
immunohistochemical expression and clinicopathologcial parameters
of patients.
| Variables | Total | IHC score (−/+) | IHC score
(++/+++) | P-value |
|---|
| Gender | | | | |
| Male | 42 | 27 | 15 | 0.5155 |
| Female | 25 | 18 | 7 | |
| Age (years) | | | | |
| ≥50 | 44 | 32 | 12 | 0.1799 |
| <50 | 23 | 13 | 10 | |
| KPS | | | | |
| >70 | 43 | 33 | 10 | 0.0254 |
| ≤70 | 24 | 12 | 12 | |
| WHO grade | | | | |
| High (III–IV) | 41 | 23 | 18 | 0.0180 |
| Low (I–II) | 26 | 22 | 4 | |
The aberrant expression and prognostic
value of KIF1B is validated in the databases
To further investigate KIF1B expression and its
clinical significance in gliomas, we used two databases to examine
the differential expression and prognostic value of KIF1B in
glioma. Analysis based on a set of Oncomine data showed that KIF1B
mRNA expression was markedly upregulated in anaplastic astrocytoma
(n=19), diffuse astrocytoma (n=7), GBM (grade n=81) and
oligodendroglioma (grade n=50) than in non-tumor controls (n=23;
each P<0.001; Fig. 2A).
According to the data extracted from REMBRANDT, patients with high
KIF1B mRNA-expressing astrocytoma (P=0.0369; Fig. 2B) and oligodendroglioma (P=0.0275;
Fig. 2C) showed poorer prognosis
compared to patients with low KIF1B mRNA-expressing ones. Although
there is only a tendency towards poorer prognosis in GBM (P=0.0769;
Fig. 2D), a significant prognostic
difference still remains in all glioma (P=0.0352; Fig. 2E).
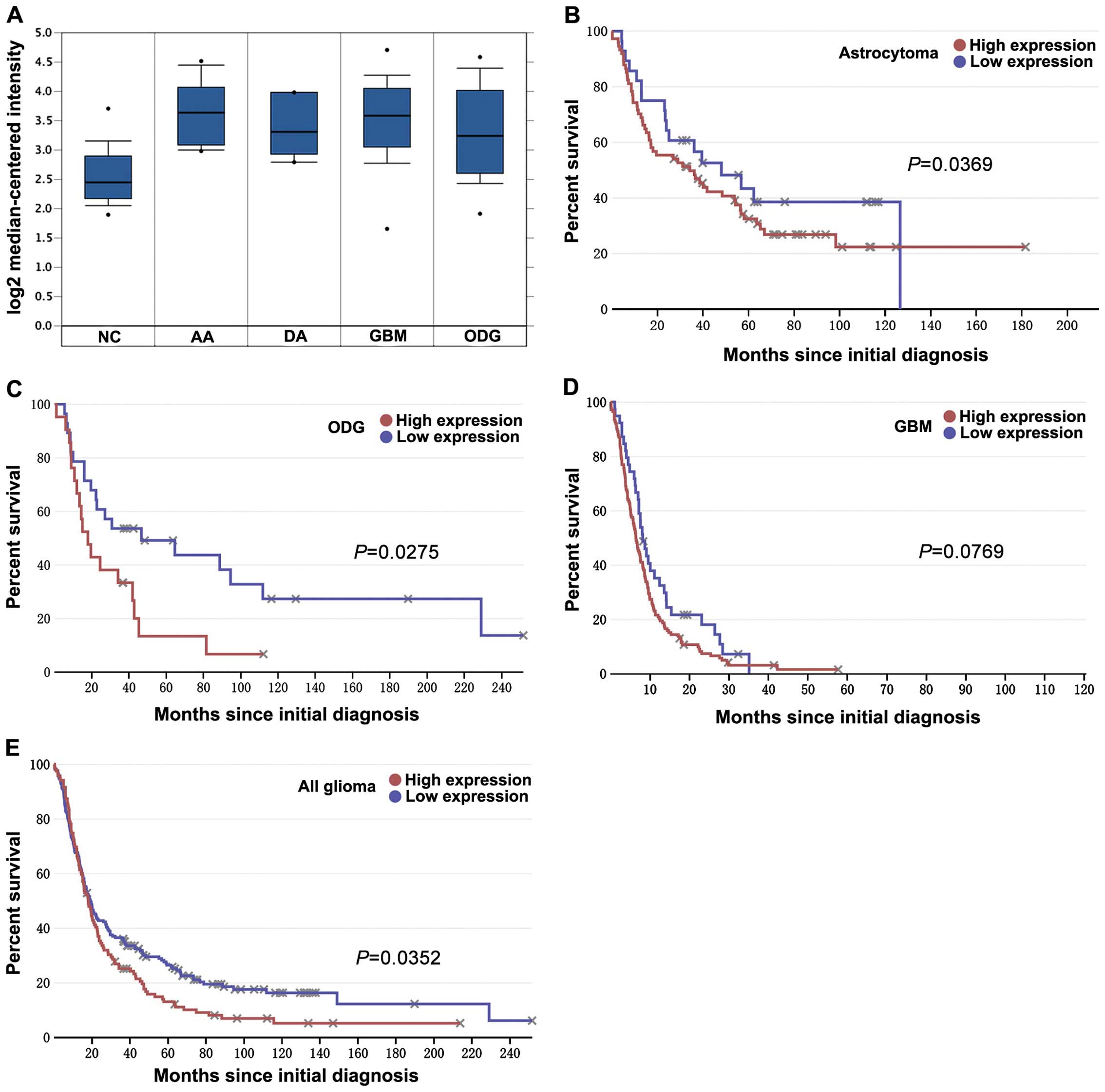 | Figure 2Expression and prognostic value of
KIF1B in gliomas of the databases. (A) Oncomine microarray data
analysis for KIF1B expression in different gliomas vs. normal brain
tissue is shown. The Student's t-tests were conducted using the
Oncomine software; compared with normal brain, each type of glioma
has a P-value <0.001. NC represents non-tumor controls (n=23),
AA represents anaplastic astrocytoma (grade III, n=19), DA
represents diffuse astrocytoma (grade II, n=7), GBM represents
glioblastoma (grade IV, n=81) and ODG represents oligodendroglioma
(grade II, n=50). The boxes represent the 25th through 75th
percentiles; the horizontal lines represent the medians; the points
represent the end of the ranges. (B–E) According to different KIF1B
mRNA expression for overall survival of glioma patients,
Kaplan-Meier curves were plotted (using data extracted from the
REMBRANDT database at http://www.betastasis.com/). Briefly, (B) atrocytoma,
(C) oligodendroglioma (ODG), (D) glioblastoma (GBM) and (E) all
gliomas. P-values were obtained from log-rank tests. |
Silencing KIF1B inhibits invasion and
migration of glioma cells, not affecting cell viability
KIF1B was aberrantly expressed in glioma tissue
samples and correlated with pathological classification and KPS of
patients. It was revealed that the pathological classification of
glioma is related to invasiveness (21). Moreover, we previously proved that
KIF1B promoted invasiveness of gastric cancer cells (15). Hence, KIF1B was antagonized by siRNA
in vitro in U87 as well as A172 glioma cell lines. The
silencing efficacy of selected KIF1B siRNA was verified by qRT-PCR
(data not shown). Compared with control siRNA treatment, knocking
down KIF1B significantly inhibited both invasion (P<0.001;
Fig. 3A) and migration (P<0.05;
Fig. 3B) of U87 glioma cells.
Silencing KIF1B in A172 cells also remarkably inhibited their
invasiveness (P<0.001; Fig. 3C)
and migratory (P<0.01; Fig. 3D)
ability. KIF1B-siRNA was transfected into U87 glioma cells and did
not affect cell viability after 24, 48 and 72 h (P=0.25; Fig. 3E). Knocking down KIF1B in A172
glioma cells showed similar results (data not shown).
Silencing KIF1B inhibits glioma invasion
and migration through downregulation of membranal MT1-MMP
MT1-MMP is transported to cell surface in order to
exert its biological function (4).
Our previous co-immunoprecipitation data have demonstrated that
MT1-MMP and KIF1B interacts with each other (15). Considering the important role of
KIF1B on intracellular transport and the effects of MT1-MMP on
tumor invasion, we speculated that KIF1B may affect the cell
localization of MT1-MMP, and then influence cell migration and
invasion in glioma. After knocking down KIF1B (P<0.001; Fig. 4A and B), the protein levels of
MT1-MMP in the whole U87 glioma cell lysate were not affected
(P=0.485; Fig. 4A and C). However,
the expression of membranal MT1-MMP was remarkably lower in the
KIF1B-siRNA treating group (P<0.01; Fig. 4A and C). Additionally, knocking down
KIF1B did not affect the cytoskeleton of U87 glioma cells (Fig. 4D). In conclusion, KIF1B promoted
glioma cell invasion via intracellular transport of MT1-MMP.
Discussion
In the present study, we revealed that malignant
gliomas express high levels of KIF1B both at the levels of mRNA and
protein. Aberrant expression of KIF1B correlated with the WHO
pathological classification and KPS. Unfortunately, we failed to
follow up a number of included patients. Then we accessed the
REMBRANDT database and discovered that glioma aberrantly expressing
KIF1B correlated with poorer prognosis. Moreover, we verified that
gliomas have increased expression of KIF1B compared to non-tumor
tissue samples. Previous studies have revealed that KIF2C (22) and KIF14 (23) are aberrantly expressed in gliomas
and they are independent markers for prognosis. KIF23 was
aberrantly expressed in glioma tissue samples, and knockdown of
KIF23 suppressed glioma cell proliferation (24). These KIFs are crucial for the
regulation of cell cycle and mitosis, which are emerging targets
for human cancer control. However, the known function of KIF1B is
intracellular transportation.
Accumulating evidence shows that specific members of
the 23 known human matrix metalloproteinases (MMPs), especially
MT1-MMP, alter tumor cell behavior and stimulate cancer
progression. In terms of glioma, expression of MT1-MMP both
correlates with and increases with histological grade of malignancy
(25). Moreover, it is known that
MT1-MMP is vital in glioma cell malignant behavior including
growth, invasion, migration and angiogenesis (25). Besides, MT1-MMP may be a key
regulator of CD133+ glioma stem cells (26). Previously, our group demonstrated
that leptin promotes MT1-MMP expression as well as its cell surface
localization in a KIF1B-dependent manner in gastric cancer
(15). According to the above
evidence, we presumed that MT1-MMP is also the key regulator in
KIF1B-mediated glioma cell invasion and migration.
Malignant gliomas are notorious for their
invasiveness. In the Transwell assay, knocking down KIF1B via siRNA
inhibited invasion and migration of U87 and A172 glioma cells. The
possibility that KIF1B may influence cell viability was excluded
through MTT assay. Similarly, silencing KIF1B did not affect the
overall expression of MT1-MMP protein in U87 glioma cells. As a
crucial metalloproteinase in tumor invasion, MT1-MMP is regulated
at various levels, including intracellular trafficking. It is known
that the localization on the cell surface is required for protease
activity of MT1-MMP and activation of subsequent MMPs. Then we
examined the membranal MT1-MMP level, and our results showed the
membranal MT1-MMP was downregulated in U87 glioma cells. On the
other hand, siRNA-mediated KIF1B knockdown did not obviously change
the cytoskeleton of glioma cells. Therefore, our results suggested
that the membranal MT1-MMP was the key point in KIF1B-mediated
glioma cell invasion.
Recent clinical as well as basic evidence indicates
that glioma's escape from antiangiogenic therapies (e.g.
bevacizumab) at least in part correlates with augmented invasion
(27). What is more, glioma
overexpresses MT1-MMP in order to enhance angiogenesis via
upregulation of VEGF (28). Based
on our findings, anti-invasive therapies targeting KIF1B is an
option in treating glioma, particularly in combination with
antiangiogenic therapies. For instance, siRNA therapy is believed
to have the potential to effectively reduce expression of
cancer-specific genes (29,30). Knockdown of KIF1B by siRNA may
suppress glioma progression. Alternatively, specific inhibitors
have been developed to downregulate other KIFs (8). The same strategy is another choice in
the case of KIF1B.
In conclusion, we discovered gliomas overexpressed
KIF1B, which correlated with worse prognosis. Moreover, aberrant
expression of KIF1B was associated to both pathological grade and
KPS. To the best of our knowledge, we showed for the first time
that KIFs were involved in glioma invasion. KIF1B played a key role
in glioma invasiveness via membranal MT1-MMP. Our data indicated
that KIF1B may be a promising target for glioma.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furnari FB, Fenton T, Bachoo RM, Mukasa A,
Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al:
Malignant astrocytic glioma: Genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lucio-Eterovic AK, Piao Y and de Groot JF:
Mediators of glioblastoma resistance and invasion during
antivascular endothelial growth factor therapy. Clin Cancer Res.
15:4589–4599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poincloux R, Lizárraga F and Chavrier P:
Matrix invasion by tumour cells: A focus on MT1-MMP trafficking to
invadopodia. J Cell Sci. 122:3015–3024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fillmore HL, VanMeter TE and Broaddus WC:
Membrane-type matrix metalloproteinases (MT-MMPs): Expression and
function during glioma invasion. J Neurooncol. 53:187–202. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo P, Imanishi Y, Cackowski FC, Jarzynka
MJ, Tao HQ, Nishikawa R, Hirose T, Hu B and Cheng SY: Up-regulation
of angiopoietin-2, matrix metalloprotease-2, membrane type 1
metalloprotease, and laminin 5 gamma 2 correlates with the
invasiveness of human glioma. Am J Pathol. 166:877–890. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Yuan J, Tu Y, Mao X, He S, Fu G,
Zong J and Zhang Y: Co-expression of MMP-14 and MMP-19 predicts
poor survival in human glioma. Clin Transl Oncol. 15:139–145. 2013.
View Article : Google Scholar
|
|
8
|
Rath O and Kozielski F: Kinesins and
cancer. Nat Rev Cancer. 12:527–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang CQ, Qu X, Zhang XY, Zhou CJ, Liu GX,
Dong ZQ, Wei FC and Sun SZ: Overexpression of Kif2a promotes the
progression and metastasis of squamous cell carcinoma of the oral
tongue. Oral Oncol. 46:65–69. 2010. View Article : Google Scholar
|
|
10
|
Wang J, Ma S, Ma R, Qu X, Liu W, Lv C,
Zhao S and Gong Y: KIF2A silencing inhibits the proliferation and
migration of breast cancer cells and correlates with unfavorable
prognosis in in breast cancer. BMC Cancer. 14:4612014. View Article : Google Scholar
|
|
11
|
Liu Z, Rebowe RE, Wang Z, Li Y, Wang Z,
DePaolo JS, Guo J, Qian C and Liu W: KIF3a promotes proliferation
and invasion via Wnt signaling in advanced prostate cancer. Mol
Cancer Res. 12:491–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nangaku M, Sato-Yoshitake R, Okada Y, Noda
Y, Takemura R, Yamazaki H and Hirokawa N: KIF1B, a novel
microtubule plus end-directed monomeric motor protein for transport
of mitochondria. Cell. 79:1209–1220. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lyons DA, Naylor SG, Scholze A and Talbot
WS: Kif1b is essential for mRNA localization in oligodendrocytes
and development of myelinated axons. Nat Genet. 41:854–858. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao C, Takita J, Tanaka Y, Setou M,
Nakagawa T, Takeda S, Yang HW, Terada S, Nakata T, Takei Y, et al:
Charcot-Marie-Tooth disease type 2A caused by mutation in a
microtubule motor KIF1Bbeta. Cell. 105:587–597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Z, Xu X, Du L, Yang Y, Cheng H, Zhang
X, Li Z, Wang L, Li J, Liu H, et al: Leptin-mediated regulation of
MT1-MMP localization is KIF1B dependent and enhances gastric cancer
cell invasion. Carcinogenesis. 34:974–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang YM, Feng AL, Zhou CJ, Liang XH, Mao
HT, Deng BP, Yan S, Sun JT, Du LT, Liu J, et al: Aberrant
expression of chemokine receptor CCR4 in human gastric cancer
contributes to tumor-induced immunosuppression. Cancer Sci.
102:1264–1271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao P, Li XG, Yang M, Shao Q, Wang D, Liu
S, Song H, Song B, Zhang Y and Qu X: Hypoxia suppresses the
production of MMP-9 by human monocyte-derived dendritic cells and
requires activation of adenosine receptor A2b via cAMP/PKA
signaling pathway. Mol Immunol. 45:2187–2195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Sima N, Kong D, Luo A, Gao Q, Liao
S, Li W, Han L, Wang J, Wang S, et al: Selective targeting of
HPV-16 E6/E7 in cervical cancer cells with a potent oncolytic
adenovirus and its enhanced effect with radiotherapy in vitro and
vivo. Cancer Lett. 291:67–75. 2010. View Article : Google Scholar
|
|
20
|
Liu Q, Li G, Li R, Shen J, He Q, Deng L,
Zhang C and Zhang J: IL-6 promotion of glioblastoma cell invasion
and angiogenesis in U251 and T98G cell lines. J Neurooncol.
100:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palfi S, Swanson KR, De Boüard S, Chrétien
F, Oliveira R, Gherardi RK, Kros JM, Peschanski M and Christov C:
Correlation of in vitro infiltration with glioma histological type
in organotypic brain slices. Br J Cancer. 91:745–752.
2004.PubMed/NCBI
|
|
22
|
Bie L, Zhao G, Wang YP and Zhang B:
Kinesin family member 2C (KIF2C/MCAK) is a novel marker for
prognosis in human gliomas. Clin Neurol Neurosurg. 114:356–360.
2012. View Article : Google Scholar
|
|
23
|
Wang Q, Wang L, Li D, Deng J, Zhao Z, He
S, Zhang Y and Tu Y: Kinesin family member 14 is a candidate
prognostic marker for outcome of glioma patients. Cancer Epidemiol.
37:79–84. 2013. View Article : Google Scholar
|
|
24
|
Takahashi S, Fusaki N, Ohta S, Iwahori Y,
Iizuka Y, Inagawa K, Kawakami Y, Yoshida K and Toda M:
Downregulation of KIF23 suppresses glioma proliferation. J
Neurooncol. 106:519–529. 2012. View Article : Google Scholar
|
|
25
|
Ulasov I, Yi R, Guo D, Sarvaiya P and
Cobbs C: The emerging role of MMP14 in brain tumorigenesis and
future therapeutics. Biochim Biophys Acta. 1846:113–120.
2014.PubMed/NCBI
|
|
26
|
Annabi B, Laflamme C, Sina A, Lachambre MP
and Béliveau R: A MT1-MMP/NF-kappaB signaling axis as a checkpoint
controller of COX-2 expression in CD133+ U87
glioblastoma cells. J Neuroinflammation. 6:82009. View Article : Google Scholar
|
|
27
|
Hardee ME and Zagzag D: Mechanisms of
glioma-associated neovascularization. Am J Pathol. 181:1126–1141.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deryugina EI, Soroceanu L and Strongin AY:
Up-regulation of vascular endothelial growth factor by
membrane-type 1 matrix metalloproteinase stimulates human glioma
xenograft growth and angiogenesis. Cancer Res. 62:580–588.
2002.PubMed/NCBI
|
|
29
|
Akita H, Kogure K, Moriguchi R, Nakamura
Y, Higashi T, Nakamura T, Serada S, Fujimoto M, Naka T, Futaki S,
et al: Nanoparticles for ex vivo siRNA delivery to dendritic cells
for cancer vaccines: programmed endosomal escape and dissociation.
J Control Release. 143:311–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yagi N, Manabe I, Tottori T, Ishihara A,
Ogata F, Kim JH, Nishimura S, Fujiu K, Oishi Y, Itaka K, et al: A
nanoparticle system specifically designed to deliver short
interfering RNA inhibits tumor growth in vivo. Cancer Res.
69:6531–6538. 2009. View Article : Google Scholar : PubMed/NCBI
|


















