Introduction
Epigenetic studies have demonstrated that long-term
intake of cruciferous vegetables, such as broccoli, carrots and
cauliflower, effectively reduced the risk of cancer, as the variety
of natural anticancer compounds contained in the crucifers could be
absorbed in human body. 3,3′-Diindolylmethane (DIM) is one of the
most important natural compounds with anticancer properties
extracted from crucifers (1).
Presently, the natural compounds with anticancer effects causing
only slight side-effects are of great interests to medical
researchers. Several studies have reported a series of natural
compounds, which were extracted from natural materials, with the
anticancer effects of inhibiting tumor cell growth and division
(2–4), or with anticancer effects of
inhibiting tumor metastasis in animal experiments (5,6).
Besides, several natural compounds were applied in combination with
radiotherapy or chemotherapy in tumor treatment, and enhanced
effects of treatments were observed (5,7–9).
DIM was reported as an effective cell proliferation
inhibitor and apoptosis inducer in a variety of tumors, such as
breast (3), prostate (10,11),
pancreatic (8), ovarian (12) and thyroid cancers (13). However, the majority of the studies
focused on the short-term killing effects of DIM on tumor cells.
Similarly to most of the researches, in our previous study,
short-term high-dose manner was designed, and we found that DIM
effectively induced cell death in nasopharyngeal carcinoma (NPC)
cells both in vitro and in vivo, and no obviously
toxic effects were observed on the normal tissues and organs
(14). While, whether this type of
model could induce similar response in the human body with
long-term dietary intake of DIM was unclear, more likely the
different key effectors activated in the long-term low-dose manner,
thus, in the present study, a long-term low concentration of DIM
was chosen to treat the NPC cells, the selection criteria of the
concentration referred to several published results (15,16),
the detectable concentration of DIM in serum or tissue of human or
animal was reported to be 20 µmol/l, whereas, no significant
inhibitory effects on NPC cell proliferation were observed with 20
µmol/l DIM in the short-term response in our previous study
(14).
We further explored the antitumor effects of DIM in
long-term low-dose manner on NPC cells, as well as the potential
targets or effectors playing important roles in the long-term
low-dose manner of DIM, which would provide reliable technical
indications for clinical use of DIM in long-term low-dose
manner.
Materials and methods
Reagents
DIM was purchased from Sigma-Aldrich, dissolved in
dimethylsulfoxide (both from Sigma-Aldrich St. Louis, MO, USA) and
was diluted to 20 µM in complete medium (HyClone Corp.,
Logan, UT, USA). Cell Counting Kit-8 (CCK-8; Dojindo Laboratories,
Tokyo, Japan), the antibodies used in the present study were
purchased from Cell Signaling Technology Inc. (Danvers, MA,
USA).
Cell culture
Human NPC cell lines CNE-2, and 5-8F were maintained
in our laboratory, and were stored in liquid nitrogen. The cells
were cultured in RPMI-1640 (HyClone Corp.), supplemented with 10%
fetal bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA,
USA) and 20 µg/ml antibiotics (ampicilin and kanamycin;
Genome Biotechnology, Hangzhou, China), at 37°C, 5% CO2.
Cells in logarithmic growth phase were used in the experiment. The
NPC cells CNE-2, and 5-8F were treated with 20 µM DIM for
over a month then renamed as CNE-2/DIMLL and
5-8F/DIMLL.
Cell proliferation assay
Cells were seeded into 96-well plates with
1×103 cells/well for normal culture. Six double wells
were set. After 24, 48, 72 and 96 h culture, 10 µl of CCK-8
was added in each well, and were incubated at 37°C for 1 h, and
absorbance value was measured at 450 nm.
Transwell assay
Transwell assay was performed using polycarbonate
membrane Transwell (Corning Inc., Corning, NY, USA). The bottom
chamber included medium (0.5 ml) containing 5% FBS. The cell
density seeded was 3.0×105/ml in the upper chamber,
incubated 24 h at 37°C, 5% CO2. Membranes were then
washed, fixed and stained by Methyl violet (Guge Biotechnology,
Wuhan, China). The invasion ability of the cells was determined by
counting the cells that had pass through to the lower side of the
filter with a microscope.
Flow cytometry
Annexin V/PI apoptosis kit (cat. no. LK-AP101-100;
Lianke Biotech Co., Ltd., Hangzhou, China) was used for the
detection. Cells were harvested and washed twice with cold
phosphate-buffered saline (Genom Biotechnology). The cells were
resuspended in Annexin V binding buffer, and were then stained with
5 µl of Annexin V-FITC solution and 10 µl of
propidium iodide (PI) solution for 15 min in the dark. Fluorescence
was analyzed on a FACSCanto™ II spectrometer (BD Biosciences, San
Jose, CA, USA).
Animal feeding and grouping
The animal experiment was approved by the Ethics
Committee of Renmin Hospital of Wuhan University. Forty-eight
female BALB/c nude mice (4–6 weeks old) were purchased from Beijing
HuaFukang Biological Technology Co. Ltd. (HFK Bioscience, Beijing,
China), and underwent adaptive feeding one week before the
experiment. The NPC cells at logarithmic phase were collected,
resuspended in serum-free medium, then the cell concentration was
adjusted to 1×107/ml. Syringe (1 ml) was used for
injection of 200 µl cell suspension for each animal
(14). After the inoculation, the
animals were raised for 8 weeks. The short and long diameter of
transplanted tumors at 1–8 weeks after the inoculation were
measured, 8 weeks after inoculation, animals were sacrificed.
Hematoxylin and eosin (H&E) staining
and immunohistochemistry (IHC)
Specimens of transplanted tumors were fixed,
embedded and sliced. The H&E staining was performed following
the protocol of the H&E staining kit (Guge Biotechnology). Then
neutral balsam was used for mounting and the section was observed
and photographed under a microscope. The IHC was performed with the
method of SABC, following the protocol of the IHC kit (Guge
Biotechnology).
Western blotting
Cells were harvested and lysed in buffer containing
1% Nonidet-P40 supplemented with complete protease inhibitor
'cocktail' (Roche) and 2 mM dithiothreitol. Lysates were resolved
by 10% SDS-PAGE, transferred to polyvinylidene defluoride (PVDF)
(Immobilon-FL; Millipore, Billerica, MA, USA) membranes and
immunoblotted with primary antibodies. After immunoblotting with
the secondary antibody, donkey anti-rabbit immunoglobulin G (cat.
no. 926-3221), the membranes were scanned with Odyssey CLx Infrared
Imaging System (both from LI-COR, Lincoln, NE, USA).
Statistical analysis
The values are expressed as the mean ± SD.
Statistical analyses were carried out by one-way ANOVA performed
using the SPSS statistical software (SPSS, Inc., Chicago, IL, USA).
Probability values of (P-value) <0.05 were considered as
statistically significant.
Results
DIM in the long-term low-dose manner
significantly reduces the proliferation and migration without
influence of apoptosis
To evaluate the effects of long-term low-dose DIM on
the proliferation of NPC cells, the proliferation ability was
detected with CCK-8 assay. As shown in Fig. 1B, compared to 5-8F group, the
proliferation rate of 5-8F treated with long-term low concentration
DIM (5-8F/DIMLL) group decreased by 35% (P<0.05),
while the proliferation rate of CNE-2 treated with long-term low
concentration DIM (CNE-2/DIMLL) group had no significant
difference from that of the CNE-2 cell group (P>0.05). To
further explore the effects of long-term low-dose DIM on apoptosis
of NPC cells, the apoptotic rates were detected with flow
cytometric assay. The results in Fig.
1C and D show that the apoptotic rates of 5-8F,
5-8F/DIMLL, CNE-2 and CNE-2/DIMLL were
(2.7±0.5%), (2.9±0.4%), (1.0±0.4%) and (1.0±0.6%), respectively.
There was no statistically significant difference between the
treatments (P>0.05). In addition, the migration ability was
explored to evaluate the effects of long-term low-dose DIM on NPC
cells. Transwell assay for migration was performed, as shown in
Fig. 1E and F, after 36 h
incubation in the Transwell chamber, the numbers of the
membrane-penetrating cells in the four cell groups were 67.3±8.9,
52.4±10.2, 24.8±6.3 and 21.2±7.1, respectively. Apparently, the
number of the membrane-penetrating cells was significantly reduced
in the CNE-2/DIMLL and 5-8F/DIMLL cell groups
as compared to those of the CNE-2 and 5-8F cell groups
(P<0.01).
 | Figure 1Long-term low-dose of DIM results in
the change of proliferative, apoptosis and migration in NPC cells.
(A) Molecular formula of DIM; the proliferative capacity was
detected by CCK-8 assay (B), compared to 5-8F,
5-8F/DIMLL group was decreased, while the proliferation
rate of CNE-2/DIMLL group had no apparently difference
when compared to CNE-2. The apoptosis rates of 5-8F,
5-8F/DIMLL, CNE-2 and CNE-2/DIMLL (C and D)
were detected by a flow cytometric assay, and there was no
statistically significant difference between 5-8F and
5-8F/DIMLL, similar result was observed between group
CNE-2 and CNE-2/DIMLL (C and D). Transwell assay was
chosen for the detection of the migration ability, the numbers of
the membrane-penetrating cells of 5-8F/DIMLL, 5-8F,
CNE-2/DIMLL, CNE-2 are shown (E). The histogram of the
statistically significant difference between the four groups is
shown (F). |
DIM in the long-term low-dose manner
significantly reduces metastasis in vivo
The in vitro experiments demonstrated that
the treatment with long-term low concentration DIM significantly
decreased the proliferation and migration in NPC cells. Therefore,
an animal experiment was conducted by establishing a subcutaneous
xenograft tumor model in nude mice with 5-8F, CNE-2,
5-8F/DIMLL and CNE-2/DIMLL cells. Eight weeks
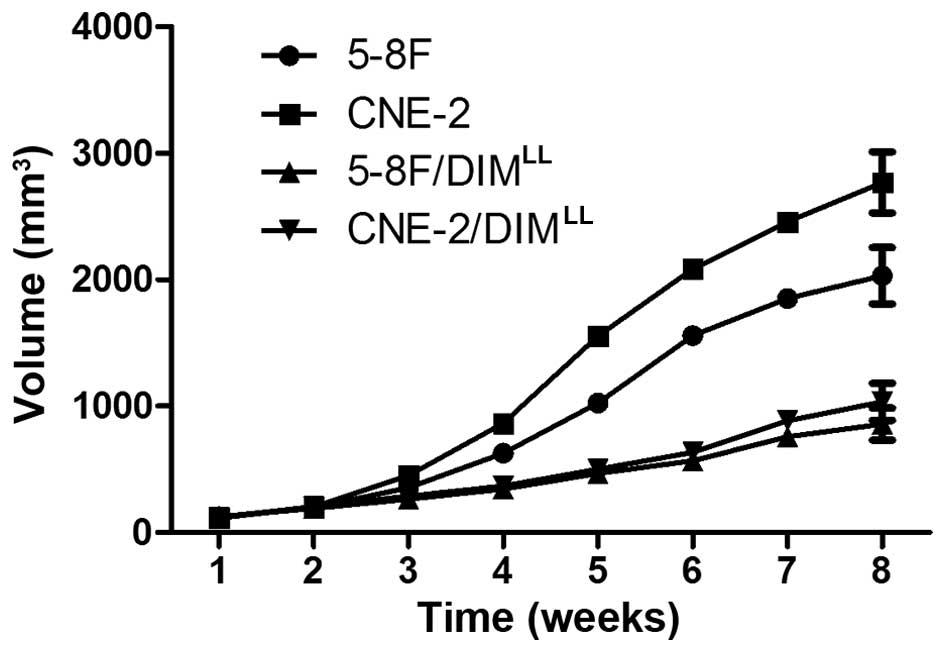
after the NPC cell inoculations, the average volume of the
xenograft tumor in the 5-8F/DIMLL and
CNE-2/DIMLL animal groups were 857.8±126.1 and
1034.4±147.1 mm3, as shown in Fig. 2, respectively. In comparison, the
average volume of the xenograft tumor in the 5-8F and CNE-2 animal
groups were significantly larger, 2032.1±223.3 and 2769.1±241.3
mm3, respectively (P<0.05). Eight weeks after the NPC
cells inoculation, the tumor formation rates of the xenografts in
the 5-8F and CNE-2 animal groups were 12/12 and 12/12,
respectively. The lymph node metastasis rates were 10/12 and 11/12,
respectively. In comparison, the 5-8F/DIMLL and
CNE-2/DIMLL animal groups had a lower tumor formation
rates of the xenografts (9/12 and 10/12), and a significantly
decreased lymph node metastatic rates (2/9 and 3/10), respectively
(P<0.01), and the results are shown in Table I.
 | Table ILong-term low-dose of DIM resulted in
the change of proliferative and metastasis in vivo. |
Table I
Long-term low-dose of DIM resulted in
the change of proliferative and metastasis in vivo.
| Group | No. of mice | Xenograft tumor | Lymph node
metastasis |
|---|
| 5-8F | 12 | 12/12 (100%) | 11/12 (91.7%) |
| CNE-2 | 12 | 12/12 (100%) | 10/12 (83.3%) |
| 5-8F/DIM | 12 | 9/12 (75%) | 2/9 (22.2%) |
| CNE-2/DIM | 12 | 10/12 (83.3%) | 3/10 (30%) |
Proliferation and metastasis-related
protein are altered in the long-term low-dose DIM manner
In the subcutaneous xenograft tumor model of the
nude mice, the decrease of proliferation and metastasis of the 5-8F
and CNE-2 treated with long-term low-dose DIM were observed, then,
to further identify the related effectors involved in the effects
of anti-proliferation and antimetastasis of long-term low-dose DIM,
we detected the expression of proliferation related PCNA and Ki-67
as well as metastasis related E-cadherin and vimentin in the
xenograft of the nude mice through immunohistochemical assay. As
shown in Fig. 3B and C, the
relative expression intensities of PCNA, Ki-67 and vimentin were
significantly lower (P<0.01), while the E-cadherin was
significantly higher (P<0.01) in the 5-8F/DIMLL and
CNE-2/DIMLL groups than the 5-8F and CNE-2 groups.
ERK signal pathway is significantly
decreased in both the short-term high-dose manner and the long-term
low-dose manner of DIM
As indicated above, the NPC cell lines 5-8F and
CNE-2 underwent significant changes in proliferation, migration, as
well as metastasis in the long-term low-dose manner of DIM,
therefore, we investigated the key signaling pathways involved in
the occurrence and development of NPC, the protein expression in
relation to cell proliferation, migration and metastasis was
detected by western blotting. Since the short-term high-dose manner
of DIM was reported in our previous study (14), the key signaling pathways were
detected and compared with the previous results, and the results
are shown in Fig. 4, the ERK
signaling showed similar changes, while in the short-term high-dose
manner (14), the PI3K/Akt, NF-κB,
JNK pathways which were significantly reduced, and the P38 pathway,
which was significantly increased, showed no obvious change,
indicating the ERK signaling may be the main effector involved in
the long-term low-dose manner of DIM.
 | Figure 4Long-term low-dose of DIM results in
the change of proliferation and metastasis-related protein
expression in NPC cells. Western blotting was chosen for the
detection. The expression of proteins related to the PI3K, NF-κB,
MAPK signal pathway were detected, and there were no significant
changes between the 5-8F/DIMLL, CNE-2/DIMLL
and 5-8F, CNE-2 groups in the PI3K, NF-κB, P38, JNK pathway,
however, the ERK pathway showed a significant decreasing trend in
the 5-8F/DIMLL, CNE-2/DIMLL groups compared
to the 5-8F and CNE-2 group. |
Discussion
DIM is a natural compound extracted from cruciferous
vegetables, and widely recognized for its antitumor effects.
Several in vitro studies had reported the pro-apoptosis
effects in tumor cells in short-term high-dose manner of DIM
(2,5,8–10). In
our previous study, short-term high concentration of DIM
effectively inhibited the proliferation and apoptosis of NPC cells
in a dose-dependent manner. Moreover, the results of animal
experiments showed that DIM had certain effects on NPC prevention
and inhibition, including slowing down the tumor progression and
reducing the incidence of tumor metastasis (14). In the present study, we raised the
suggestion that, treatment with high-dose of DIM would induce an
acute or transient response, as a result, the experiments based on
the high-dose DIM may only show an acute or transient response in
the body, whereas the continued dietary intake of DIM resulted in a
relatively steady-state, the response in vivo would be
different, therefore the long-term low-dose was designed for the
study.
Long-term intake of edible crucifers effectively
prevents the occurrence of specific tumors. The possible mechanism
is thought to be that, a certain dose of DIM was persistently
identified in the blood in the condition of sustaining intake of
crucifers, the early mutations which resulted in the proliferation
induction or the apoptosis inhibition of the tumor cells were
thereby effectively inhibited. Studies have shown that colon cancer
spontaneously occurred in heterozygous TRAMP mice, while oral
administration of DIM significantly decreased its incidence and
alleviated the severity of colon lesions in the transgenic mice.
These findings indicated that DIM effectively inhibited the
occurrence of colon cancer and prevented its progression (17). In another experiment, Howells et
al found that DIM was detectable in the blood samples with a
concentration up to 20 µmol/l (16,18).
Similarly, Moiseeva et al (19) found that a continued treatment with
low concentration of DIM significantly changed the specific gene
expression of the breast cancer MD-MBA-231 cells and affected their
biological behavior, such as the doubling time, motility and
ability to repair DNA damage. Therefore, in the present study, we
conducted in vivo and in vitro experiments in order
to examine the effect of long-term low-dose DIM treatment on the
NPC cells; moreover, the related underlying molecular mechanism was
further investigated.
Our previous results showed that after 48–72 h of
treatment with 20 µmol/l of DIM, no obvious changes were
observed in the NPC cells, neither proliferation inhibition nor
apoptosis induction (14), however,
the continued treatments with 20 µmol/l DIM for over a month
resulted in significant changes in proliferation, migration and
metastasis. While, there was a slight difference between the two
NPC cell lines. For example, after being treated with 20
µmol/l of DIM for over 30 days, the 5-8F cell line had a
significant reduction in proliferation ability, migration and
metastasis, however, the CNE-2 cell line had no evident decrease in
the proliferation ability, but showed a decreased ability for
migration and metastasis. Such variations could be related to the
differences of gene expression profiles or epigenetic properties
between various cancer cell lines.
The signaling pathways PI3K/Akt, MAPK and NF-κB,
which played an important role in the occurrence, pathogenesis and
metastasis processes of NPC (20–24),
were reported in regulation of cell proliferation, migration and
metastasis. In addition, they were detected by western blot assay
in the study, aimed to explore the possible targets of DIM (20
µmol/l, over a month) in NPC. It turned out that the ERK
pathway was significantly decreased during the treatment, while the
other primary signal pathways were not obviously changed. Compared
to the short-term high-dose manner in our previous study, the
expression changes of relevant signaling pathway proteins
differed.
ERK signaling pathway was vital in vivo, a
number of important biological process were under the regulation of
ERK signaling pathway, such as proliferation, differentiation,
apoptosis, cancerization and other biological reaction. The ERK1/2
was located in the cytosol in an inactivated state, when
phosphorylated to activate, translocated from the cytosol to the
nucleus, and affected the multiple biological processes through the
regulation of the activity of transcription factors. ERK was
reported activated in a majority of NPC (25–27).
Since we observed that the ERK signaling showed similar changes,
while the PI3K/Akt, NF-κB, P38 and JNK pathways which were
significantly changed in the short-term high-dose manner (14) showed no obvious change, indicated
the ERK signaling may be the main effector involved in the
long-term low-dose manner of DIM treatment. Based on the
differences of the activated signals (Fig. 5), we speculated that the PI3K/Akt,
NF-κB, P38, JNK pathways which were activated/inactivated during
the short-term high-dose manner of DIM were only thought to be the
drug toxic reaction of the high-dose DIM, and also considered to be
the reason that apoptosis increased in the high-dose of DIM, by
contrast, apoptosis was not changed in the low-dose of DIM, these
results confirmed that ERK may be the real target of DIM in the
long-term low-dose treatment of DIM in NPC.
In conclusion, a long-term low-dose DIM treatment
(20 µmol/l) inhibited the proliferation, migration, as well
as the in vivo metastasis in NPC cells, further, the ERK
signaling pathway may be the main effector in the long-term
low-dose DIM. Our results provide evidence that the use of
long-term low-dose DIM suppresses the activation of the ERK
pathway, which would add support for the use of DIM in preclinical
and clinical settings in the management of NPC patients.
Acknowledgments
The present study was supported by the grants from
the National Natural Science Foundation of China (no. 81372880),
the Independent Research Project of Wuhan University (nos.
2042014kf0184 and 2042014kf0119), the Doctoral Program of Higher
Education Research Fund (nos. 20130141120093 and 20110141110062),
and the Natural Science Foundation of Hubei Province (no.
2012FFA045).
References
|
1
|
Safe S, Papineni S and Chintharlapalli S:
Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane
and synthetic analogs. Cancer Lett. 269:326–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chinnakannu K, Chen D, Li Y, Wang Z, Dou
QP, Reddy GP and Sarkar FH: Cell cycle-dependent effects of
3,3′-diindolyl-methane on proliferation and apoptosis of prostate
cancer cells. J Cell Physiol. 219:94–99. 2009. View Article : Google Scholar
|
|
3
|
Jin Y, Zou X and Feng X:
3,3′-Diindolylmethane negatively regulates Cdc25A and induces a
G2/M arrest by modulation of microRNA 21 in human breast cancer
cells. Anticancer Drugs. 21:814–822. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang YQ, Chen C, Chen Z, Xu Y, Wang Y,
Xiao BK, Chen SM and Tao ZZ: Indole-3-carbinol inhibits cell
proliferation and induces apoptosis in Hep-2 laryngeal cancer
cells. Oncol Rep. 30:227–233. 2013.PubMed/NCBI
|
|
5
|
Ahmad A, Kong D, Wang Z, Sarkar SH,
Banerjee S and Sarkar FH: Down-regulation of uPA and uPAR by
3,3′-diin-dolylmethane contributes to the inhibition of cell growth
and migration of breast cancer cells. J Cell Biochem. 108:916–925.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim EJ, Shin M, Park H, Hong JE, Shin HK,
Kim J, Kwon DY and Park JH: Oral administration of
3,3′-diindolylmethane inhibits lung metastasis of 4T1 murine
mammary carcinoma cells in BALB/c mice. J Nutr. 139:2373–2379.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali S, Banerjee S, Ahmad A, El-Rayes BF,
Philip PA and Sarkar FH: Apoptosis-inducing effect of erlotinib is
potentiated by 3,3′-diindolylmethane in vitro and in vivo using an
orthotopic model of pancreatic cancer. Mol Cancer Ther.
7:1708–1719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banerjee S, Wang Z, Kong D and Sarkar FH:
3,3′-Diindolylmethane enhances chemosensitivity of multiple
chemotherapeutic agents in pancreatic cancer. Cancer Res.
69:5592–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahman KMW, Banerjee S, Ali S, Ahmad A,
Wang Z, Kong D and Sakr WA: 3,3′-Diindolylmethane enhances
taxotere-induced apoptosis in hormone-refractory prostate cancer
cells through survivin down-regulation. Cancer Res. 69:4468–4475.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith S, Sepkovic D, Bradlow HL and Auborn
KJ: 3,3′-Diindolyl-methane and genistein decrease the adverse
effects of estrogen in LNCaP and PC-3 prostate cancer cells. J
Nutr. 138:2379–2385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vivar OI, Lin CL, Firestone GL and
Bjeldanes LF: 3,3′-Diindolyl-methane induces a G1 arrest
in human prostate cancer cells irrespective of androgen receptor
and p53 status. Biochem Pharmacol. 78:469–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu EL, Chen N, Westbrook A, Wang F, Zhang
R, Taylor RT and Hankinson O: CXCR4 and CXCL12 down-regulation: A
novel mechanism for the chemoprotection of 3,3′-diindolylmethane
for breast and ovarian cancers. Cancer Lett. 265:113–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajoria S, Suriano R, George A, Shanmugam
A, Schantz SP, Geliebter J and Tiwari RK: Estrogen induced
metastatic modulators MMP-2 and MMP-9 are targets of
3,3′-diindolylmethane in thyroid cancer. PLoS One. 6:e158792011.
View Article : Google Scholar
|
|
14
|
Chen C, Chen SM, Xu B, Chen Z, Wang F, Ren
J, Xu Y, Wang Y, Xiao BK and Tao ZZ: In vivo and in vitro study on
the role of 3,3′-diindolylmethane in treatment and prevention of
nasopharyngeal carcinoma. Carcinogenesis. 34:1815–1821. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manach C, Williamson G, Morand C, Scalbert
A and Rémésy C: Bioavailability and bioefficacy of polyphenols in
humans. I. Review of 97 bioavailability studies. Am J Clin Nutr.
81(Suppl 1): 230S–242S. 2005.PubMed/NCBI
|
|
16
|
Howells LM, Moiseeva EP, Neal CP, Foreman
BE, Andreadi CK, Sun YY, Hudson EA and Manson MM: Predicting the
physiological relevance of in vitro cancer preventive activities of
phytochemicals. Acta Pharmacol Sin. 28:1274–1304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho HJ, Park SY, Kim EJ, Kim JK and Park
JH: 3,3′-Diindolyl-methane inhibits prostate cancer development in
the transgenic adenocarcinoma mouse prostate model. Mol Carcinog.
50:100–112. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heath EI, Heilbrun LK, Li J, Vaishampayan
U, Harper F, Pemberton P and Sarkar FH: A phase I dose-escalation
study of oral BR-DIM (BioResponse 3,3′- Diindolylmethane) in
castrate-resistant, non-metastatic prostate cancer. Am J Transl
Res. 2:402–411. 2010.PubMed/NCBI
|
|
19
|
Moiseeva EP, Almeida GM, Jones GDD and
Manson MM: Extended treatment with physiologic concentrations of
dietary phytochemicals results in altered gene expression, reduced
growth, and apoptosis of cancer cells. Mol Cancer Ther.
6:3071–3079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koon HK, Chan PS, Wong RNS, Wu ZG, Lung
ML, Chang CK and Mak NK: Targeted inhibition of the EGFR pathways
enhances Zn-BC-AM PDT-induced apoptosis in well-differentiated
nasopharyngeal carcinoma cells. J Cell Biochem. 108:1356–1363.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horikawa T, Yoshizaki T, Kondo S, Furukawa
M, Kaizaki Y and Pagano JS: Epstein-Barr Virus latent membrane
protein 1 induces Snail and epithelial-mesenchymal transition in
metastatic nasopharyngeal carcinoma. Br J Cancer. 104:1160–1167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: MiR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong VC, Chen H, Ko JM, Chan KW, Chan YP,
Law S, Chua D, Kwong DL, Lung HL, Srivastava G, et al: Tumor
suppressor dual-specificity phosphatase 6 (DUSP6) impairs cell
invasion and epithelial-mesenchymal transition (EMT)-associated
phenotype. Int J Cancer. 130:83–95. 2012. View Article : Google Scholar
|
|
24
|
Yang F, Qian XJ, Qin W, Deng R, Wu XQ, Qin
J, Feng GK and Zhu XF: Dual phosphoinositide 3-kinase/mammalian
target of rapamycin inhibitor NVP-BEZ235 has a therapeutic
potential and sensitizes cisplatin in nasopharyngeal carcinoma.
PLoS. 8:e598792013. View Article : Google Scholar
|
|
25
|
Xie YQ, Wu XB and Tang SQ: Curcumin
treatment alters ERK-1/2 signaling in vitro and inhibits
nasopharyngeal carcinoma proliferation in mouse xenografts. Int J
Clin Exp Med. 7:108–114. 2014.PubMed/NCBI
|
|
26
|
Chen LC, Liu HP, Li HP, Hsueh C, Yu JS,
Liang CL and Chang YS: Thymidine phosphorylase mRNA stability and
protein levels are increased through ERK-mediated cytoplasmic
accumulation of hnRNP K in nasopharyngeal carcinoma cells.
Oncogene. 28:1904–1915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng C, Liu HY, Zhou M, Zhang LM, Li XL,
Shen SR and Li GY: BRD7 suppresses the growth of nasopharyngeal
carcinoma cells (HNE1) through negatively regulating beta-catenin
and ERK pathways. Mol Cell Biochem. 303:141–149. 2007. View Article : Google Scholar : PubMed/NCBI
|