Introduction
Angiogenesis plays an essential role in promoting
tumor growth. Tumor development beyond 1–2 mm is dependent on the
formation of a functional blood supply system for nutrient delivery
(1–3). Based on previous studies involving
intact established cell lines or vessels, the blood vessels of
tumors and those of normal tissues differ in regards to
permeability, composition of the basement membrane, extracellular
matrix and cellular composition. When compared to normal blood
vessels, tumor vessels are tortuous, exhibit poorly organizational
characteristics, high permeability and are inclined to leak
allowing macromolecules of the tumor microenvironment into blood
circulation (4–6). The study of the mechanism of the
development of tumor blood vessels plays a major role in tumor
diagnosis and therapy. However, endothelial cells (ECs) comprise
only 1–2% of the total amount of tumor tissues, and they are
embedded in matrix components and tightly surrounded by various
other cell types (7). Therefore, it
is particularly difficult to isolate endothelial cells from tumor
tissues.
In recent years, purification of ECs for culture and
molecular profiling has gained more and more interest, and
different techniques have been employed (8–10).
However, these techniques are all prone to obtain a mixed sample
with unwanted cells. This study reports a new purification method
by which to obtain numerous endothelial cells in superior
conditions.
Materials and methods
Cells and animals
C57BL/6 mice were obtained from Vital River Company
(Beijing, China) and were housed and cared for in accordance with
the Federation of European Laboratory Animal Science Association
guidelines, and all protocols were approved by the Animal Ethics
Committee of Guangxi Medical University (Nanning, Guangxi, China).
Mouse lung carcinoma cells (Lewis) (1×106) were injected
into the right flank of mice. Tumors were excised for study 45 days
after injection.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues were
utilized. Dewaxed sections of Hep1–6 xenografts were blocked with
3% hydrogen peroxide and 10% normal serum from the secondary
antibody species, and then incubated at 4°C with the primary
antibody mCD105 (Abcam, Cambridge, UK) overnight. This was then
followed by biotin labeled secondary antibody for 30 min and
horseradish peroxidase-conjugated ultrastreptavidin-labeling
reagent for 30 min. Color was developed with 3,3′-diaminobenzidine
(DAB) solution.
Endothelial cell isolation
Tumors were removed and placed in cold PBS solution
with 50 U/ml heparin. Peripheral and necrotic tissues were excised
and the remaining tumor was minced using a scalpel. Dissociation of
0.1×0.1×0.1 cm3 minced tissue was performed in an enzyme
cocktail of 10 mg collagenase type I, 20 ml Dulbecco's modified
Eagle's medium (DMEM) and 2 ml FBS at room temperature for 60 min
of constant mixing with a vortex. The cell suspension was passed
through 80 mesh strainer, washed with PBS solution, and then the
cells were resuspended in 100 µl buffer [PBS (pH 7.2), 0.5%
FBS, 2 nM ethylenediaminetetraacetic acid]. Single cells were
magnetically labeled with anti-CD105 microbeads (Miltenyi Biotec,
Bergisch Gladbach, Germany) in the dark at 4°C for 30 min and
applied to the prepared MS Column (Miltenyi Biotec, Bergisch
Gladbach). CD105− cells were collected in the
flow-through of the column, while CD105+ cells bound to
the beads were flushed out by applying the plunger supplied with
the column. Sorted CD105+ cells were plated into 6-well
plates and cultured in endothelial cell medium (ScienCell,
USA).
Fluorescence-activated cell sorting
(FACS)
For flow cytometry, the cells were stained at the
concentration of 1×106 cells/90 µl buffer and 10
µl phycoerythrin-conjugated anti-CD105 (eBioscience, San
Diego, CA, USA) at 4°C for 25 min before FACS analysis. All data
were analyzed by EXPO32 software.
Tube formation assay
To analyze the capillary-like tube formation ability
of CD105+ cells, 50 µl/well of growth
factor-reduced Matrigel (BD Biosciences, San Jose, CA, USA) was
laid into 96-well plates to solidify. Cells were seeded into
96-well plates. After 12 h, the tube formation was assessed with
microscopy.
Dil-Ac-LDL uptake assay
CD105+ cells were plated into 6-well
plates at 5×104 cells/dish. At 75% confluency, the
culture medium was replaced with serum-free DMEM for 24 h, followed
by incubation with 2 µg/ml Dil-ac-LDL for 5 h at 37°C in 5%
CO2. Then, the cells were washed and fixed with 4%
paraformaldehyde at 4°C for 30 min, followed by DAPI staining for 3
min. The Dil-ac-LDL uptake was assessed using microscopy.
Statistical analysis
Data are expressed as mean ± SEM. The significance
of differences between groups was assessed by a t-test. All
analyses were performed with GraphPad Prism program version 5
(GraphPad Software, La Jolla, CA, USA). A P-value of <0.05 was
considered to indicate a statistically significant result.
Results
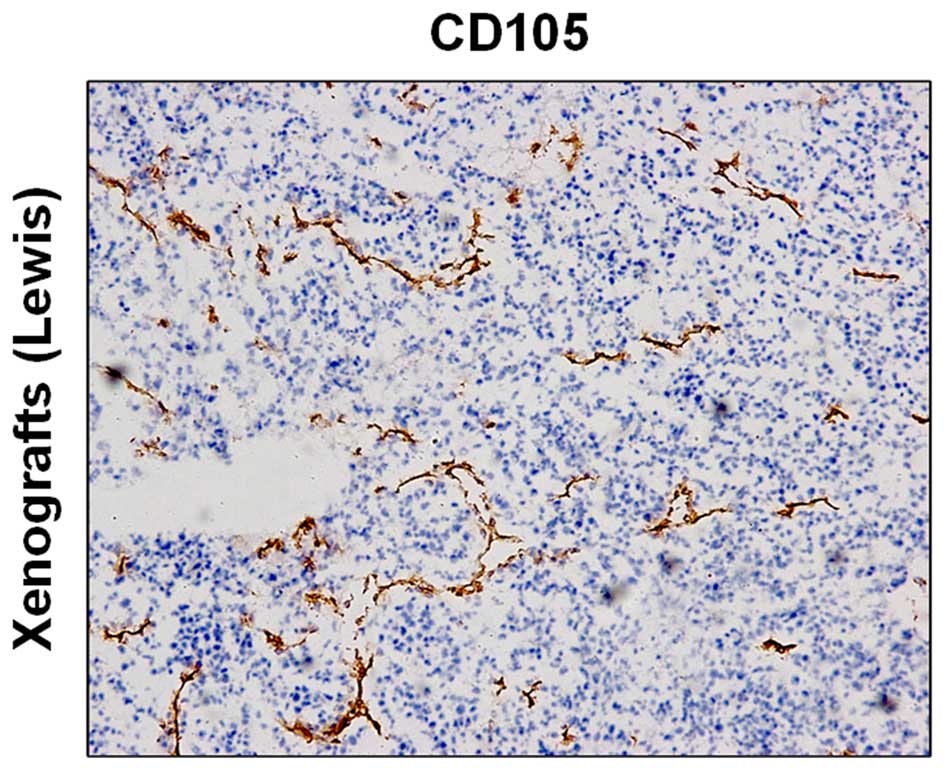
CD105 expression of vascular endothelial
cells in tumor tissues
Immunohistochemistry revealed high expression of
CD105 in the tumor tissue (Fig.
1).
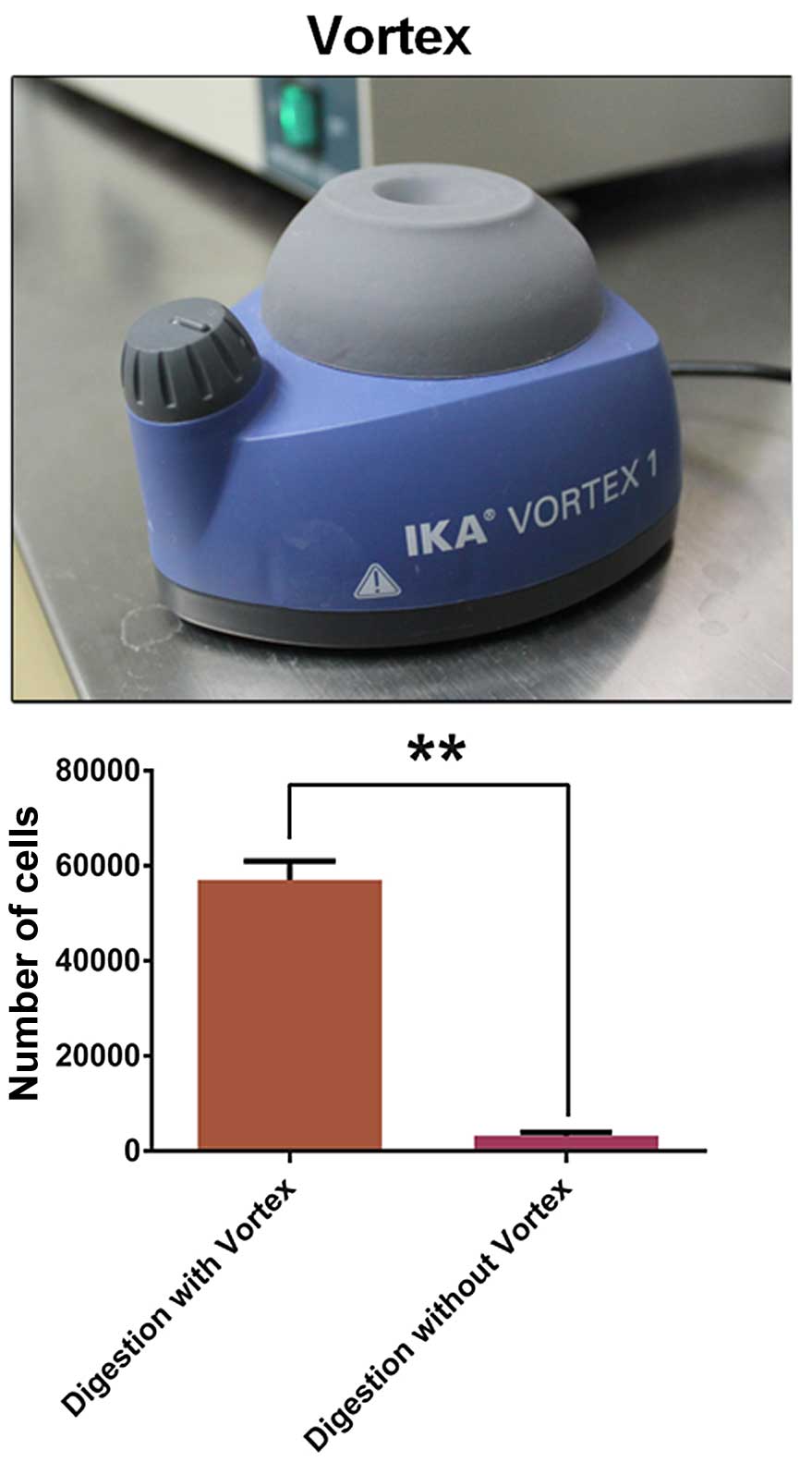
Enrichment and purity of
CD105+ cells
After magnetic separation, the number of
CD105+ cells digested with a vortex
(5.7±0.23×104) was much more than the number without a
vortex (0.32±0.04×104) (Fig.
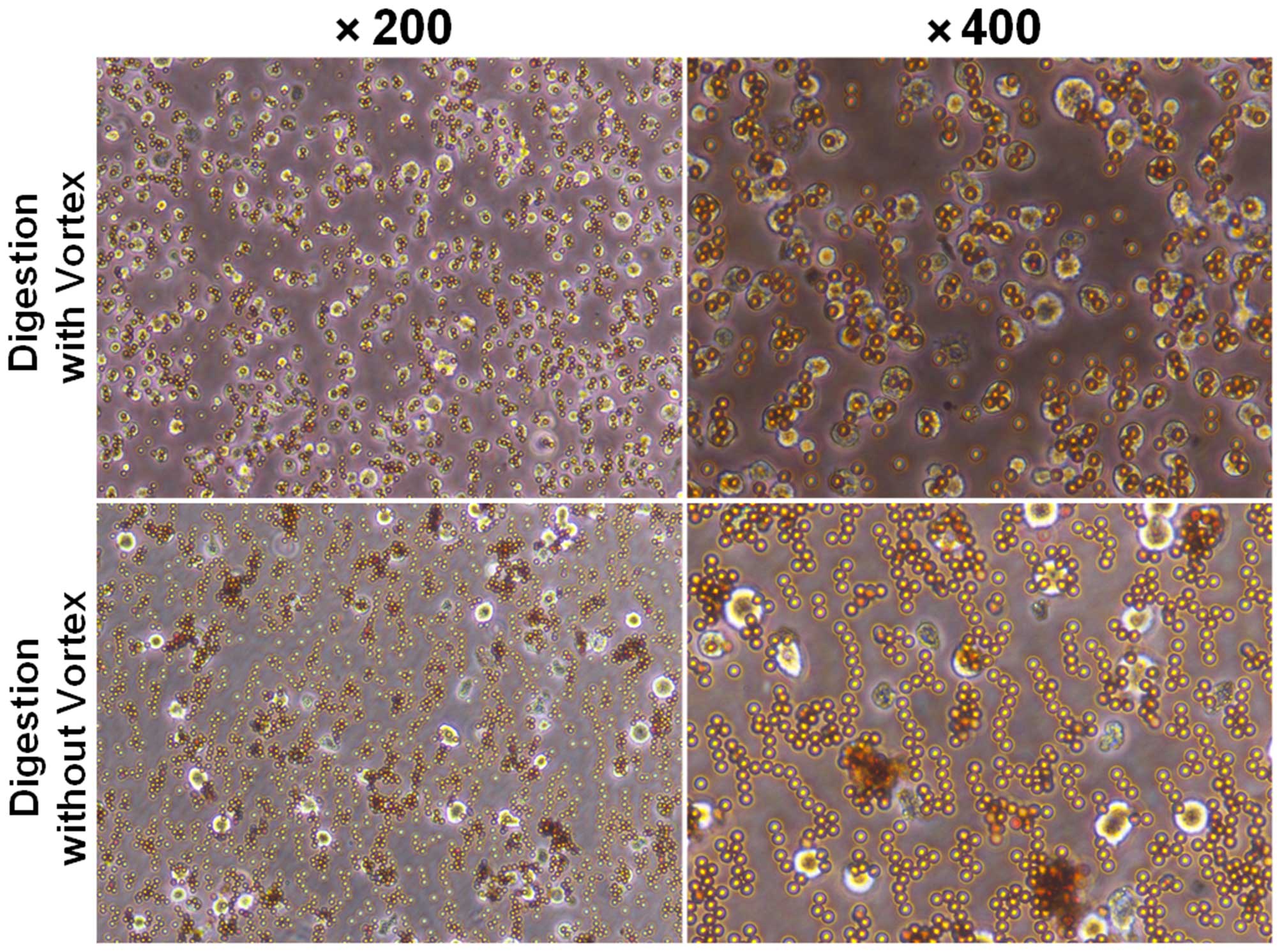
2). After magnetic cell separation (MACS), we showed that
CD105+ cells combined with microbeads as detected under
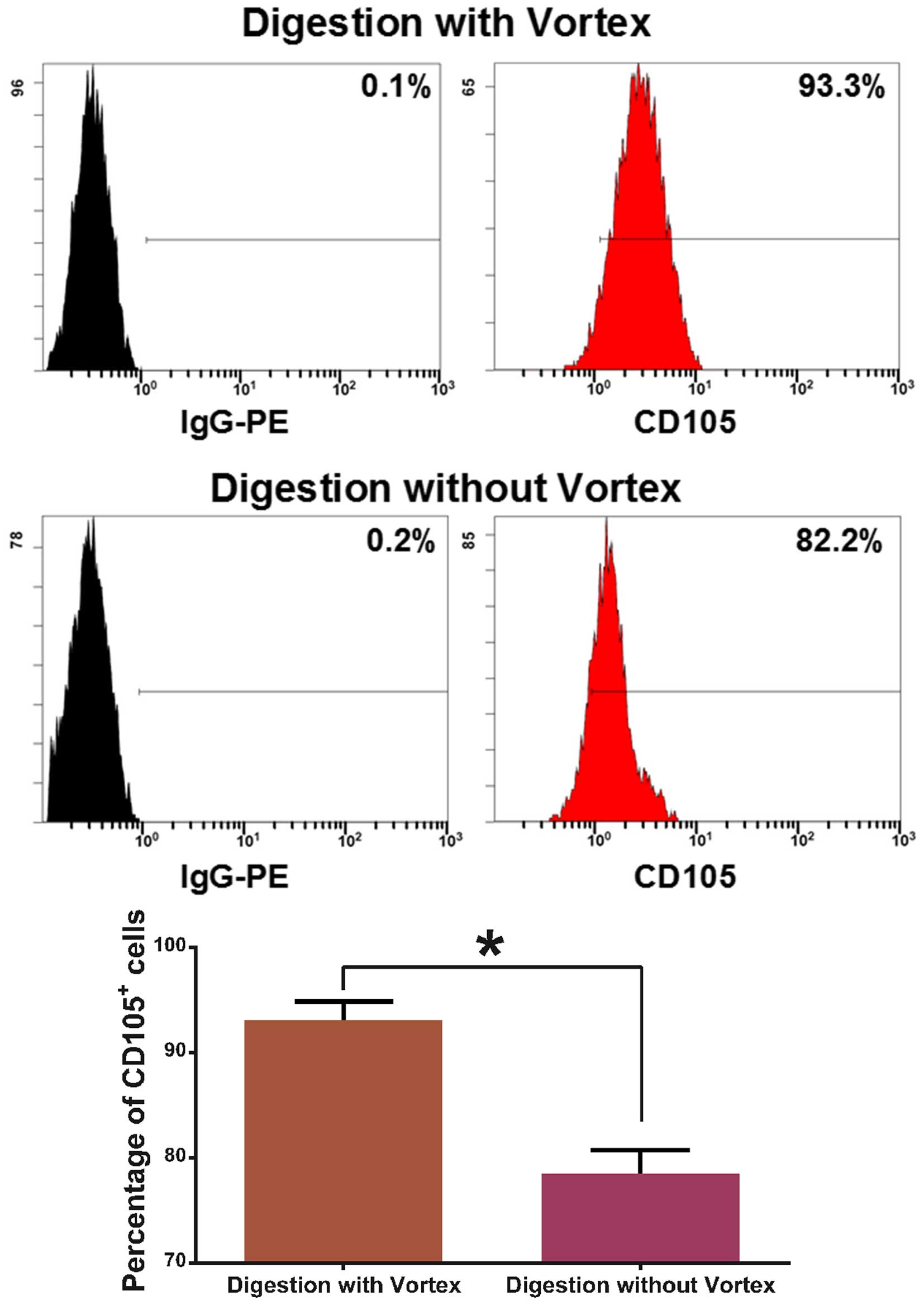
a microscope (Fig. 3). The purity
of the CD105+ cells was 93.07±1.7% as established by
digestion with a vortex, while the purity was 78.53±2.2% as
established by digestion without a vortex (Fig. 4).
Detection of CD105+
endothelial cell activity
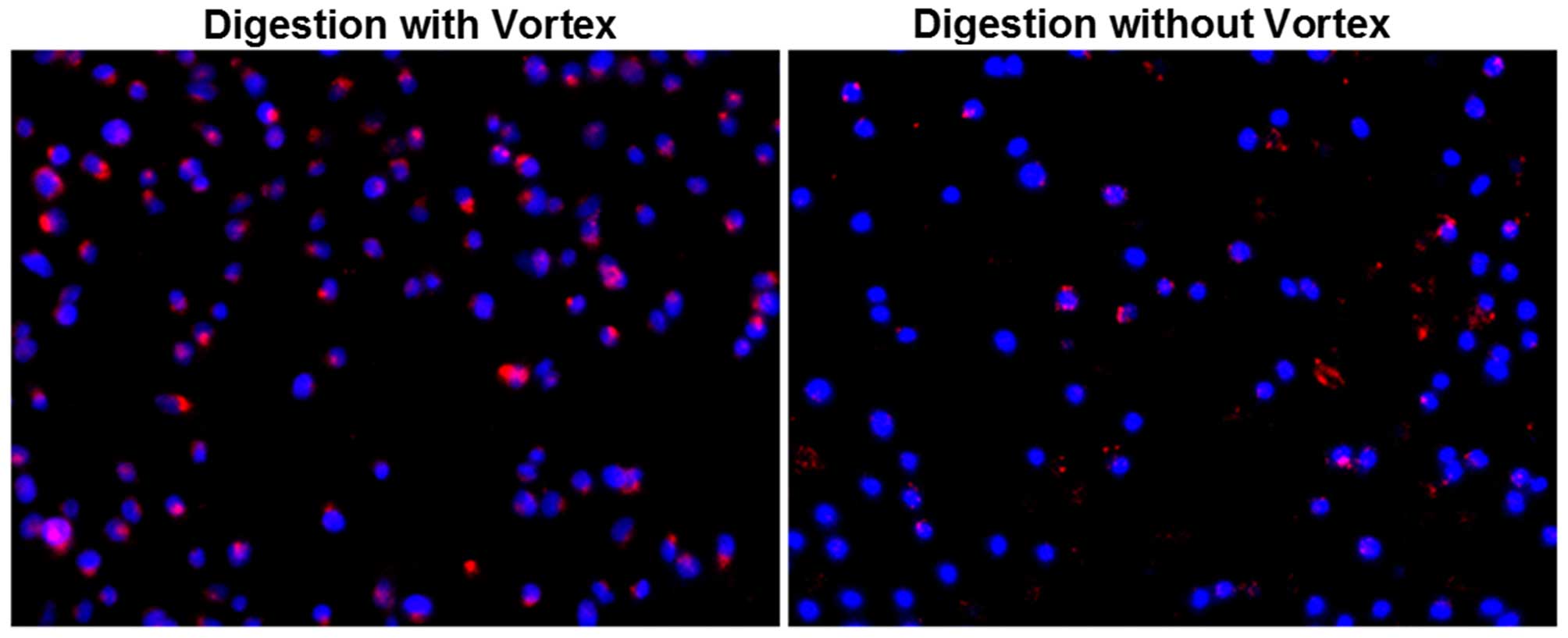
Dil-ac-LDL uptake assay showed that
CD105+ cells which were digested without a vortex
exhibited red fluorescence, and CD105+ cells which were
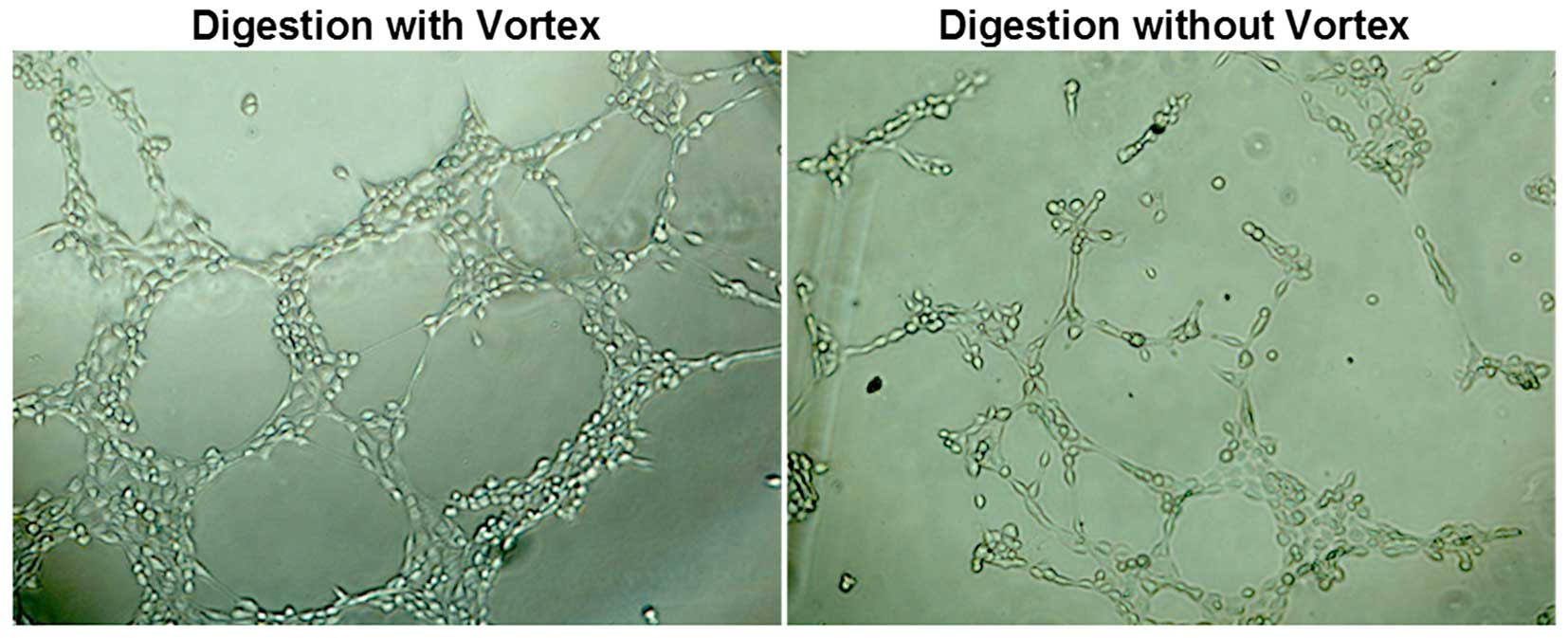
digested with a vortex exhibited stronger fluorescence (Fig. 5). Cells which were digested without
a vortex and seeded onto Matrigel formed capillary-like tube
structures within 12 h, while cells which were digested with a
vortex formed more capillary-like tube structures (Fig. 6).
Discussion
In the process of tumor growth, tumor angiogenesis
and apoptosis have emerged as important aspects. Morover,
proangiogenic factors are always dominant during the process of
tumor development. It is well known that during tumor angiogenesis,
endothelial cells undergo cellular and molecular changes that
accompany the phenotypic appearance of angiogenic vessels (11,12).
Angiogenesis is necessary for tumor growth and
metastasis (13,14). CD105 is an important marker in
angiogenesis but is also essential for the proliferation of
endothelial cells and the stimulation of the active phase of
angiogenesis (15–17).
In view of the known heterogeneity of endothelial
cells, it would appear logical to study endothelial cells derived
from tumor tissues when studying the mechanisms of tumor
progression (18–20). Even though methods have been
described for the isolation of endothelial cells, the efficiency
and purity of sorting have not been described.
In the present study, we described a method to
isolate endothelial cells, a rare cell population found in tumor
tissue, through digestion with a vortex. The subsequent isolation
of endothelial cells was achieved using anti-CD105 antibody-coated
microbeads. This purification technique produces isolated cells
with a purity in excess of 93%, and a higher number of
CD105+ cells than that produced by digestion without a
vortex. These cells express surface markers consistent with their
endothelial cell origin, and maintain the ability of capillary
tube-like structure formation and the uptake of acetylated LDL.
This technology has a significantly important role in tumor
angiogenesis research.
Acknowledgments
This study was supported, in part, by grants from
the National Natural Scientific Foundation of China (nos. 81430055,
81172139, 81172138 and 81372452), the International Cooperation
Project of the Ministry of Science and Technology of China (no.
2015DFA31320), the Project for Innovative Research Team in Guangxi
Natural Science Foundation (2015GXNSFFA139001) and the Project of
Science and Technology of Guangxi (nos. 14125008-2-12 and
1599005-2-10).
References
|
1
|
Folkman J: Tumor angiogenesis. Adv Cancer
Res. 43:175–203. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holzer TR, Fulford AD, Nedderman DM,
Umberger TS, Hozak RR, Joshi A, Melemed SA, Benjamin LE, Plowman
GD, Schade AE, et al: Tumor cell expression of vascular endothelial
growth factor receptor 2 is an adverse prognostic factor in
patients with squamous cell carcinoma of the lung. PLoS One.
8:e802922013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Francescone R, Ngernyuang N, Yan W,
Bentley B and Shao R: Tumor-derived mural-like cells coordinate
with endothelial cells: Role of YKL-40 in mural cell-mediated
angiogenesis. Oncogene. 33:2110–2122. 2014. View Article : Google Scholar :
|
|
4
|
Heuser LS and Miller FN: Differential
macromolecular leakage from the vasculature of tumors. Cancer.
57:461–464. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerlowski LE and Jain RK: Microvascular
permeability of normal and neoplastic tissues. Microvasc Res.
31:288–305. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hori K, Suzuki M, Tanda S and Saito S: In
vivo analysis of tumor vascularization in the rat. Jpn J Cancer
Res. 81:279–288. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Griffioen AW and Molema G: Angiogenesis:
Potentials for pharmacologic intervention in the treatment of
cancer, cardiovascular diseases, and chronic inflammation.
Pharmacol Rev. 52:237–268. 2000.PubMed/NCBI
|
|
8
|
Shi J, Wan Y, Shi S, Zi J, Guan H, Zhang
Y, Zheng Z, Jia Y, Bai X, Cai W, et al: Expression, purification,
and characterization of scar tissue neovasculature endothelial
cell-targeted rhIL10 in Escherichia coli. Appl Biochem Biotechnol.
175:625–634. 2015. View Article : Google Scholar
|
|
9
|
Cai G, Satoh T and Hoshi H: Purification
and characterization of an endothelial cell-viability maintaining
factor from fetal bovine serum. Biochim Biophys Acta. 1269:13–18.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Behdani M, Zeinali S, Karimipour M,
Khanahmad H, Asadzadeh N, Azadmanesh K, Seyed N, Baniahmad SF and
Anbouhi MH: Expression, purification, and characterization of a
diabody against the most important angiogenesis cell receptor:
Vascular endothelial growth factor receptor 2. Adv Biomed Res.
1:342012. View Article : Google Scholar
|
|
11
|
van Beijnum JR, Dings RP, van der Linden
E, Zwaans BM, Ramaekers FC, Mayo KH and Griffioen AW: Gene
expression of tumor angiogenesis dissected: Specific targeting of
colon cancer angiogenic vasculature. Blood. 108:2339–2348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B, et al: Genes expressed in human tumor endothelium.
Science. 289:1197–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joshi S, Singh AR, Zulcic M and Durden DL:
A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1α
and HIF2α stability and tumor growth, angiogenesis, and metastasis.
Mol Cancer Res. 12:1520–1531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ostapoff KT, Awasthi N, Cenik BK, Hinz S,
Dredge K, Schwarz RE and Brekken RA: PG545, an angiogenesis and
heparanase inhibitor, reduces primary tumor growth and metastasis
in experimental pancreatic cancer. Mol Cancer Ther. 12:1190–1201.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nassiri F, Cusimano MD, Scheithauer BW,
Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K and Lloyd RV:
Endoglin (CD105): A review of its role in angiogenesis and tumor
diagnosis, progression and therapy. Anticancer Res. 31:2283–2290.
2011.PubMed/NCBI
|
|
16
|
Goumans MJ, Lebrin F and Valdimarsdottir
G: Controlling the angiogenic switch: A balance between two
distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med.
13:301–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slevin M, Krupinski J and Badimon L:
Controlling the angiogenic switch in developing atherosclerotic
plaques: Possible targets for therapeutic intervention. J
Angiogenes Res. 1:42009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aird WC: Endothelial cell heterogeneity.
Crit Care Med. 31(Suppl 4): S221–S230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lehmann I, Brylla E, Sittig D,
Spanel-Borowski K and Aust G: Microvascular endothelial cells
differ in their basal and tumour necrosis factor-alpha-regulated
expression of adhesion molecules and cytokines. J Vasc Res.
37:408–416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Craig LE, Spelman JP, Strandberg JD and
Zink MC: Endothelial cells from diverse tissues exhibit differences
in growth and morphology. Microvasc Res. 55:65–76. 1998. View Article : Google Scholar : PubMed/NCBI
|