Introduction
Hypoxia is present in the majority of primary and
secondary solid human tumours and occurs as a consequence of the
rapid tumour growth and the poorly organised vasculature (1). Tumour hypoxia represents a significant
clinical problem since it increases resistance to radiotherapy and
many conventional chemotherapeutic agents, in addition to promoting
malignant progression and formation of metastases (2,3).
Notably, these hypoxic regions are a unique feature of solid
tumours that does not occur in normal tissues and thereby, they are
potentially exploitable in the development of cancer-specific
therapies. This has led to the development of bioreductive drugs
that are preferentially toxic to the hypoxic cells known as the
'hypoxia-activated prodrugs'. Agents currently under investigation
include the dinitrobenzamide mustard drug PR-104 (Proacta), the
nitroimidazole drug TH-302 (Threshold Pharmaceuticals), the
dinitroazetidine compounds (4) and
the lead hypoxia-selective Tirapazamine Analogue SN30000 (5).
A further class of hypoxia-activated prodrugs is
exemplified by Banoxantrone (AQ4N), which is an aliphatic
N-oxide (AstraZeneca) and its recently developed analogue
OCT1002 (OncoTherics) (6). AQ4N
exhibits minimal toxicity in normoxic cells but under hypoxic
conditions, the drug undergoes two sequential 2e−
reductions, via the mono N-oxide AQ4M, to give the toxic
metabolite AQ4 (7). The latter is a
stable, potent topoisomerase II inhibitor and DNA intercalator. It
is also unaffected by tumour reoxygenation, does not undergo futile
cycling and has been shown to be efficient in exerting bystander
cell killing (8).
AQ4N is well-documented in murine and human
pre-clinical models as a very effective enhancer of radiotherapy
(9,10), chemo(cisplatin)-radiotherapy
(10) and cyclophosphamide
chemotherapies (11). Although
hypoxia is a pre-requisite, enzymatic reduction is the key process
controlling the activation and toxicity of AQ4N (12). Cytochrome P450 enzymes are important
activators of the prodrug, leading to enhanced response of tumours
to AQ4N (13,14). Unfortunately, the levels of these
enzymes can vary considerably within tumours (15). Recently, we and other researchers
have made the observation that an alternative oxygen-dependent
enzyme, known as inducible nitric oxide synthase (iNOS) is an
efficient activator of AQ4N (8,16).
iNOS is commonly overexpressed in tumours compared
to normal tissues and its expression levels have been linked to
tumour grade (17–19), making it a potential biomarker for
targeted therapies. The enzyme is a homodimer protein in which each
monomer contains an oxygenase domain at its amino-terminal and a
reductase domain at its carboxy-terminal end (20). The iNOS reductase domain shares
considerable homology with NADPH: cytochrome P450 reductase
(21), whereas the oxygenase domain
is responsible for the conversion of L-arginine to citrulline with
the release of nitric oxide (NO) in the presence of oxygen
(22). NO has direct cytotoxic
properties and its role as an efficient radiosensitiser is
well-established (23,24).
The purpose of the present study was to investigate
the potential of exploiting the dual role of iNOS as a prodrug
activator and NO generator in enhancing tumour response to
anticancer therapies. This was achieved by assessing the in
vitro toxicity of AQ4N alone or combined with radiation in cell
lines with variable levels of iNOS activity.
Materials and methods
Chemicals and gases
Mixtures of varying oxygen concentrations balanced
with nitrogen/5% CO2 were purchased from the British
Oxygen Company (London, UK). (AQ4N,
1,4-Bis-{[2-(dimethylamino-N-oxide)ethyl)amino-5,8-dihydroxyanthracene-9,10-dione)}
was kindly provided by AstraZeneca (formally KuDOS Pharmaceuticals
Ltd., Cambridge, UK). Recombinant murine interferon-γ (IFN-γ) was
purchased from R&D Systems (Abingdon, UK). All other reagents
were of analytical grade and were purchased from Sigma Chemical Co.
(Poole, Dorset, UK).
Cell culture
Human fibrosarcoma HT1080 cells were obtained from
the European Collection of Animal Cell Cultures (ECACC; Salisbury,
UK). Cells were routinely cultured in RPMI-1640 medium supplemented
with 2 mM L-glutamine (both from Gibco, Paisley, UK) and 10% fetal
calf serum (LabTech International, Ringmer, UK).
Puromycin-resistant iNOS clones were cultured under identical
conditions in the presence of puromycin (2.5 µg/ml) and 100
µM N-methyl-l-arginine (NMLA). The cells were incubated
under normoxic (21% O2) conditions at 37°c in a
humidified atmosphere of 95% air: 5% CO2. Anoxic
incubations were carried out at <0.01% O2 in a
catalyst induced hypoxic environment (Bactron Anaerobic Chamber,
Sheldon Manufacturing, Inc., Cornelius, Or, USA). Hypoxic (0.1 and
1% O2) exposures were achieved by placing cells in
sealed containers and flowing the appropriate gas mixture (0.1 or
1% O2, plus 5% CO2 in N2) for up
to 24 h.
Generation of stable HT1080 cells
overexpressing iNOS
The mammalian expression vector, pEF iNOS12-puro
containing the iNOS and puromycin resistant genes was constructed
and transfected into HT1080 parental cells, as previously described
(25). The stability of transfected
clones was frequently monitored by growing cells in the presence of
puromycin (2. 5 µg/ml) and checking the iNOS reductase and
oxygenase activities.
Induction of iNOS expression using
cytokines
Exponentially growing cells were harvested, seeded
into 10 cm dishes (Falcon, Becton-Dickinson), and incubated for 24
h. Cells were then exposed to the combination of IFN-γ (100 ng/ml)
and LPS (50 µg/ml) in serum-free medium for 24 h. After
treatment, LPS and IFN-γ were washed out from cultures, and serum
was returned into the medium.
Measurements of iNOS oxygenase and
reductase activity
Determination of enzymatic activity was made in cell
lysates prepared from exponentially growing cultures. The oxygenase
activity of iNOS was measured by monitoring the conversion of
L-[U-14C) arginine to L-[U-14C) citrulline
under fully aerobic conditions (25).
The iNOS reductase activity was determined by the
NADPH-dependent reduction of cytochrome c, as previously
described (26, 27).
Protein determination
The amount of total protein in the cell lysates was
determined by the Pierce bicinchoninic acid (BCA) assay (28) using bovine serum albumin as a
protein standard.
Drug sensitivity studies
Cells with or without prior treatment with cytokines
for 24 h were washed in phosphate-buffered saline (PBS), harvested
and seeded at different densities (500–5×104 cells) into
6 cm dishes (Falcon, Becton-Dickinson). Cells were then exposed to
different oxygen conditions (normoxia, 1% oxygen, 0.1% oxygen or
anoxia) for 24 h before treatment with AQ4N for 90 min. After
treatment, the cells were washed and fresh medium containing 100
µM of the iNOS inhibitor NMLA was added. Survival was
measured by colony formation assay 8–10 days later and individual
colonies containing >50 cells were scored. Clonogenic survival
was calculated for each drug dose after correcting for plating
efficiency and values of ic10, the concentration of the
drug required to result in 10% survival, were calculated from
clonogenic survival curves.
AQ4N-radiation combination studies
Cells with or without prior treatment with cytokines
were seeded and exposed to the different oxygen concentrations as
described above. The cells were then treated with varying
concentrations of AQ4N in the presence or absence of NMLA and X-ray
was delivered at 0.75 Gy/min. The cells were then washed and fresh
medium containing NMLA was added. Survival was measured by colony
formation assay 8–10 days later. The sensitizer enhancement ratio
(SER) for radiation was calculated by dividing the AQ4N dose that
gives a surviving fraction of 0.1 (IC10) for
unirradiated cells by the AQ4N dose for cells treated with AQ4N
plus radiation. The contribution of NO to the SER was calculated by
dividing the IC10 for AQ4N for irradiated cells in
presence of NMLA by the IC10 for AQ4N obtained in the
absence of NMLA.
Statistical analysis
All data shown are from at least three independent
experiments. Drug and radiation survival curves were plotted after
correction for the plating efficiency of untreated cells.
Statistical analysis was carried out with unpaired two-tailed
t-tests and a P-value ≤0.05 was considered to indicate a
statistically significant result.
Results
Upregulation of iNOS activity in human
tumour cells
We have employed an expression vector containing the
cdNA for human iNOS to transfect HT1080 tumour cells. A clone
(NOS12) was selected based on stable expression of high
iNOS activity as determined by the conversion of
14C-L-arginine to citrulline (71.39 pmol/min/mg compared
to 0.94 pmol/min/mg for parental cells). Catalytic activity of the
reductase domain as determined by the NADPH-dependent reduction of
cytochrome c was also elevated (16.12 nmol/min/mg in
HT1080-NOS12 compared to 3.71 nmol/min/mg for parental
cells).
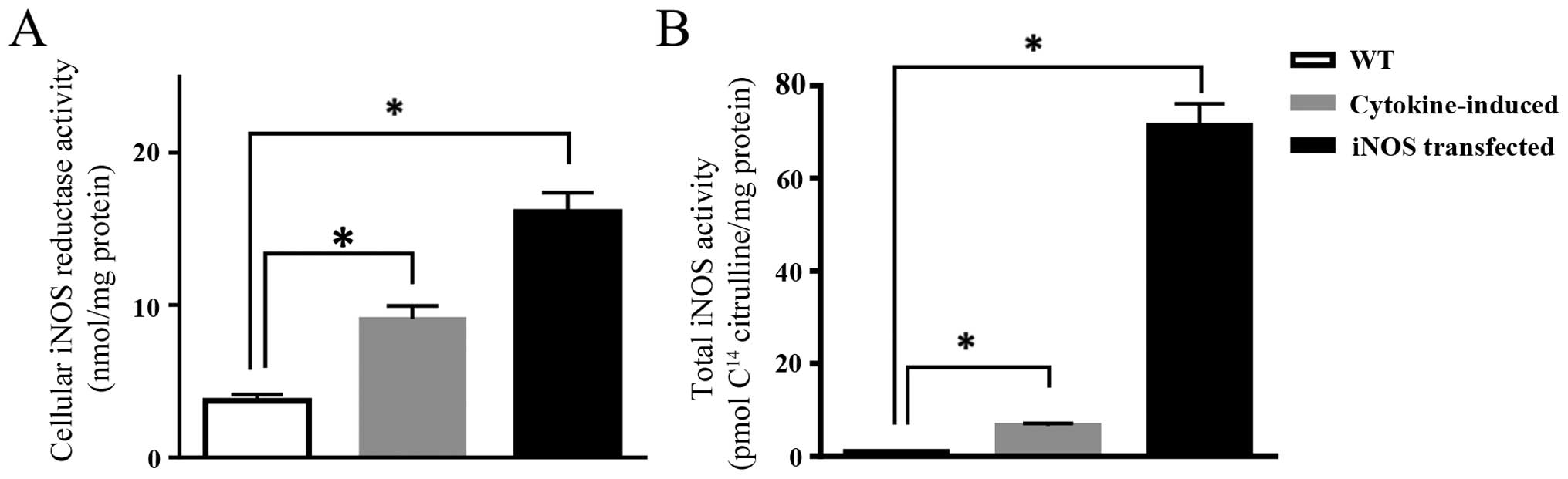
Induction of the iNOS protein using a cocktail of
cytokines resulted in significant increase in enzymatic activity
with a 2.5- and 7-fold increase in reductase and oxygenase
activities, respectively, when compared to parental cells (Fig. 1).
Induction of the iNOS oxygenase activity reflects an
enhanced potential for generating NO and as previously shown, NO
production is oxygen-dependent (24) and this provides the basis for
carrying out the subsequent drug/radiation experiments at different
oxygen tensions.
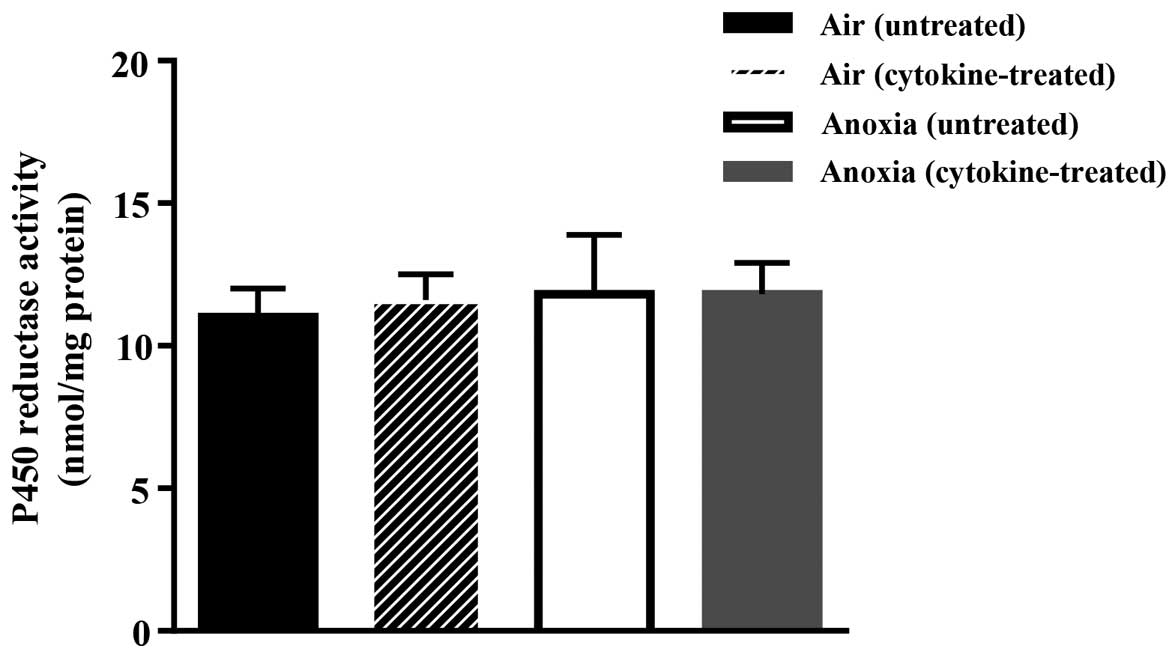
Exposure of the cells to the cytokine cocktail did
not affect the activity of cytochrome P450 reductase as shown in
Fig. 2. This, therefore, discounts
the possibility of additional contribution of P450 reductase to
drug activation following treatment with cytokines.
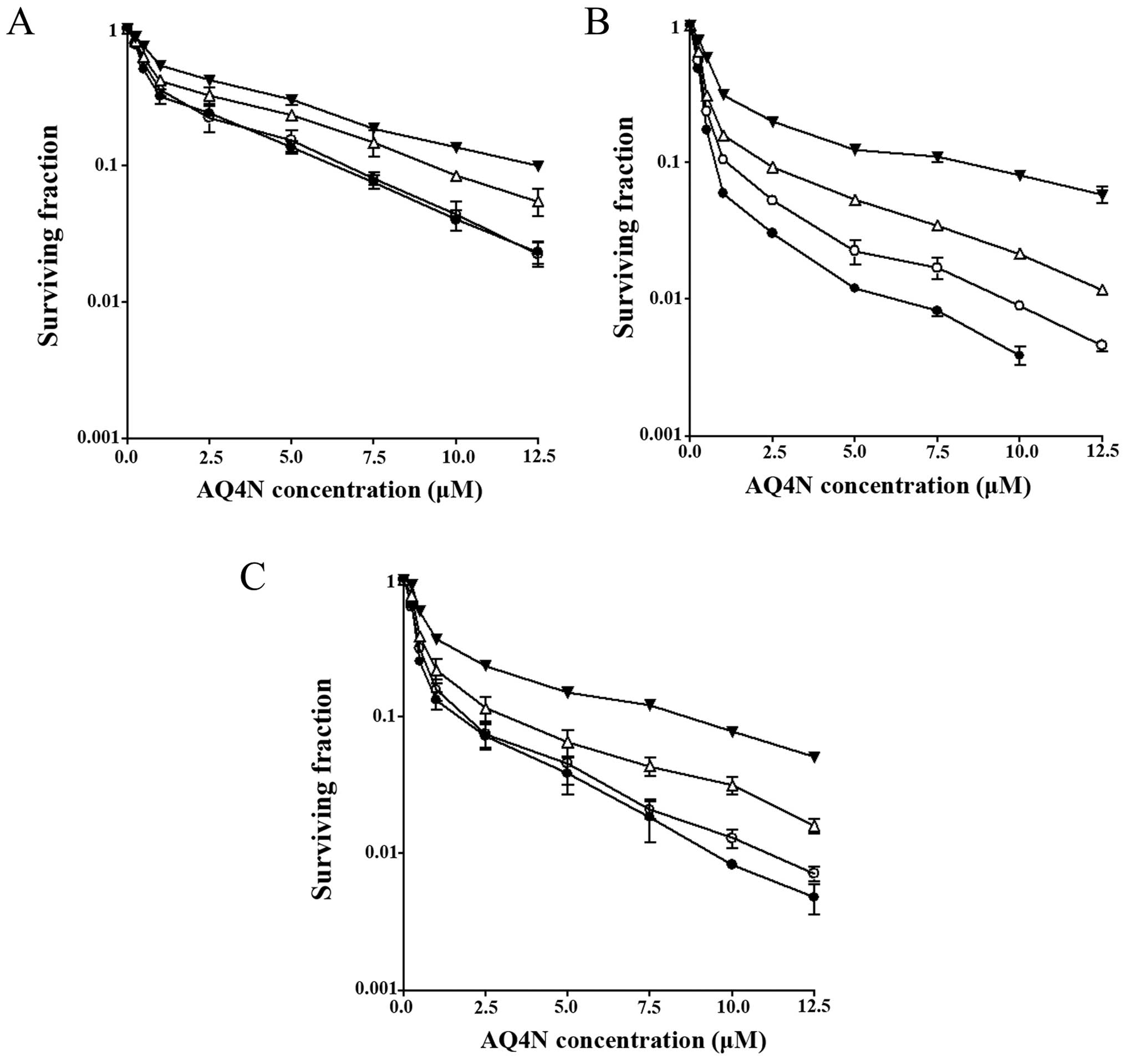
Increased iNOS activity selectively
enhances the cellular toxicity of AQ4N in hypoxia
Toxicity of AQ4N to HT1080 parental and
cytokine-induced cells, and the iNOS12 clone was
determined by exposing the cells to the drug for 90 min under
different oxygen conditions (normoxia, 1 and 0.1% hypoxia and
anoxia). At this stage, the cells were cultured in medium
containing the iNOS inhibitor NMLA to preclude any contribution to
toxicity by NO. Cytotoxicity was then measured using clonogenic
survival assay and the data are presented in Fig. 3A–C.
The normoxic IC10 value of AQ4N (12.4
µM) in HT1080 parental cells did not significantly change
following induction with cytokines or transfection with the iNOS
gene (~9 µM in both cell lines). In fact, sensitivity of the
three cell lines to AQ4N was dependent on both hypoxia and the
level of iNOS activity with HT1080 iNOS12 cells
exhibiting the highest sensitivity to the drug under conditions of
hypoxia and this corresponds to the higher level of iNOS activity
in these cells.
For example, under conditions of 1% oxygen, there
was a 1.3-fold decrease in the IC10 value of HT1080
parental cells, a 2.7-fold decrease in the IC10 value of
cytokine-induced cells and a 3.4-fold decrease in the
IC10 value of HT1080 iNOS12 cells in
comparison to their corresponding normoxic IC10 values.
Under more severe hypoxic conditions (0.1% oxygen level), there was
a 1.9-, 4.7- and 7.1-fold increase in AQ4N cytotoxicity in HT1080
parental, cytokine-induced cells and HT1080 iNOS12
cells, respectively. In anoxia, these values further increased to
2, 5.1 and 10.9 in each of the cell lines.
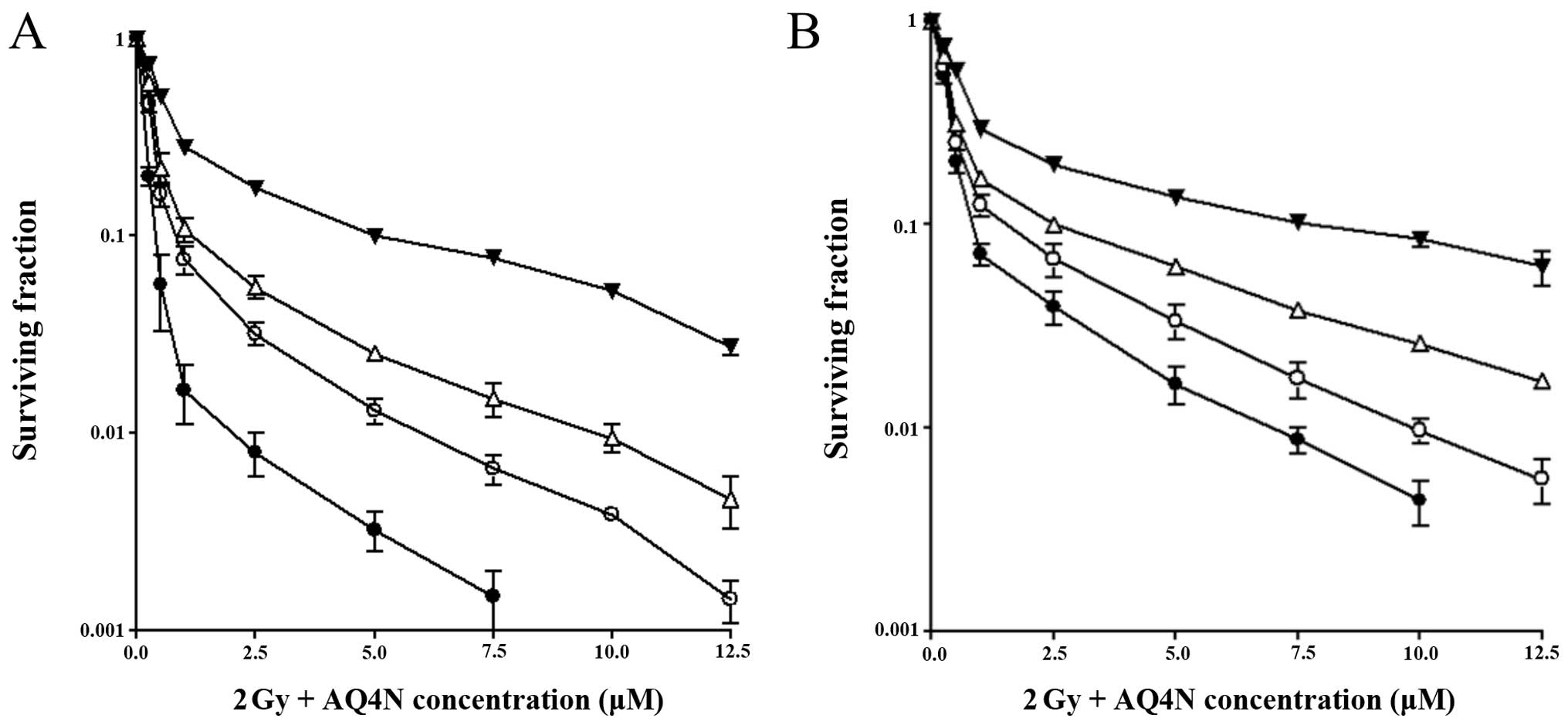
Radiation enhances the chemotherapeutic
effects of AQ4N in iNOS-expressing cell lines
The effect of a low radiation dose (2 Gy) on the
cellular toxicity of AQ4N was examined in the two cell lines
expressing different levels of iNOS activity: HT1080
iNOS12 and cytokine-induced HT1080 cells. NMLA was also
present in the cultures during the course of these experiments to
exclude any contribution by NO.
We found that cytotoxicity of AQ4N was enhanced by
radiation in both cell lines when compared to exposure to the drug
alone. This enhancement was significant only under anoxic and
hypoxic conditions (Fig. 4). The
drug dose required to achieve 10% survival of HT1080
iNOS12 cells in anoxia was 0.75 µM. However, when
the cells were exposed to a radiation dose of 2 Gy immediately
after AQ4N treatment, the dose required to achieve 10% cell
survival was further reduced to 0.38 µM, representing a
2-fold enhancement in cytotoxicity of AQ4N. Under 0.1% oxygen
conditions, 1.15 µM of AQ4N was required to achieve 10% cell
survival, whereas, when AQ4N was combined with radiation, the drug
concentration required fell to 0.55 µM, leading to another
2-fold increase in toxicity of the prodrug. Again, under 1% oxygen
conditions, radiation led to a 1.8-fold increase in sensitivity of
the cells to AQ4N (Fig. 4A).
A similar pattern was observed with the
cytokine-treated cells, although higher drug concentrations were
required to achieve similar results which could be explained by
their lower ability to activate AQ4N. For example, under anoxic
conditions, 1.68 µM of AQ4N was required to achieve 10%
survival in anoxia, which further decreased to 0.75 µM when
the cells were exposed to radiation, leading to a 2.2-fold
enhancement in drug toxicity. In 1% oxygen conditions, a
concentration of 3.2 µM of AQ4N was needed to achieve 10%
cell survival, and this requirement was again reduced to 1.6
µM after irradiation, representing a 2-fold enhancement
(Fig. 4B).
NO enhances the radiosensitising effects
of AQ4N
The effect of endogenously produced NO on the
sensitivity of cells to the combination therapy of radiation (2 Gy)
and AQ4N was assessed in HT1080 iNOS12 clones and
cytokine-induced HT1080 cells. We demonstrated that AQ4N was most
potent when NO release was allowed prior to irradiation. The
results suggest that this increase in potency is due to the
NO-mediated 'fixing' of the radiation-induced damage which was
observed in intermediate oxygen conditions only. There was a 1.4-
and 2.2-fold increase in sensitivity of HT1080 iNOS12
cells to AQ4N/radiation treatment when NO was present under
conditions of 0.1% and 1% oxygen, respectively (Fig. 5A). Therefore, compared to the drug
alone, 2.8- and 5.26-fold enhancement in AQ4N sensitisation was
achieved with combination therapy of AQ4N/radiation/NO under 0.1%
and 1% oxygenation levels, respectively.
This effect was comparable in HT1080
cytokine-induced cells (Fig. 5B).
The sensitisation enhancement ratios for NO were 1.41 and 2.13 in
0.1 and 1% oxygen levels, respectively. Therefore, combination
therapy of AQ4N/radiation/NO resulted in up to 4-fold increase in
cell sensitisation when compared to AQ4N monotherapy under hypoxic
conditions.
Evidence for the involvement of NO in this
enhancement was provided by the addition of the NO inhibitor NMLA,
which completely reversed the previously observed effect. As
expected, no enhancement in cellular toxicity of either cell line
was observed in anoxia since NO production is very limited. In
addition, under normoxic conditions, iNOS expression and subsequent
production of NO did not have any additive effect to oxygen in
radiosensitisation of the cells.
Discussion
AQ4N is an attractive hypoxia-activated prodrug
since it penetrates deep into tumour tissue (29), and its final toxic metabolite AQ4 is
stable and generally localises to the hypoxic tumour regions as
shown in both pre-clinical and clinical studies (10,30).
However, its cytotoxicity is disadvantaged by the limited in
vitro bioactivation in several cancer cell lines incubated
under hypoxic conditions (31). In
fact, significant cytotoxicity of AQ4N was only observed when
hypoxic cells were incubated with NADPH supplemented microsomes
(32). Although, enzymes such as
cytochrome P450 reductases are useful in activating bioreductive
prodrugs in human cell cultures, their expression in tumours tend
to be low and generally does not overlap with biomarkers of hypoxia
such as carbonic anhydrase IX (33). Therefore, our aim here was to mimic
an in vivo situation where enzymatic activity is abundant.
We chose nitric oxide (NO) synthase since the enzyme is widely
expressed and often upregulated in multiple tumour tissues with an
expression that correlates with tumour grade, high incidence of
metastasis and poor prognosis (18,19,34–37).
Immunohistochemical staining of breast tumour biopsies revealed
that the expression of the NOS enzyme was mainly localised in areas
between viable and necrotic regions of the tumour (presumably
hypoxic regions) (25). Therefore,
considerable benefit could be gained by exploiting the inherent
differences in NOS expression that can distinguish between normal
and malignant tissues as well as its ability to produce a hypoxic
cytotoxin and generate the strong radiosensitiser NO.
We initially examined the ability of iNOS to
activate AQ4N and improve its toxicity in vitro in the human
fibrosarcoma cells HT1080. AQ4N was shown to produce an
oxygen-dependent effect as there was a clear difference in
clonogenic cell kill between normoxic and hypoxic conditions with
the greatest cell kill occurring in anoxia. AQ4N treatment targeted
to the iNOS expressing cells led to further reductions in the drug
dose required to achieve similar toxicity under all oxygen
concentrations except normoxia. Our results are in agreement with
the fact that both enzymatic activity and hypoxia are
pre-requisites for the efficient activation of AQ4N (12).
Combination therapy of AQ4N and radiation
experiments demonstrate that enhancement of cytotoxicity was only
observed in hypoxia without affecting well-oxygenated tissues.
Therefore, combining the two therapeutic agents can increase
toxicity to the hypoxic tumour cells without doing so in the
critical normal tissues.
Although the role of NO as a radiosensitiser was
first reported in hypoxic bacteria and human cells not long after
oxygen (23,38), it was not until recently that its
potential radiosensitising properties have been rediscovered with
the development of several NO-generating agents. These compounds
showed that NO was almost as efficient as oxygen in improving the
radiosensitivity of cancer cells (39–41).
In the present study, we found that NO exhibited important
cell-sensitisation characteristics to the combination of AQ4N and
radiotherapy under hypoxic conditions. The fact that this
enhancement was abolished by an iNOS inhibitor confirms the role of
NO in mediating this effect. Under normoxic conditions, we believe
that oxygen on its own is able to mediate maximal radiation-induced
damage, hence, masking any radiosensitising effects of NO (24). Using different oxygen conditions to
model the varied oxygenation level usually found in tumour tissues,
we were able to examine not only the cytotoxicity effects under
different oxygen concentrations, but more importantly the changes
in the level of radiosensitivity when NO was generated
endogenously.
Obviously, rationale design of novel bioreductive
drugs based on knowledge of their enzymology and heterogeneity of
the tumour microenvironment is a vital approach in the development
of anticancer therapeutics. Importantly, identifying a clinical
cohort that is most likely to benefit from these prodrugs is
essential in ensuring their success in the clinic. In the present
study, we demonstrate that increased iNOS expression in hypoxic
tumour cells presents a viable target to improve treatment outcomes
with AQ4N and radiation without sensitising the healthy
well-oxygenated tissues. Hopefully, lessons will be learned from
previous failures of bioreductive drugs in clinical trials and a
more personalised therapy approach will be adopted in the
future.
Acknowledgments
The present study was supported by grant from the
KuDOS Pharmaceuticals Ltd. (now AstraZeneca) to I.J.S., the Medical
Research Council (I.J.S. and K.J.W.), and the EU 6th and 7th
Framework Programmes Euroxy (I.J.S.) and Metoxia (K.J.W.)
respectively, and are gratefully acknowledged.
Abbreviations:
|
CYPs
|
cytochromes
|
|
IFN-γ
|
interferon-γ
|
|
iNOS
|
inducible nitric oxide synthase
|
|
IRF-1
|
interferon regulatory factor-1
|
|
LPS
|
lipopolysccharides
|
|
NMLA
|
N-methyl-L-arginine
|
|
NO
|
nitric oxide
|
|
NOS
|
nitric oxide synthase
|
References
|
1
|
Brown JM: Exploiting the hypoxic cancer
cell: Mechanisms and therapeutic strategies. Mol Med Today.
6:157–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hockel M, Schlenger K, Aral B, Mitze M,
Schaffer U and Vaupel P: Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.
Cancer Res. 56:4509–4515. 1996.PubMed/NCBI
|
|
3
|
Brizel DM, Sibley GS, Prosnitz LR, Scher
RL and Dewhirst MW: Tumor hypoxia adversely affects the prognosis
of carcinoma of the head and neck. Int J radiat Oncol Biol Phys.
38:285–289. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ning S, Bednarski M, Oronsky B, Scicinski
J, Saul G and Knox SJ: Dinitroazetidines are a novel class of
anticancer agents and hypoxia-activated radiation sensitizers
developed from highly energetic materials. Cancer Res.
72:2600–2608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hicks KO, Siim BG, Jaiswal JK, Pruijn FB,
Fraser AM, Patel R, Hogg A, Liyanage HD, Dorie MJ, Brown JM, et al:
Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and
SN29751 as tirapazamine analogues with improved tissue penetration
and hypoxic cell killing in tumors. Clin Cancer Res. 16:4946–4957.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith P: Cytomics of the tumour
microenvironment: Therapeutic targeting. J Inflamm. 12(Suppl 1):
O102015. View Article : Google Scholar
|
|
7
|
McKeown SR, Cowen RL and Williams KJ:
Bioreductive drugs: From concept to clinic. Clin Oncol (R Coll
Radiol). 19:427–442. 2007. View Article : Google Scholar
|
|
8
|
Mehibel M, Singh S, Chinje EC, Cowen RL
and Stratford IJ: Effects of cytokine-induced macrophages on the
response of tumor cells to banoxantrone (AQ4N). Mol Cancer Ther.
8:1261–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McKeown SR, Hejmadi MV, Mcintyre IA,
McAleer JJ and Patterson LH: AQ4N: An alkylaminoanthraquinone
N-oxide showing bioreductive potential and positive interaction
with radiation in vivo. Br J Cancer. 72:76–81. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams KJ, Albertella MR, Fitzpatrick B,
Loadman PM, Shnyder SD, Chinje EC, Telfer BA, Dunk CR, Harris PA
and Stratford IJ: In vivo activation of the hypoxia-targeted
cytotoxin AQ4N in human tumor xenografts. Mol Cancer Ther.
8:3266–3275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friery OP, Gallagher R, Murray MM, Hughes
CM, Galligan ES, Mcintyre IA, Patterson LH, Hirst DG and McKeown
SR: Enhancement of the anti-tumour effect of cyclophosphamide by
the bioreductive drugs AQ4N and tirapazamine. Br J Cancer.
82:1469–1473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patterson LH and McKeown SR: AQ4N: A new
approach to hypoxia-activated cancer chemotherapy. Br J Cancer.
83:1589–1593. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mccarthy HO, Yakkundi A, Mcerlane V,
Hughes CM, Keilty G, Murray M, Patterson LH, Hirst DG, McKeown SR
and Robson T: Bioreductive GDEPT using cytochrome P 450 3A4 in
combination with AQ4N. Cancer Gene Ther. 10:40–48. 2003. View Article : Google Scholar
|
|
14
|
Mcerlane V, Yakkundi A, Mccarthy HO,
Hughes CM, Patterson LH, Hirst DG, Robson T and McKeown SR: A
cytochrome P450 2B6 meditated gene therapy strategy to enhance the
effects of radiation or cyclophosphamide when combined with the
bioreductive drug AQ4N. J Gene Med. 7:851–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rooseboom M, Commandeur JN and Vermeulen
NP: Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol
Rev. 56:53–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishida CR and Ortiz de Montellano PR:
Reductive heme-dependent activation of the N-oxide prodrug AQ4N by
nitric oxide synthase. J Med Chem. 51:5118–5120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fitzpatrick B, Mehibel M, Cowen RL and
Stratford IJ: iNOS as a therapeutic target for treatment of human
tumors. Nitric Oxide. 19:217–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cobbs CS, Brenman JE, Aldape KD, Bredt DS
and Israel MA: Expression of nitric oxide synthase in human central
nervous system tumors. Cancer Res. 55:727–730. 1995.PubMed/NCBI
|
|
19
|
Gallo O, Masini E, Morbidelli L, Franchi
A, Fini-Storchi I, Vergari WA and Ziche M: Role of nitric oxide in
angiogenesis and tumor progression in head and neck cancer. J Natl
Cancer Inst. 90:587–596. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hemmens B and Mayer B: Enzymology of
nitric oxide synthases. Methods Mol Biol. 100:1–32. 1998.
|
|
21
|
Bredt DS, Hwang PM, Glatt CE, Lowenstein
C, Reed RR and Snyder SH: Cloned and expressed nitric oxide
synthase structurally resembles cytochrome P-450 reductase. Nature.
351:714–718. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Griffith OW and Stuehr DJ: Nitric oxide
synthases: Properties and catalytic mechanism. Annu Rev Physiol.
57:707–736. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gray LH, Green FO and Hawes CA: Effect of
nitric oxide on the radiosensitivity of tumour cells. Nature.
182:952–953. 1958. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh S, Cowen RL, Chinje EC and Stratford
IJ: The impact of intracellular generation of nitric oxide on the
radiation response of human tumor cells. Radiat Res. 171:572–580.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chinje EC, Williams KJ, Telfer BA, Wood
PJ, van der Kogel AJ and Stratford IJ: 17beta-Oestradiol treatment
modulates nitric oxide synthase activity in MDA231 tumour with
implications on growth and radiation response. Br J Cancer.
86:136–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chinje EC, Cowen RL, Feng J, Sharma SP,
Wind NS, Harris AL and Stratford IJ: Non-nuclear localized human
NOSII enhances the bioactivation and toxicity of tirapazamine
(SR4233) in vitro. Mol Pharmacol. 63:1248–1255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patterson AV, Barham HM, Chinje EC, Adams
GE, Harris AL and Stratford IJ: Importance of P450 reductase
activity in determining sensitivity of breast tumour cells to the
bioreductive drug, tirapazamine (SR 4233). Br J Cancer.
72:1144–1150. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith PK, Krohn RI, Hermanson GT, Mallia
AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ and
Klenk DC: Measurement of protein using bicinchoninic acid. Anal
Biochem. 150:76–85. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trédan O, Garbens AB, Lalani AS and
Tannock IF: The hypoxia-activated ProDrug AQ4N penetrates deeply in
tumor tissues and complements the limited distribution of
mitoxantrone. Cancer Res. 69:940–947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Albertella MR, Loadman PM, Jones PH,
Phillips RM, Rampling R, Burnet N, Alcock C, Anthoney A, Vjaters E,
Dunk CR, et al: Hypoxia-selective targeting by the bioreductive
prodrug AQ4N in patients with solid tumors: Results of a phase I
study. Clin Cancer Res. 14:1096–1104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manley E Jr and Waxman DJ: Impact of tumor
blood flow modulation on tumor sensitivity to the bioreductive drug
banoxantrone. J Pharmacol Exp Ther. 344:368–377. 2013. View Article : Google Scholar :
|
|
32
|
Patterson LH: Rationale for the use of
aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA
binding agents: A new class of bioreductive agent. Cancer
Metastasis Rev. 12:119–134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guise CP, Abbattista MR, Tipparaju SR,
Lambie NK, Su J, Li D, Wilson WR, Dachs GU and Patterson AV:
Diflavin oxidoreductases activate the bioreductive prodrug PR-104A
under hypoxia. Mol Pharmacol. 81:31–40. 2012. View Article : Google Scholar
|
|
34
|
Swana HS, Smith SD, Perrotta PL, Saito N,
Wheeler MA and Weiss RM: Inducible nitric oxide synthase with
transitional cell carcinoma of the bladder. J Urol. 161:630–634.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song ZJ, Gong P and Wu YE: Relationship
between the expression of iNOS, VEGF, tumor angiogenesis and
gastric cancer. World J Gastroenterol. 8:591–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lagares-Garcia JA, Moore RA, Collier B,
Heggere M, Diaz F and Qian F: Nitric oxide synthase as a marker in
colorectal carcinoma. Am Surg. 67:709–713. 2001.PubMed/NCBI
|
|
37
|
Thomsen LL, Miles DW, Happerfield L,
Bobrow LG, Knowles RG and Moncada S: Nitric oxide synthase activity
in human breast cancer. Br J Cancer. 72:41–44. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Howard-Flanders P: Effect of nitric oxide
on the radiosensitivity of bacteria. Nature. 180:1191–1192. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Griffin RJ, Makepeace CM, Hur WJ and Song
CW: Radiosensitization of hypoxic tumor cells in vitro by nitric
oxide. Int J radiat Oncol Biol Phys. 36:377–383. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mitchell JB, Cook JA, Krishna MC, DeGraff
W, Gamson J, Fisher J, Christodoulou D and Wink DA: Radiation
sensitisation by nitric oxide releasing agents. Br J Cancer Suppl.
27:S181–S184. 1996.PubMed/NCBI
|
|
41
|
Verovski VN, Van den Berge DL, Soete GA,
Bols BL and Storme GA: Intrinsic radiosensitivity of human
pancreatic tumour cells and the radiosensitising potency of the
nitric oxide donor sodium nitroprusside. Br J Cancer. 74:1734–1742.
1996. View Article : Google Scholar : PubMed/NCBI
|