Introduction
Despite the development of early diagnostics,
therapeutic regimens, and targeted drugs, breast cancer is the most
common female cancer and the second leading cause of cancer death
in women worldwide (1).
Approximately 70–75% of breast cancer tumors express the estrogen
receptor (ER), progesterone receptor (PR), and/or
estrogen-responsive and ER-dependent gene products (2). Generally, tamoxifen, an ER antagonist
in the breast, is used as the chemotherapeutic agent for
ER-positive breast cancer patients and bound ER complex
competitively prevents the interaction of estrogen with ER
(2,3). However, a substantial proportion of
the patients who receive tamoxifen treatment are limited by
intrinsic and acquired resistance (4,5).
Therefore, many researchers have been investigating a variety of
strategies to overcome or bypass tamoxifen resistance. Recently, we
also reported that unlike gefitinib, neratinib prevents the more
effective EGFR and HER2 signaling pathways as well as triggers
apoptotic cell death of tamoxifen-resistant cells (6).
Interleukin-8 (IL-8, CXCL8) is a multifunctional
pro-inflammatory chemokine that is upregulated by hypoxia,
cytokines and other environmental stresses through activation of
transcription factors such as NF-κB and AP-1 (7,8). Serum
IL-8 binds to its receptors CXCR1 and CXCR2, resulting in
metastatic invasiveness, early recurrence and worse outcomes
(9–11). All of the malignant breast cancer
specimens express CXCR1 and CXCR2, whereas only 50% of the benign
breast tissue samples express these receptors (12). In addition, IL-8 is highly expressed
in estrogen receptor-negative [ER−] and HER2+
breast cancers (13,14). The ER significantly decreases IL-8
promoter activity through an estrogen-independent pathway (15). The complex of IL-8 and CXCR2
stimulates VEGF expression in endothelial cells and enhances
production of MMP-2 and MMP-9 (16,17).
The aim of the present study was to determine
whether IL-8 is a prognostic marker for tamoxifen resistance and to
understand the mechanisms underlying the role of IL-8 expression in
tamoxifen-resistant cells. We found that the level of IL-8
expression was directly associated with the survival of luminal A
type breast cancer patients and was significantly increased in TamR
cells. Moreover, IL-8 expression was upregulated through the
MEK/ERK-dependent pathway in TamR cells. Finally, we observed that
anchorage-independent growth of TamR cells was completely
suppressed by the specific CXCR1/2 inhibitor SB225002. Therefore,
we suggest that IL-8 and its receptors may be a promising
therapeutic target for overcoming tamoxifen resistance.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was
purchased from Thermo Scientific (hemel hempstead, UK). Fetal
bovine serum (FBS) was purchased from HyClone Laboratories (Logan,
UT, USA). Phenol red-free DMEM and penicillin (100 U/ml) and 100
mg/ml streptomycin were purchased from Life Technologies
(Rockville, MD, USA). UO126 was purchased from Selleck Chemicals
(Houston, TX, USA). SB253580 and LY294002 were purchased from
Tocris Bioscience (Ellisville, MO, USA). 4-Hydroxytamoxifen (4-OHT)
was purchased from Sigma (St. Louis, MO, USA). The secondary
horseradish peroxidase (HRP)-conjugated and mouse monoclonal
anti-β-actin antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit polyclonal
anti-MEK and anti-Akt antibodies (total and phospho-form) were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Recombinant human IL-8 was purchased from R&D Systems
(Minneapolis, MN, USA). The ECL prime reagents were purchased from
Amersham (Buckinghamshire, UK).
Microarray data analysis
We downloaded expression data from a public database
[Kaplan-Meier plotter database (http://kmplot.com/breast)] and analyzed the clinical
value of IL-8 expression in luminal A and B type breast cancer
patients.
Establishment of tamoxifen-resistant
MCF-7 breast cancer cells
TamR was established using a previously reported
methodology (6,18). Briefly, to establish TamR, MCF-7
cells were washed with PBS, and the culture medium was changed to
phenol red-free DMEM containing 10% charcoal-stripped
steroid-depleted FBS and 0.1 mM 4-hydroxytamoxifen (4-OHT). The
cells were continuously exposed to this treatment regimen for two
weeks, and the 4-OHT concentration was increased gradually to up to
3 mM over a 9-month period. Initially, cell growth rates were
depressed. However, after exposure to the medium for 9 months, the
rate of cell growth increased gradually, indicating the
establishment of tamoxifen-resistant cells.
Soft agar colony formation assay
TamS and TamR cells were seeded at a density of
5×104 cells/well in 6-well plates in growth medium
containing 0.7% agar (1.5 ml/well) on top of a layer of growth
medium containing 1.4% agar (2 ml/well). Growth medium (500
µl) with 10% FBS was added on top of the agar. The cell
suspension was plated and cultured in a 37°C incubator for two
weeks. After two weeks, viable colonies were stained with 0.01%
crystal violet and were then observed using a CK40 inverted
microscope (Olympus, Tokyo, Japan) (6).
Flow cytometric analysis (FACS)
Apoptosis assays were performed with the Annexin
V-fluorescein isothiocyanate (FITC) apoptosis kit-I (BD
Biosciences, San Diego, CA, USA), according to the manufacturer's
protocol. Briefly, cells (1×106 cells/ml) were collected
and washed twice with PBS and then resuspended in 500 µl of
staining solution containing 5 µl FITC-conjugated Annexin V
and 5 µl propidium iodide (PI). After incubation for 15 min
at room temperature (RT) in the dark, cells were immediately
analyzed on a flow cytometer. Apoptotic cells were double stained
with Annexin V and PI and were then analyzed using the FACS Vantage
system (Becton-Dickinson, San Diego, CA, USA). The percentage of
cells undergoing apoptosis was determined (6).
Real-time PCR
The total RNA was extracted from the cells using the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer's instructions. Isolated RNA samples were then used
for RT-PCR. Samples (1 µg of total RNA) were
reverse-transcribed into cDNA in 20 µl reaction volumes
using a first-strand cDNA synthesis kit for RT-PCR, according to
the manufacturer's instructions (MBI Fermentas, Hanover, MD,
USA).
The gene expression was quantified by real-time PCR
using a SensiMix SYBR kit (Bioline Reagents Ltd., London, UK) and
100 ng of cDNA per reaction. The sequences of the primer sets used
for this analysis were: human IL-8 (forward,
5′-AGGGTTGCCAGATGCAATAC-3′; reverse, 5′-AAACCAAGGCACAGTGGAAC-3′)
and GAPDH as an internal control (forward,
5′-ATTGTTGCCATCAATGACCC-3′ and reverse,
5′-AGTAGAGGCAGGGATGATGT-3′). An annealing temperature of 60°C was
used for all of the primers. The PCR was performed in a standard
384-well plate format with an ABI 7900HT real-time PCR detection
system. For data analysis, the raw threshold cycle
(CT) value was first normalized to the
housekeeping gene for each sample to get the ΔCT.
The normalized ΔCT was then calibrated to the
control cell samples to get the ΔΔCT.
IL-8 ELISA
Protein levels of IL-8 were measured using an ELISA
kit for human IL-8 (KomaBiotech, Seoul, Korea), according to the
manufacturer's instructions, and then a microtiter plate reader was
used to read the plate at a 450 nm wavelength.
Western blotting
The cell culture media (supernatants) and cell
lysates were used in the immunoblot analysis for MEK and β-actin.
The proteins were boiled for 5 min in Laemmli sample buffer and
then electrophoresed in 8 or 10% SDS-PAGE gels, respectively. The
separated proteins were transferred to PVDF membranes and the
membranes were then blocked with 10% skim milk in TBS with 0.01%
Tween-20 for 15 min. The blots were incubated with anti-MEK,
anti-Akt (total and phospho-form), anti-p38 (phospho-form), and
β-actin antibodies (1/1,000 dilution) in 1% TBS/T buffer (0.01%
Tween-20 in TBS) at 4°C overnight. The blots were washed three
times in TBS with 0.01% Tween-20 and they were subsequently
incubated with anti-rabbit peroxidase-conjugated antibody (1/2,000
dilution) in TBS/T buffer. After 1 h of incubation at room
temperature (RT), the blots were washed three times and ECL prime
reagents were used for development.
Adenovirus induction
The empty (Lac Z) and adenoviral human CA-MEK cDNA
were gifts from Dr Hyunil Ha (Korea Institute of Oriental Medicine,
Daejeon, Korea). Recombinant adenovirus-expressing human CA-MEK was
reproduced into 293A cells and the level of MEK phosphorylation was
confirmed by western blotting. Adeniviral vectors were transfected
into BT474 cells for 24 h and then further incubated with fresh
serum-free media for 48 h to detect the level of IL-8 and
phospho-MEK and ERK expression.
Statistical analysis
Statistical significance was determined using the
Student's t-test. Data are presented as the mean ± SEM. All quoted
P-values are two-tailed and differences were considered significant
for P-values <0.05. Microsoft Excel was used for the statistical
analyses.
Results
Elevated IL-8 expression results in poor
prognosis in luminal A type breast cancer patients
Clinically, we analyzed whether elevated IL-8 levels
confer a poor prognosis for human breast cancer patients using a
Kaplan-Meier plotter database (http://kmplot.com/breast). In luminal A type breast
cancer patients, patients with high expression of IL-8 showed
poorer relapse-free survival in comparison to patients with low
expression of IL-8 (P=0.0325, Fig.
1A). However, relapse-free survival by IL-8 expression was not
significantly different between luminal B type breast cancer
patients (P=0.65, Fig. 1B). Based
on these results, we demonstrated that IL-8 expression may be
associated with tamoxifen resistance.
In the present study, we investigated the functional
role and regulatory mechanism of IL-8 expression in
tamoxifen-resistant breast cancer. We established a
tamoxifen-resistant breast cancer in vitro model and
analyzed differential characteristics of tamoxifen-sensitive (TamS)
and -resistant (TamR) breast cancer cell lines. In a recent study,
we reported the morphological findings that TamS cells stacked up
to form colonies and TamR cells were scattered, loosely packed
colonies, with many branches (6).
The tumorigenicity of TamS and TamR cells by tamoxifen treatment
was analyzed using soft agar colony formation assays. As shown in
Fig. 2B, the anchorage-independent
growth of TamS cells was completely inhibited by 3 µM
tamoxifen treatment while growth of TamR cells was maintained. In
addition, we also analyzed the apoptotic cell death of TamS and
TamR cells after tamoxifen treatment. TamS and TamR cells were
treated with or without 3 µM tamoxifen for 24 h. The
apoptotic cell population of TamS cells after tamoxifen treatment
significantly increased by 47.23% over the control level. However,
the apoptotic cell population of TamR cells was not significantly
different from that of TamS cells (Fig.
2C).
The level of IL-8 expression is
significantly increased in TamR cells
In a previous study, Shi et al (19) reported that elevated IL-6 and IL-8
expression contributes to multidrug resistance in human breast
cancer cells. Therefore, we also compared the level of IL-8
expression between TamS and TamR cells. Our results showed that the
levels of IL-8 mRNA and protein expression were significantly
increased in TamR cells when compared with TamS cells (Fig. 3). The level of IL-8 mRNA expression
in TamR cells was 62.8±5.4-fold higher than that of TamS cells
(Fig. 3A). In addition, IL-8
protein expression was also increased by 42.3±7.3 ng/ml of the
control level (14.0±2.5 ng/ml) in TamR cells (Fig. 3B). Therefore, we demonstrated that
induction of IL-8 may be associated with tamoxifen resistance in
luminal type breast cancer.
IL-8 expression is upregulated by a
MEK-dependent pathway in TamR cells
To verify the regulatory mechanism of IL-8
expression, we analyzed the phosphorylation degree in a variety of
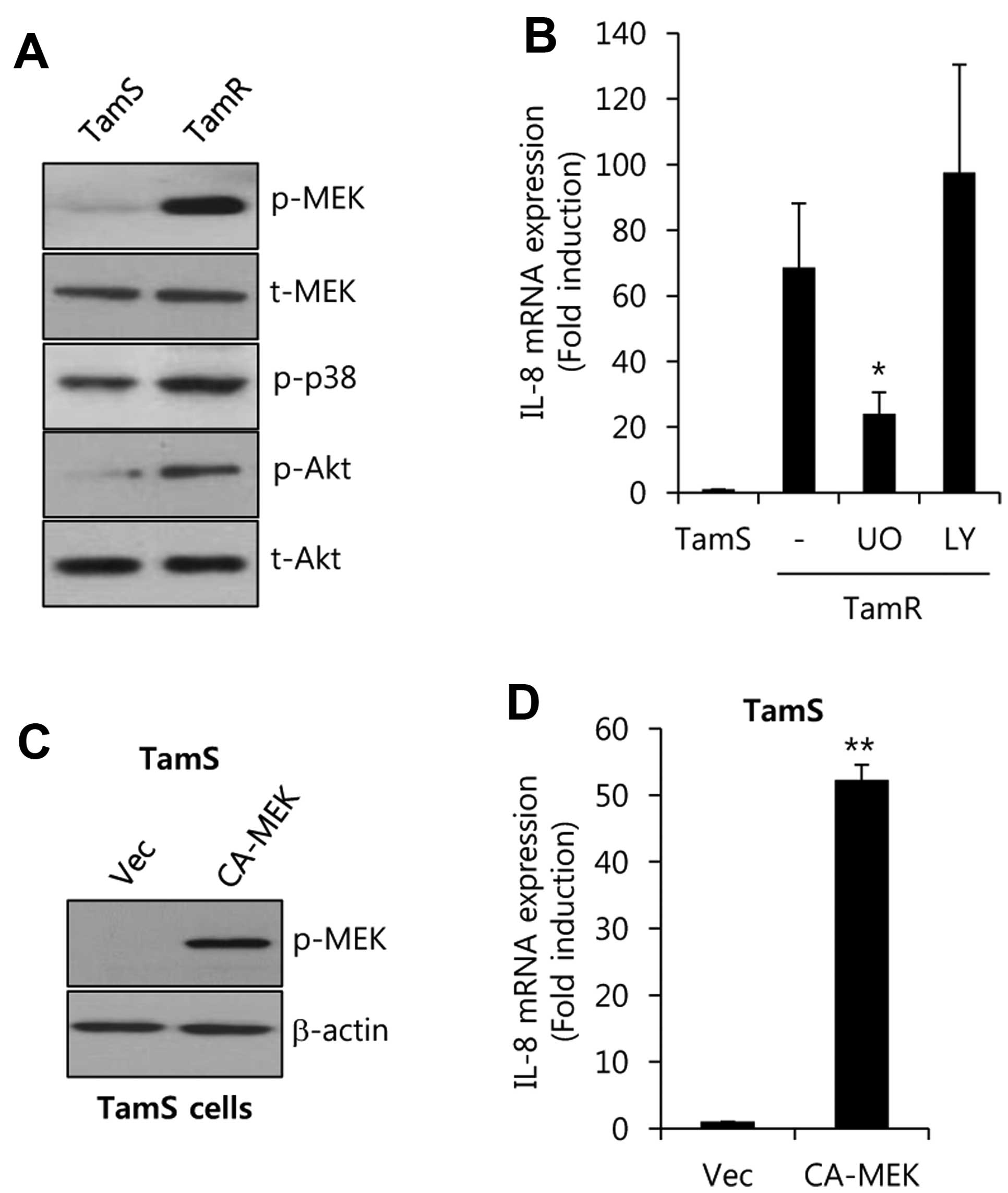
signaling molecules in TamS and TamR cells. As shown in Fig. 4A, the activities of MEK and Akt were
significantly increased in TamR cells. However, the activity of p38
was not critically different between TamR and TamS cells.
Furthermore, cells were treated using specific inhibitors such as
the MEK 1 inhibitor UO126, and the PI3-K inhibitor LY294002, for 48
h. After 48 h, the cell lysates were harvested to detect the levels
of IL-8 mRNA expression. The levels of IL-8 mRNA expression were
significantly increased by 68.6±19.7-fold over that of TamS cells
(Fig. 4B). On the contrary,
induction of IL-8 was decreased by UO (UO126, a specific MEK
inhibitor), but not by LY294002, in TamR cells (Fig. 4B).
Next, we examined whether MEK directly regulates
IL-8 expression in TamS cells. We overexpressed
adenovirus-delivered constitutively active-MEK (CA-MEK) into TamS
cells. As shown in Fig. 4C, we
observed that the phosphorylation of MEK was enhanced in
CA-MEK-overexpressing cells. Under the same condition, the level of
IL-8 mRNA expression was significantly increased by 52.3±2.3-fold
over the vector-alone levels with CA-MEK overexpression. Therefore,
we demonstrated that the level of IL-8 expression is upregulated
through a MEK/ERK-dependent pathway in tamoxifen-resistant breast
cancer cells.
Inhibition of CXCR1/2 completely
abolishes anchorage-independent growth of TamR cells
To inhibit IL-8-induced tumor cell growth, we
treated TamR cells with the specific MEK1/2 inhibitor Uo126 and the
specific CXCR1/2 inhibitor SB225002. As shown in Fig. 5A, anchorage-independent growth of
TamR cells was significantly decreased by SB225002 treatment, but
not by UO126 treatment. In this study, we suggest that UO126 plays
an important role in regulating IL-8 transcriptional activity,
while it did not heavily affect anchorage-independent growth of
TamR cells.
Next, we investigated the co-relation between
SB225002 and apoptotic cell death. We treated TamR cells with
SB225002 at the indicated concentration for 24 h and then harvested
whole cell lysates to detect the levels of PARP-1 expression. As
expected, the level of full-length PARP-1 protein expression was
dose-dependently decreased by SB225002 (Fig. 5B). Furthermore, there were serious
changes in cell morphology and the number of apoptotic cells was
significantly increased by SB225002 (Fig. 5C). The number of apoptotic cells was
669.5% greater than that of the control levels after the SB225002
treatment (Fig. 5C). Therefore, we
have demonstrated that the complex of IL-8 and its receptors play a
pivotal role in the survival of TamR cells.
Discussion
Although tamoxifen is a powerful drug for the
treatment of premenopausal breast cancer, a substantial proportion
of ER-positive breast cancer patients are resistant to tamoxifen
therapy (4). Until now, many
researchers have sought to understand the mechanism underlying
anti-estrogen resistance. Recently, we reported that the level of
EGFR expression is significantly increased in tamoxifen-resistant
MCF-7 cells and the pan-EGFR inhibitor, neratinib, suppresses
growth of tamoxifen-resistant MCF-7 cells (6). Furthermore, IL-6 and IL-8 can activate
the ER pathway through an estrogen-independent mechanism in ovarian
cancer cells (20). In the present
study, we found that IL-8 expression is increased in TamR cells
when compared with TamS cells. Therefore, we suggest that elevated
IL-8 levels may be associated with tamoxifen resistance.
Inflammatory cytokines, including IL-8 and IL-6,
regulate breast cancer stem cell self-renewal (21,22).
Induction of IL-8 expression by chemotherapy contributes to the
enhancement of cancer stem-cell populations and survival, while
these phenomena are suppressed by IL-8 directed drugs (22). Moreover, TNBC patients with high B
cell and low IL-8 activity have ~84% recurrence-free survival at
five-years (23). Serum IL-8 levels
are increased in 67% of patients with early and metastatic breast
cancer (24). IL-8-overexpressing
breast cancer cells are favored at bone metastasis sites (25). Consistent with these reports, we
also found that high expression of IL-8 is directly associated with
poor prognosis in luminal A type breast cancer patients, but not in
luminal B type breast cancer patients. In addition, the levels of
IL-8 mRNA and protein expression are significantly increased in an
in vitro model for tamoxifen resistance. Therefore, we
demonstrated that IL-8 expression or the IL-8 signaling pathway
would be a cause of tamoxifen resistance. IL-8 and its receptors
may be therapeutic targets to overcome tamoxifen resistance.
Constitutive IL-8 expression has been found in many
human cancers, including breast and colon cancer, and it is also
regulated by a variety of stimuli such as lipopolysaccharide (LPS),
phorbol-12-myristate-13-acetate, IL-1, TNF, hypoxia and nitric
oxide (26–28). The core IL-8 promoter region
contains the binding sites for AP-1, NF-κB, CAAT/enhancer-binding
protein (C/EBP) and NF-IL-6-like factors (29). Scherle et al (30) reported that LPS-induced IL-8
expression is blocked by UO126 treatment in monocytes. TNF-α and
IL-1 upregulate IL-8 expression through the activation of NF-κB in
fibroblast-like synoviocytes (31).
In this study, we observed that the activity of MEK and IL-8
expression is significantly increased in TamR cells. On the
contrary, increased IL-8 levels are completely suppressed by the
MEK1/2 inhibitor, UO126, in TamR cells. In contrast, CA-MEK
overexpression triggers IL-8 mRNA expression in TamS cells.
Therefore, we demonstrate that MEK activity plays an important role
in regulating IL-8 expression in TamR cells.
Recently, CXC chemokine receptor 2 (CXCR2), one of
the IL-8 receptors, was found to be associated with poor prognosis
in intrahepatic cholangiocellular carcinoma (ICC) (32). Tumorigenesis and metastasis are
significantly suppressed by both CXCR2 siRNA and the specific CXCR2
antagonist, SB225002 (32).
Furthermore, CXCR1-specific blocking antibody or the CXCR1/2
inhibitor, repertaxin, are able to suppress the cancer stem cell
population in human breast cancer xenografts, retarding tumor
growth and reducing metastasis (33). In accordance with these reports, our
results show that anchorage-independent growth of
tamoxifen-resistant cells is completely suppressed by SB225002
treatment. The number of apoptotic cells is also increased in
response to SB225002. Therefore, we suggest that CXCR1 and/or CXCR2
inhibitors such as repertaxin and SB225002 may be promising drugs
for treating tamoxifen-resistant breast cancer patients.
In conclusion, we investigated the regulation of
IL-8 expression and the effect inhibiting the IL-8 receptor in TamR
cells. Clinically, elevated IL-8 expression is associated with poor
prognosis in luminal A type breast cancer patients, but not in
luminal B type breast cancer patients. In addition, the level of
IL-8 expression and MEK activity is significantly increased in TamR
cells. Elevated IL-8 expression is completely suppressed by the
specific MEK inhibitor Uo126, whereas, the basal level of IL-8
expression is increased by CA-MEK overexpression. Therefore, we
demonstrate that a MEK-dependent pathway plays an important role in
regulating IL-8 expression in TamR cells. Finally, we found that
the specific CXCR1/2 inhibitor SB225002 completely suppresses
anchorage-independent growth and triggers apoptotic cell death in
TamR cells. Finally, we suggest that a variety of specific CXCR1/2
inhibitors like SB225002 may be promising drug candidates for
overcoming or bypassing tamoxifen resistance.
Acknowledgments
The present study was supported by a grant from the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute (KHIDI), funded by the Ministry of
Health & Welfare, Republic of Korea (HI14C3418) and by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education
(2015R1D1A1A01057585).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan VC and O'Malley BW: Selective
estrogen-receptor modulators and antihormonal resistance in breast
cancer. J Clin Oncol. 25:5815–5824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jordan VC and Murphy CS: Endocrine
pharmacology of antiestrogens as antitumor agents. Endocr Rev.
11:578–610. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ring A and Dowsett M: Mechanisms of
tamoxifen resistance. Endocr Relat Cancer. 11:643–658. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davies C, Godwin J, Gray R, Clarke M,
Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, et al Early
Breast Cancer Trialists' Collaborative Group (EBCTCG): Relevance of
breast cancer hormone receptors and other factors to the efficacy
of adjuvant tamoxifen: Patient-level meta-analysis of randomised
trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim S, Lee J, Oh SJ, Nam SJ and Lee JE:
Differential effect of EGFR inhibitors on tamoxifen-resistant
breast cancer cells. Oncol Rep. 34:1613–1619. 2015.PubMed/NCBI
|
|
7
|
Maxwell PJ, Gallagher R, Seaton A, Wilson
C, Scullin P, Pettigrew J, Stratford IJ, Williams KJ, Johnston PG
and Waugh DJ: HIF-1 and NF-kappaB-mediated upregulation of CXCR1
and CXCR2 expression promotes cell survival in hypoxic prostate
cancer cells. Oncogene. 26:7333–7345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collins TS, Lee LF and Ting JP: Paclitaxel
up-regulates interleukin-8 synthesis in human lung carcinoma
through an NF-kappaB- and AP-1-dependent mechanism. Cancer Immunol
Immunother. 49:78–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kassim SK, El-Salahy EM, Fayed ST, Helal
SA, Helal T, Azzam ED and Khalifa A: Vascular endothelial growth
factor and interleukin-8 are associated with poor prognosis in
epithelial ovarian cancer patients. Clin Biochem. 37:363–369. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayerhofer K, Bodner K, Bodner-Adler B,
Schindl M, Kaider A, Hefler L, Zeillinger R, Leodolter S, Joura EA
and Kainz C: Interleukin-8 serum level shift in patients with
ovarian carcinoma undergoing paclitaxel-containing chemotherapy.
Cancer. 91:388–393. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller LJ, Kurtzman SH, Wang Y, Anderson
KH, Lindquist RR and Kreutzer DL: Expression of interleukin-8
receptors on tumor cells and vascular endothelial cells in human
breast cancer tissue. Anticancer Res. 18(1A): 77–81.
1998.PubMed/NCBI
|
|
13
|
Chavey C, Bibeau F, Gourgou-Bourgade S,
Burlinchon S, Boissière F, Laune D, Roques S and Lazennec G:
Oestrogen receptor negative breast cancers exhibit high cytokine
content. Breast Cancer Res. 9:R152007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freund A, Chauveau C, Brouillet JP, Lucas
A, Lacroix M, Licznar A, Vignon F and Lazennec G: IL-8 expression
and its possible relationship with estrogen-receptor-negative
status of breast cancer cells. Oncogene. 22:256–265. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freund A, Jolivel V, Durand S, Kersual N,
Chalbos D, Chavey C, Vignon F and Lazennec G: Mechanisms underlying
differential expression of interleukin-8 in breast cancer cells.
Oncogene. 23:6105–6114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin D, Galisteo R and Gutkind JS:
CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF)
expression and the autocrine activation of VEGFR2 in endothelial
cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1)
complex. J Biol Chem. 284:6038–6042. 2009. View Article : Google Scholar :
|
|
17
|
Li A, Varney ML, Valasek J, Godfrey M,
Dave BJ and Singh RK: Autocrine role of interleukin-8 in induction
of endothelial cell proliferation, survival, migration and MMP-2
production and angiogenesis. Angiogenesis. 8:63–71. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Knowlden JM, Hutcheson IR, Jones HE,
Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE and Nicholson
RI: Elevated levels of epidermal growth factor receptor/c-erbB2
heterodimers mediate an autocrine growth regulatory pathway in
tamoxifen-resistant MCF-7 cells. Endocrinology. 144:1032–1044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q,
Zhu J, Chen JJ, Huang RC, Chen ZS and Huang RP: Enhanced
chemosensitization in multidrug-resistant human breast cancer cells
by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat.
135:737–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang J, Wang Y, Gao Y, Shao J, Zhang XJ
and Yao Z: Reciprocal regulation of 17beta-estradiol, interleukin-6
and interleukin-8 during growth and progression of epithelial
ovarian cancer. Cytokine. 46:382–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sansone P, Storci G, Giovannini C,
Pandolfi S, Pianetti S, Taffurelli M, Santini D, Ceccarelli C,
Chieco P and Bonafé M: p66Shc/Notch-3 interplay controls
self-renewal and hypoxia survival in human stem/progenitor cells of
the mammary gland expanded in vitro as mammospheres. Stem Cells.
25:807–815. 2007. View Article : Google Scholar
|
|
22
|
Korkaya H, Paulson A, Charafe-Jauffret E,
Ginestier C, Brown M, Dutcher J, Clouthier SG and Wicha MS:
Regulation of mammary stem/progenitor cells by
PTEN/Akt/beta-catenin signaling. PLoS Biol. 7:e10001212009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rody A, Karn T, Liedtke C, Pusztai L,
Ruckhaeberle E, Hanker L, Gaetje R, Solbach C, Ahr A, Metzler D, et
al: A clinically relevant gene signature in triple negative and
basal-like breast cancer. Breast Cancer Res. 13:R972011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benoy IH, Salgado R, Van Dam P, Geboers K,
Van Marck E, Scharpé S, Vermeulen PB and Dirix LY: Increased serum
interleukin-8 in patients with early and metastatic breast cancer
correlates with early dissemination and survival. Clin Cancer Res.
10:7157–7162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh B, Berry JA, Vincent LE and Lucci A:
Involvement of IL-8 in CoX-2-mediated bone metastases from breast
cancer. J Surg Res. 134:44–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh JK, Simões BM, Howell SJ, Farnie G
and Clarke RB: Recent advances reveal IL-8 signaling as a potential
key to targeting breast cancer stem cells. Breast Cancer Res.
15:2102013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan A, Yang PC, Yu CJ, Chen WJ, Lin FY,
Kuo SH and Luh KT: Interleukin-8 messenger ribonucleic acid
expression correlates with tumor progression, tumor angiogenesis,
patient survival, and timing of relapse in non-small-cell lung
cancer. Am J Respir Crit Care Med. 162:1957–1963. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mukaida N, Okamoto S, Ishikawa Y and
Matsushima K: Molecular mechanism of interleukin-8 gene expression.
J Leukoc Biol. 56:554–558. 1994.PubMed/NCBI
|
|
30
|
Scherle PA, Jones EA, Favata MF, Daulerio
AJ, Covington MB, Nurnberg SA, Magolda RL and Trzaskos JM:
Inhibition of MAP kinase kinase prevents cytokine and prostaglandin
E2 production in lipopolysaccharide-stimulated monocytes. J
Immunol. 161:5681–5686. 1998.PubMed/NCBI
|
|
31
|
Aupperle K, Bennett B, Han Z, Boyle D,
Manning A and Firestein G: NF-kappa B regulation by I kappa B
kinase-2 in rheumatoid arthritis synoviocytes. J Immunol.
166:2705–2711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sueoka H, Hirano T, Uda Y, Iimuro Y,
Yamanaka J and Fujimoto J: Blockage of CXCR2 suppresses tumor
growth of intrahepatic cholangiocellular carcinoma. Surgery.
155:640–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ginestier C, Liu S, Diebel ME, Korkaya H,
Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum
D, et al: CXCR1 blockade selectively targets human breast cancer
stem cells in vitro and in xenografts. J Clin Invest. 120:485–497.
2010. View
Article : Google Scholar : PubMed/NCBI
|