Introduction
Triple-negative breast cancer (TNBC) is a subtype of
breast cancer, taking up 15–20% of all breast cancer cases, which
is characterized by more aggressiveness and high rate of
proliferation, metastasis, often leading to a poor prognosis and
much lower overall survival compared with other subtypes of breast
cancer (1–3). Due to its triple-negative expression
of estrogen receptor (ER), progesterone receptor (PR) and human
epithelial growth factor receptor 2 (HER2), which are the currently
available therapeutic targets, TNBC remains an important clinical
challenge (4,5). So far, the common therapeutic method
for TNBC has been limited to cytotoxic chemotherapy. Hence, there
is still a lack of full understanding towards the molecular
mechanism of TNBC development.
Cohesin, a highly evolutionary conserved
multifunctional nuclear protein complex, is worth noting. Cohesin
mainly participates in sister chromatid cohesion, which is also
involved in DNA repair (6).
Moreover, cohesin has also been shown to be involved in regulation
of transcription and cell proliferation, and the maintenance of
pluripotency (7,8). Cohesin is composed of two structural
maintenance of chromosomes proteins, structural maintenance of
chromosomes 1 (SMC1) and structural maintenance of chromosomes 3
(SMC3), and a kleisin protein like RAD21 (6). More specifically, together with SMC3,
SMC1 forms an affinitive heterodimer and associates with SCC1/RAD21
and SCC3/SA to form the cohesin complex (9). Among them, SMC1 is known for its role
in cell division, DNA repair and activation of the cell cycle
checkpoints (10–12). In particular, SMC1 has been shown to
contribute to tumor genomic instability, and ectopic expression of
cohesin subunits, including SMC1, has been found in sarcoma,
melanoma, colon and glioblastoma tumors (13,14).
To date, SMC1 has also been reported to be overexpressed in TNBC
(15), but the role of SMC1 in the
progression of TNBC is not fully understood.
Brachyury is a T-box transcription factor, which
plays an important role in the development of vertebrates,
including the formation of cervical vertebra, differentiation of
the posterior mesoderm and axial development (16). In humans, T-box transcription
factors mainly participate in regulating the progenitors and their
differentiated descendants (17).
Brachyury was also found to be overexpressed in various human
malignant neoplasms (18,19), which often contribute to the
metastasis of tumor cells (20). It
is also worth noting that Brachyury could induce
epithelial-mesenchymal transition (EMT) in human epithelial cells
through the induction of transcription factors, including Snail and
Slug (21). However, the role of
Brachyury in TNBC and its interactions with EMT remain poorly
elucidated.
EMT refers to a series of events that converts
epithelial cells into individual migratory cells, during which
cells lose epithelial characteristics, such as cell-to-cell
adhesion and cell-layer organization, along with acquiring
mesenchymal properties including metastasis and invasiveness
(22,23). Also, during the EMT process, cells
lose epithelial markers, such as E-cadherin, and gain expression of
mesenchymal markers, including N-catenin and vimentin (24,25).
Hence, EMT has been demonstrated to be the central mechanism
responsible for the metastasis and invasiveness of various cancers
(26,27). Moreover, EMT is also involved in
early embryo development, wound healing, and tissue regeneration
(28). However, few studies have
focused on its roles in TNBC progression, and no studies have
reported its association with SMC1 and Brachyury until now.
In the present study, we compared the expression of
SMC1 in TNBC and normal tissues and cells. Subsequently, SMC1 was
artificially overexpressed and silenced in TNBC cells. We further
analyzed the relationship between SMC1 and EMT, SMC1 and Brachyury,
respectively, in TNBC. The upregulation of Brachyury was also
investigated to evaluate its role in EMT.
Materials and methods
Sample collection
In total 40 TNBC and 38 adjacent non-tumor tissue
samples were acquired from the Department of Oncology, Xijing
Hospital (Xi'an, China). The tissue samples were derived from
patients who had not been subjected to preoperative chemotherapy or
radiotherapy. The samples were immediately frozen in liquid
nitrogen after dissection in preparation for use. All patients with
TNBC gave written informed consent for the use of clinical
specimens for medical research, and experiments were approved by
the Committees for Ethical Review of Research involving human
subjects of the Fourth Military Medical University (Xi'an,
China).
Cell culture
Two TNBC cell lines (hs578T and HCC1937), a human
non-tumorigenic mammary epithelial cell line (MCF10a) and an
ER+/hormone responsive breast cancer cell line (MCF7)
were acquired from the American Type Culture Collection (ATCC;
Manassas, VA, USA). All cells were incubated at 37°C in a
humidified atmosphere of 5% CO2 in the appropriate
medium supplemented with 10% fetal bovine serum (FBS); Dulbecco's
modified Eagle's medium (DMEM) for hs578T and HCC1937; DMEM/F12 for
MCF10a; and RPMI-1640 (all from Invitrogen, Carlsbad, CA, USA) for
MCF7.
Plasmid construction and transfection for
SMC1 and Brachyury overexpression
Human Brachyury and SMC1 were amplified by PCR using
cDNA from MCF7. Subsequently, cDNA was sub-cloned into eukaryotic
expression vector pcDNA3.1 (Invitrogen). Next, hs578T and HCC1937
cells were transiently transfected with the eukaryotic expression
vector (pcDNA3.1) alone or with pcDNA3.1/SMCl and
pcDNA3.1/Brachyury, respectively, using Lipofectamine 2000
(Invitrogen) according to the manufacturer's instructions. The
stable expression of SMC1 and Brachyury was assessed using
real-time quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Small interfering RNA (siRNA)
transfection for SMC1 silence
The siRNA for SMC1, in addition to non-targeting
siRNA, was purchased from GenePharma Co., Ltd. (Shanghai, China). A
scrambled siRNA also purchased from GenePharma Co., Ltd., was used
as a negative control. Subsequently, siRNA (100 nM) was transfected
into hs578T and HCC1937 cells using Lipofectamine 2000 (Invitrogen)
for an incubation of 48 h in antibiotic-free medium. The absent
expression of SMC1 was confirmed by RT-qPCR and western blot
analysis.
Evaluation of the migratory ability
Cell migration was investigated using Transwell
chambers. Briefly, medium supplemented with 10% FBS was added to
the lower chambers, and 1×104 cells in serum-free medium
were added to the upper chambers. Subsequently, chambers were
incubated for 24 h at 37°C. The non-migrating cells from the
interior of the inserts were gently removed using a cotton-tipped
swab. Next, cells on the bottom side of the filters were fixed with
methanol and stained with hematoxylin. The migratory ability was
determined by counting migratory cells in 5 randomly selected
fields under a microscope (Olympus, Tokyo, Japan). Experiments were
performed in triplicate.
Evaluation of the proliferative
ability
Cell proliferation was evaluated using the BrdU
assay. Briefly, prepared BrdU was added into medium, and the
mixtures were incubated in 5% CO2 at 37°C for 1 h.
Subsequently, cells were fixed with 70% ethanol and incubated with
primary anti-BrdU antibody. Next, cells were counterstained with
hematoxylin and counted in randomly selected fields using
fluorescence microscopy (Olympus). The labeling index was
calculated as BrdU-positive cells vs. total cells.
RT-qPCR
Total RNA from tissue samples and cells was
extracted using TRIzol reagent (Invitrogen), which was subsequently
quantified according to absorbance at 260 nm using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
cDNA was synthesized using HiScript® First Strand cDNA
Synthesis kit (Vazyme Biotech Co., Ltd., Nanjing, China) and
RT-qPCR was performed using HiScript® II Q RT SuperMix
for qPCR (Vazyme Biotech Co., Ltd) according to the manufacturer's
instructions. The cycle threshold (Ct) was recorded and the fold
induction was calculated using the 2−ΔΔCt method. Each
gene was detected in triplicate. GAPDH served as a control. The
primer sequences of this experiment are shown in Table I.
 | Table IThe primer sequences used in this
study. |
Table I
The primer sequences used in this
study.
| Gene | Sense | Antisense |
|---|
| SMC1 |
5′-GGCGGATCCATGGTTCCTGAATGAT-3′ |
5′-CCGCTCGAGCTACTGCTCATTGGGGTT-3′ |
| Brachyury |
5′-ACTGAGAATCAGCCGGACTT-3′ |
5′-CTGCACTGCAAAGAACCACT-3′ |
| E-cadherin |
5′-TTAAACTCCTGGCCTCAAGCAATC-3′ |
5′-TCCTATCTTGGGCAAAGCAACTG-3′ |
| N-cadherin |
5′-CTCCTATGAGTGGAACAGGAACG-3′ |
5′-TTGGATCAGTCATAATCAAGTGCTGTA-3′ |
| Vimentin |
5′-ATGTGGATGTTTCCAAGCCTGAC-3′ |
5′-GAGTGGGTATCAACCAGAGGGAG-3′ |
| Snail |
5′-TTCTTCGCTACTGCTGCG-3′ |
5′-GGGCAGGTATGGAGAGGAAGA-3′ |
| Slug |
5′-ATCTGACCCGTCGTGACG-3′ |
5′-CGTCACGACGGGTCAGAT-3′ |
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
5′-GGGGTCGTTGATGGCAACA-3′ |
Western blotting
Total proteins from tissue samples and cells were
extracted in RIPA Lysis and Extraction Buffer (Thermo Fisher
Scientific) and quantified using the Bradford method. A total of 50
µg of protein was isolated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by
electro-blotting onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). Subsequently, the PVDF membranes
were incubated overnight at 4°C with primary antibodies (as shown
in Table II). After incubation
with horseradish peroxidase (HRP)-conjugated secondary antibodies
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C for 2 h,
bound proteins were visualized by 4-chloro-1-naphthol (4-CN) and
detected by chemiluminescence (ECL) detection system (Amersham,
Little Chalfont, UK).
 | Table IIThe primary antibodies used in this
study. |
Table II
The primary antibodies used in this
study.
| Protein | Host | Cat no. | Commercial
source | Dilutions |
|---|
| SMC1 | Goat | sc-21078 | Santa Cruz
Biotechnology | 1:1,000 |
| Brachyury | Goat | sc-17743 | Santa Cruz
Biotechnology | 1:1,000 |
| E-cadherin | Rabbit | sc-7870 | Santa Cruz
Biotechnology | 1:1,000 |
| N-cadherin | Rabbit | sc-7939 | Santa Cruz
Biotechnology | 1:1,000 |
| Vimentin | Goat | sc-7557 | Santa Cruz
Biotechnology | 1:1,500 |
| Snail | Goat | sc-10433 | Santa Cruz
Biotechnology | 1:500 |
| Slug | Goat | sc-10436 | Santa Cruz
Biotechnology | 1:500 |
| GAPDH | Goat | sc-20357 | Santa Cruz
Biotechnology | 1:3,000 |
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(SPSS Inc., Chicago, IL, USA). Data were presented as mean ±
standard deviation (SD). The statistical differences between two
groups were determined by Student's t-test. A p-value <0.05 was
considered statistically significant.
Results
SMC1 is highly expressed in TNBC tissues
and cells
Firstly, we compared SMC1 expression in TNBC with
its expression in non-TNBC conditions. RT-qPCR was performed. As
shown in Fig. 1A, compared with
adjacent non-tumor tissues, SMC1 expression in TNBC tissues was
significantly higher. Also, the expression of SMC1 in two TNBC cell
lines, hs578T and HCC1937, was found to exceed its expression in
normal mammary epithelial cells (MCF10a) and non-TNBC breast cancer
cells (MCF7) (Fig. 1B). These
results suggested that SMC1 was abnormally elevated in TNBC
nidus.
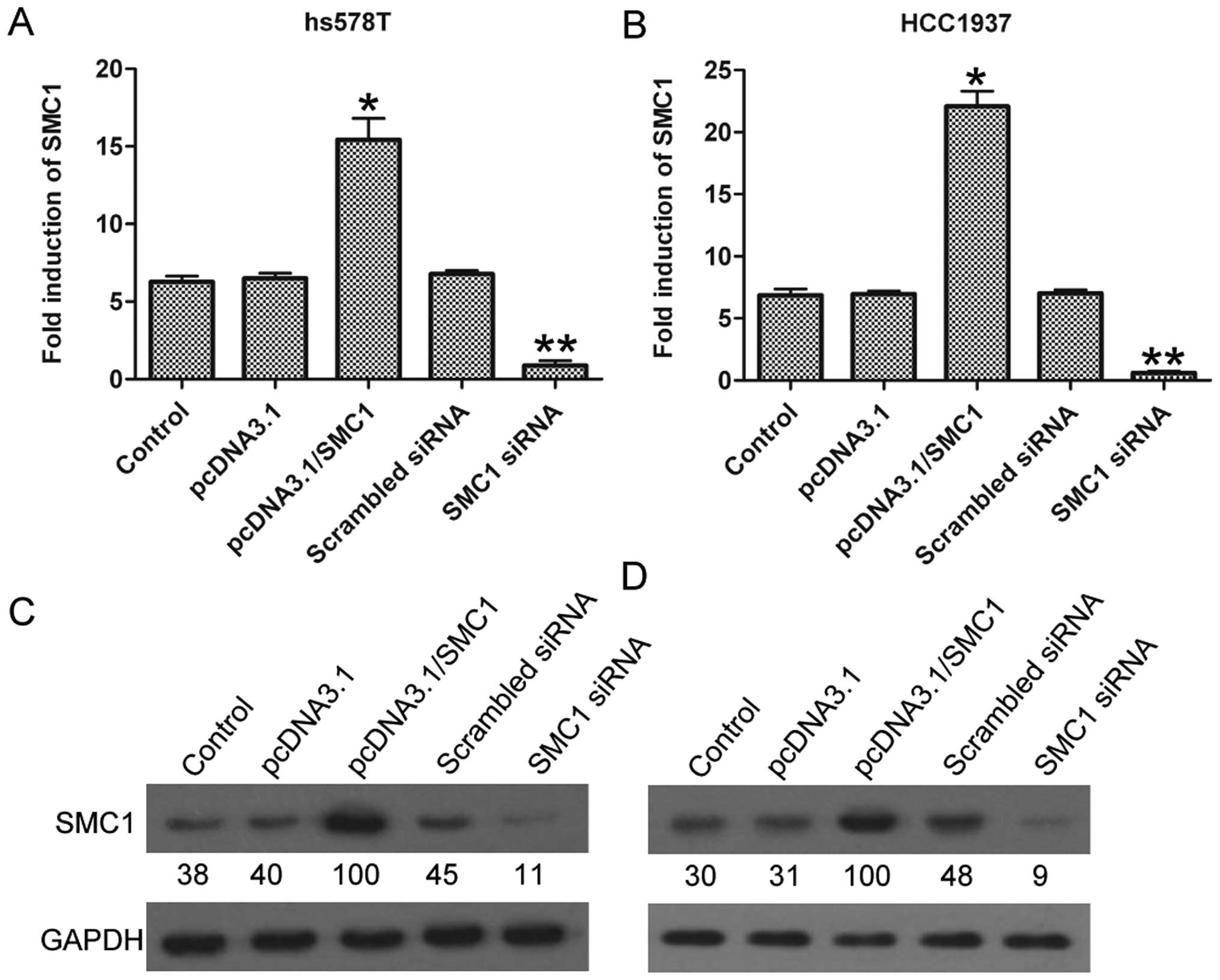
SMC1 is overexpressed after plasmid
transfection, but silenced by siRNA transfection
Next, to further investigate the effects of SMC1 on
TNBC progression, SMC1 was artificially overexpressed and silenced
through plasmid and siRNA transfection, respectively, in hs578T and
HCC1937 cells. As shown in Fig. 2A,
SMC1 showed remarkably upregulated expression of 15.4±1.38-fold
after pcDNA3.1/SMCl transfection, and markedly downregulated
expression of 0.88±0.3-fold after SMC1 siRNA transfection in hs578T
cells, compared with the control group; similarly, in HCC1937
cells, pcDNA3.1/SMCl group had a significantly improved expression
of SMC1 of 22.1±1.23-fold, and SMC1 siRNA group with an inhibited
expression of 0.6±0.15-fold (Fig.
2B). The above results were also confirmed by western blotting
at the protein level (Fig. 2C and
D). These results demonstrated the successful regulation of
SMC1 expression via exogenous transfection.
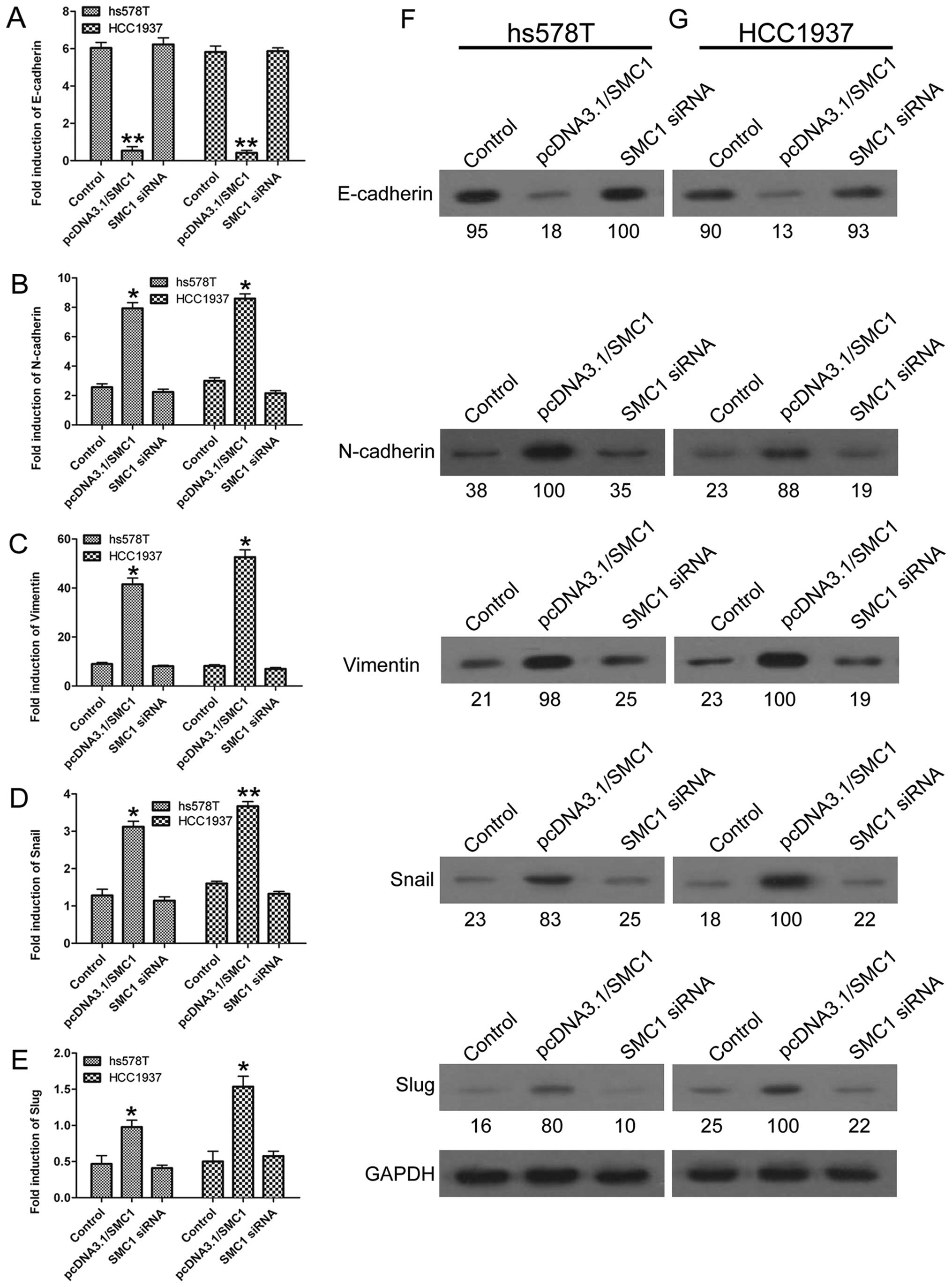
SMC1 overexpression promotes EMT
process
As EMT has been widely accepted as a major process
participating in cancer development, we next explored the role of
SMC1 in regulating EMT in TNBC cells. Upregulation of SMC1 in both
hs578T and HCC1937 cells led to decreased expression of the
epithelial marker E-cadherin (Fig.
3A) and increased expression of mesenchymal markers, such as
N-cadherin, vimentin, Snail and Slug (Fig. 3B–E). Western blotting results also
revealed that the protein levels of N-cadherin, vimentin, Snail and
Slug were promoted, whereas E-cadherin was inhibited in
SMC1-overexpressing cells compared with the control group (Fig. 3F and G). On the contrary,
downregulation of SMC1 resulted in relatively stable expression of
E-cadherin, N-cadherin, vimentin, Snail and Slug, without
statistical significance compared with the control group (Fig. 3A–E). These results indicated that
high expression of SMC1 promoted the EMT process.
SMC1 overexpression promotes metastasis
and proliferation of TNBC cells
As EMT has also been reported to be closely
correlated with the survival of tumor cells (20,29),
we next aimed to evaluate the effects of SMC1 overexpression on the
metastasis and proliferation of TNBC cells. As a result, after SMC1
overexpression, the average number of migratory cells was 73±4.8 in
hs578T and 84±4.2 in HCC1937, which was significantly higher than
that of the control group (25±2.5 and 31±2.0, respectively;
Fig. 4A). In contrast, the results
also showed that after SMC1 knockdown, the average number of
migratory cells (10±3.6 in hs578T and 15±2.5 in HCC1937) was
significantly reduced compared with the control group (Fig. 4A). The proliferative ability was
also enhanced by SMC1 overexpression, but inhibited in SMC1
silencing cells (Fig. 4B). These
data further supported the involvement of SMC1 in EMT as observed
in TNBC cells.
Brachyury expression is also elevated in
TNBC tissues and cells
This study aimed to introduce Brachyury and reveal
its relationship with TNBC. Fig. 5A
shows that Brachyury was strongly expressed in TNBC tissues. There
was also a higher expression of Brachyury in TNBC cells than in
MCF10a and MCF7 cells (Fig. 5B),
implying that TNBC was accompanied by the high expression of
Brachyury.
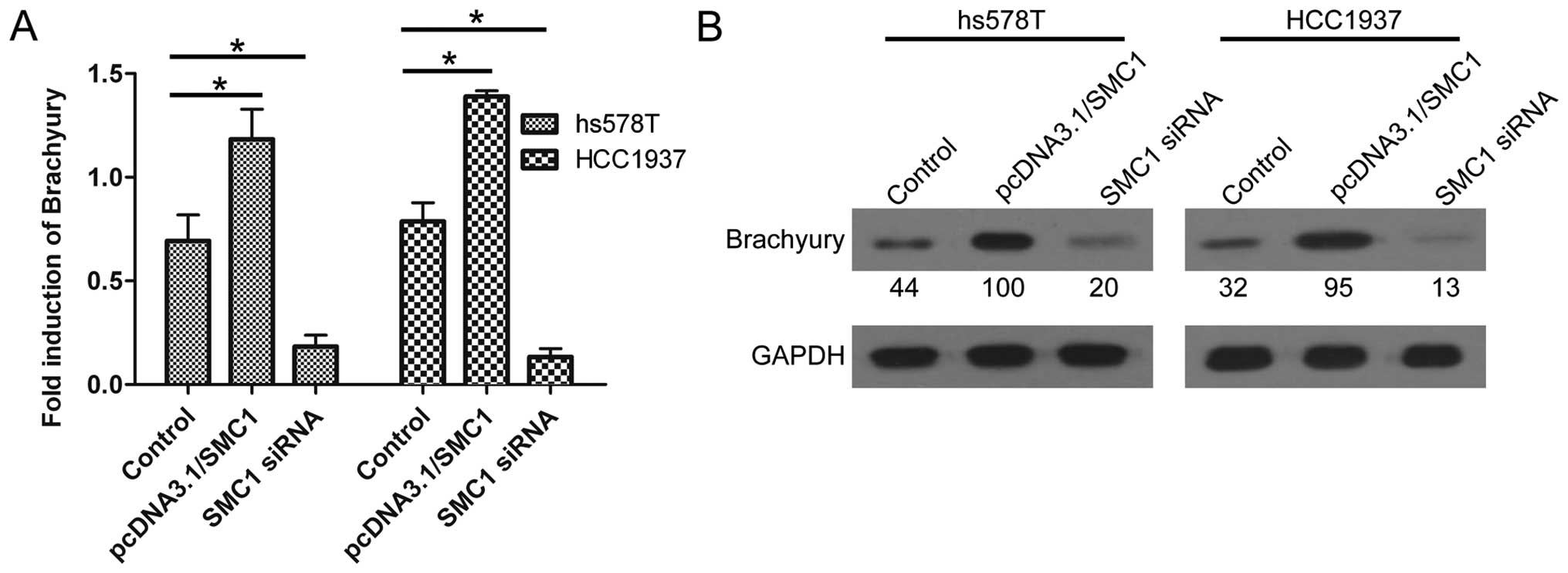
Overexpression of SMC1 indicates ectopic
expression of Brachyury
Since SMC1 and Brachyury were both highly expressed
in TNBC tissues and cells, the present study aimed to uncover the
association between SMC1 and Brachyury. As shown in Fig. 6, high expression of SMC1 usually
upregulated the expression of Brachyury; conversely, knockdown of
SMC1 led to decreased expression of Brachyury, at both the mRNA and
protein level. These results indicated that Brachyury may be a
downstream effector of the SMC1 gene.
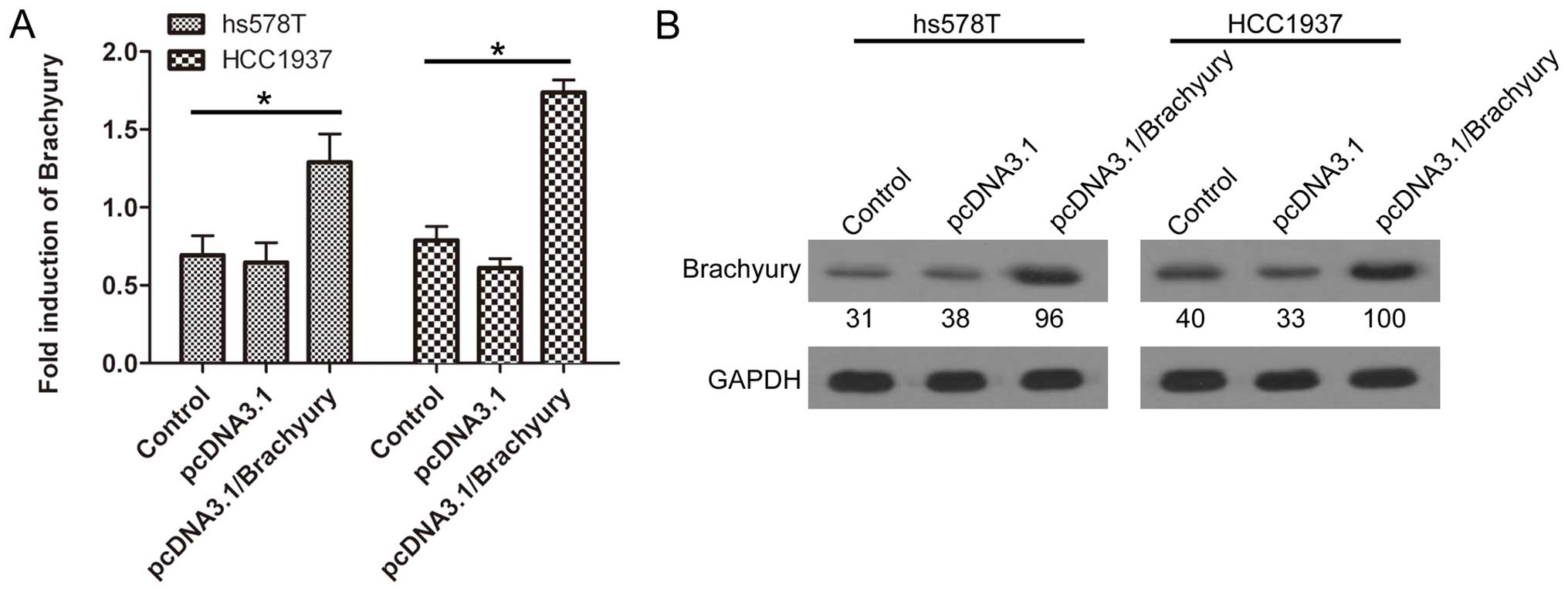
Overexpression of Brachyury promotes the
EMT process
Brachyury has previously been reported to induce the
EMT process in human epithelial cells; hence, we further
investigated whether SMC1 promoted EMT through the induction of
Brachyury expression in TNBC cells. Brachyury was overexpressed
through plasmid transfection in hs578T and HCC1937 cells (Fig. 7). Brachyury overexpression markedly
inhibited E-cadherin expression (Fig.
8A), while it remarkably promoted the expression of the two
mesenchymal markers N-cadherin (Fig.
8B) and vimentin (Fig. 8C),
indicating that Brachyury was able to promote the EMT process.
These data further demonstrated that SMC1 increased EMT in TNBC,
possibly through the induction of Brachyury expression.
Discussion
In the present study, we explored the expression of
SMC1 in TNBC; correspondingly, SMC1 was found to be highly
expressed in both TNBC tissues and cells. To further investigate
the effects of SMC1 on TNBC, SMC1 was overexpressed and silenced in
hs578T and HCC1937 cells, respectively. Interestingly, SMC1
overexpression was found to be able to induce the EMT process in
TNBC cells. Also, SMC1 was able to promote the metastasis and
proliferation of TNBC cells. Brachyury was also found to be
involved in this study. The results showed that Brachyury may be a
downstream molecule of SMC1, participating in induction of the EMT
process. The high expression of SMC1 was accompanied by the
upregulated expression of Brachyury. These consistent clues could
provide a potential target for the diagnosis and treatment of
TNBC.
As described, the increased expression of SMC1 in
TNBC was confirmed in this study. Yadav et al also focused
on the study of SMC1 and TNBC. They found that SMC1 played a role
in cell migration and drug sensitivity of TNBC cells. At the same
time, they declared that they had discovered the overexpression of
SMC1 in TNBC for the first time (15). Our results show for the first time
that the ectopic expression of SMC1 increased metastasis and the
proliferation of TNBC cells, possibly through induction of the EMT
process.
TNBC is a malignant neoplasm, which is characterized
by high capability of metastasis and invasiveness (15), thus, EMT was introduced to this
study due to its profound involvement in promoting metastasis and
invasiveness (30). Briefly, during
the EMT process, cells may lose epithelial markers and adherent
ability, and gain mesenchymal markers and invasive capability,
which can convert normal cells into tumor cells, contributing to
the metastasis and invasiveness of the tumor (29). Hence, the relationship between EMT
and TNBC needs to be urgently investigated. In the present study,
the data suggested that SMC1 promoted metastasis and proliferation
of TNBC cells through regulation of its EMT phenotype. This notion
was based on the following findings: i), mesenchymal markers were
significantly upregulated in the SMC1-overexpressed TNBC cell
lines, whereas the epithelial markers were remarkably decreased;
SMC1 knockdown showed the opposite effects; ii), western blotting
results also confirmed these alterations; and iii), Transwell and
BrdU assays demonstrated that enforced SMC1 led to increased
metastasis and proliferation, whereas decreased SMC1 expression
resulted in inhibited metastasis and proliferation of TNBC cells.
Therefore, these data indicated that SMC1 promoted TNBC metastasis
and proliferation, possibly through the induction of EMT in TNBC
cells.
Next, Brachyury was introduced into this experiment.
A previous study reported that Brachyury was highly expressed in
human tumor cells, but not in the corresponding normal cell lines
(29). Fernando et al
reported that Brachyury could induce EMT in human epithelial cells
through the repression of E-cadherin and the induction of Slug
(31). Moreover, Brachyury was also
reported to promote EMT in hepatocellular carcinoma (20), oral squamous cell carcinoma
(29) and non-small cell lung
cancer (32). In accordance with
the above results, the present study showed that Brachyury was
positively expressed in TNBC tissues and cells, whereas it was
negatively expressed in normal tissues and cells. We subsequently
aimed to investigate whether Brachyury participated in the
SMC1-induced EMT in TNBC cells. The expression of Brachyury was
found to be upregulated along with SMC1 overexpression, indicating
that Brachyury may be an effector of SMC1 in TNBC cells. Besides,
overexpression of Brachyury in TNBC cells also induced
characteristic changes of EMT, including elevated levels of
mesenchymal markers and decreased levels of epithelial markers.
Based on these data, we suggested that Brachyury expression was
linked to EMT and SMC1 expression in TNBC cells.
In conclusion, the present study demonstrated that
SMC1 could promote the EMT process through the induction of
Brachyury expression in TNBC. SMC1, in combination with Brachyury,
could serve as a novel target for the prevention and treatment of
TNBC, which will shed new light on understanding TNBC progression
and metastasis.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81072175 and
81372854).
Abbreviations:
|
SMC1
|
structural maintenance of chromosome
1
|
|
EMT
|
epithelial-mesenchymal transition
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Ossovskaya V, Wang Y, Budoff A, Xu Q,
Lituev A, Potapova O, Vansant G, Monforte J and Daraselia N:
Exploring molecular pathways of triple-negative breast cancer.
Genes Cancer. 2:870–879. 2011. View Article : Google Scholar
|
|
2
|
Metzger-Filho O, Tutt A, de Azambuja E,
Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA Jr, Ellis
P, et al: Dissecting the heterogeneity of triple-negative breast
cancer. J Clin Oncol. 30:1879–1887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu H, Scholz C, Zang C, Schefe JH, Habbel
P, Regierer AC, Schulz CO, Possinger K and Eucker J: Metformin and
the mTOR inhibitor everolimus (RAD001) sensitize breast cancer
cells to the cytotoxic effect of chemotherapeutic drugs in vitro.
Anticancer Res. 32:1627–1637. 2012.PubMed/NCBI
|
|
4
|
Stebbing J and Ellis P: An overview of
drug development for metastatic breast cancer. Br J Nurs.
21:S18–S22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brouckaert O, Wildiers H, Floris G and
Neven P: Update on triple-negative breast cancer: Prognosis and
management strategies. Int J Womens Health. 4:511–520.
2012.PubMed/NCBI
|
|
6
|
Laugsch M, Seebach J, Schnittler H and
Jessberger R: Imbalance of SMC1 and SMC3 cohesins causes specific
and distinct effects. PLoS One. 8:e651492013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Z, Scannell DR, Eisen MB and Tjian R:
Control of embryonic stem cell lineage commitment by core promoter
factor, TAF3. Cell. 146:720–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rhodes JM, McEwan M and Horsfield JA: Gene
regulation by cohesin in cancer: Is the ring an unexpected party to
proliferation? Mol Cancer Res. 9:1587–1607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nasmyth K and Haering CH: The structure
and function of SMC and Kleisin complexes. Annu Rev Biochem.
74:595–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY
and Qin J: SMC1 is a downstream effector in the ATM/NBS1 branch of
the human S-phase checkpoint. Genes Dev. 16:571–582. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michaelis C, Ciosk R and Nasmyth K:
Cohesins: Chromosomal proteins that prevent premature separation of
sister chromatids. Cell. 91:35–45. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirano T: At the heart of the chromosome:
SMC proteins in action. Nat Rev Mol Cell Biol. 7:311–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rocquain J, Gelsi-Boyer V, Adélaïde J,
Murati A, Carbuccia N, Vey N, Birnbaum D, Mozziconacci MJ and
Chaffanet M: Alteration of cohesin genes in myeloid diseases. Am J
Hematol. 85:717–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto G, Irie T, Aida T, Nagoshi Y,
Tsuchiya R and Tachikawa T: Correlation of invasion and metastasis
of cancer cells, and expression of the RAD21 gene in oral squamous
cell carcinoma. Virchows Arch. 448:435–441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yadav S, Sehrawat A, Eroglu Z, Somlo G,
Hickey R, Yadav S, Liu X, Awasthi YC and Awasthi S: Role of SMC1 in
overcoming drug resistance in triple negative breast cancer. PLoS
One. 8:e643382013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naiche LA, Harrelson Z, Kelly RG and
Papaioannou VE: T-box genes in vertebrate development. Annu Rev
Genet. 39:219–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Showell C, Binder O and Conlon FL: T-box
genes in early embryo genesis. Dev Dyn. 229:201–218. 2004.
View Article : Google Scholar
|
|
18
|
Kilic N, Feldhaus S, Kilic E, Tennstedt P,
Wicklein D, Wasielewski R, Viebahn C, Kreipe H and Schumacher U:
Brachyury expression predicts poor prognosis at early stages of
colorectal cancer. Eur J Cancer. 47:1080–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang XR, Ng D, Alcorta DA, Liebsch NJ,
Sheridan E, Li S, Goldstein AM, Parry DM and Kelley MJ: T
(brachyury) gene duplication confers major susceptibility to
familial chordoma. Nat Genet. 41:1176–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du R, Wu S, Lv X, Fang H, Wu S and Kang J:
Overexpression of brachyury contributes to tumor metastasis by
inducing epithelial-mesenchymal transition in hepatocellular
carcinoma. J Exp Clin Cancer Res. 33:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang B, Cohen JR, Fernando RI, Hamilton
DH, Litzinger MT, Hodge JW and Palena C: The embryonic
transcription factor Brachyury blocks cell cycle progression and
mediates tumor resistance to conventional antitumor therapies. Cell
Death Dis. 4:e6822013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mese nchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Savagner P: Leaving the neighborhood:
Molecular mechanisms involved during epithelial-mesenchymal
transition. BioEssays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imajyo I, Sugiura T, Kobayashi Y, Shimoda
M, Ishii K, Akimoto N, Yoshihama N, Kobayashi I and Mori Y: T-box
transcription factor Brachyury expression is correlated with
epithelial-mesenchymal transition and lymph node metastasis in oral
squamous cell carcinoma. Int J Oncol. 41:1985–1995. 2012.PubMed/NCBI
|
|
30
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: Insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
Brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu K, Liu B and Liu Y: Impact of Brachyury
on epithelial-mesenchymal transitions and chemosensitivity in
non-small cell lung cancer. Mol Med Rep. 12:995–1001.
2015.PubMed/NCBI
|