Introduction
Osteosarcoma is the most frequently diagnosed
primary malignant bone tumor in children and adolescents (1,2). The
development of new surgical and screening technologies, in
combination with neoadjuvant chemotherapy has led to great progress
in osteosarcoma therapy; however, ~40–50% of adolescent patients
eventually develop lung metastasis, and relapse is a common problem
in ~80% of patients with metastasis at diagnosis (3). Current therapies typically lead to
chemoresistance, and thus no significant change in the survival
rate has been observed in recent decades. Therefore, new
therapeutic strategies need to be developed. Molecular-targeting
drugs, which have selective inhibitory effects on a wide variety of
factors involved in tumor proliferation, migration and metastasis,
including growth factor and intracellular signaling factors have
recently been developed for other human malignancies (4).
Glycogen synthase kinase-3 (GSK-3) is a
serine/threonine protein kinase that functions in numerous
signaling pathways initiated by diverse stimuli. Two highly
homologous isoforms of GSK-3, GSK-3α and GSK-3β, are found in
mammalian cells (5). GSK-3 plays a
role in a great number of cellular and physiological processes,
including protein synthesis, glycogen metabolism, cell cycle
division, apoptosis, cell fate determination and stem cell
maintenance (6–9). The dysregulation of this protein is
associated with a variety of disorders, including neurodegenerative
diseases and cancers (5,8). It is primarily known for its role in
the Akt and Wnt signaling pathway, where it acts as a negative
regulator of the Wnt effector molecule β-catenin (7). GSK-3-mediated phosphorylation of
β-catenin has also been shown to lead to ubiquitination and
subsequently proteasomal degradation (10). GSK-3 suppresses the transcription of
several protooncogenes, such as c-myc and cyclin D1, in a wide
variety of tumors, and thus it was originally identified as a tumor
suppressor; however, the functions of GSK-3 in cancer differ
depending on cell type.
Recent research on numerous types of cancer,
including prostate, colorectal, pancreatic, ovarian, renal cell
carcinoma, multiple myeloma and leukemia, has shown that GSK-3 is
involved in tumorigenesis, and that inhibition of the expression
and activity of GSK-3 attenuates cell proliferation (11–19).
The inhibition of GSK-3 has also been shown to induce apoptosis by
suppressing the NF-κB signaling pathway, and various findings
appear to suggest that GSK-3 plays a positive role in the
regulation of NF-κB activity (13,19,20).
Thus, the biological functions and contradictory roles of GSK-3 in
apoptosis should be assessed in each type of tumor. A recent study
showed that GSK-3 activity may promote osteosarcoma cell growth
(21), but its molecular mechanisms
when using specific GSK-3 inhibitor treatment have yet to be fully
elucidated.
In the present study, with the aim of evaluating the
effects of a specific GSK-3 inhibitor on human osteosarcoma cells,
we examined whether cell proliferation was inhibited and apoptosis
was induced in human osteosarcoma cells by the GSK-3 inhibitor.
Materials and methods
Chemical reagents
SB216763, a highly specific GSK-3 inhibitor that
does not significantly inhibit other kinases (22), was purchased from Sigma Chemical Co.
(St. Louis, MO, USA), was dissolved in dimethylsulfoxide and stored
at −20°C. Anti-Akt and anti-phospho-Akt antibodies were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-mTOR and
anti-phospho-mTOR antibodies were obtained from R&D Systems
(Minneapolis, MN, USA). Anti-GSK-3β, anti-phospho-GSK-3β,
anti-NF-κB, anti-Bcl-2, anti-cleaved caspase-9, anti-cleaved
caspase-3, anti-cleaved PARP and ant-α-tubulin antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell lines and cell culture
In the present study, we used three human
osteosarcoma cell lines (KTHOS, KHOS and MG63). The KTHOS cell line
was established by Hitora et al (23), while KHOS and MG63 were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The cells were grown in Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (FBS) and 100 U/ml
penicillin (all from Sigma-Aldrich, St. Louis, MO, USA). All cells
were routinely maintained at 37°C in a humidified 5% CO2
atmosphere, and cultures were harvested at mid-log phase.
Specimens
In the present study, we used a total of eight human
bone and soft tissue tumor samples: three osteosarcomas, a
schwannoma, an enchondroma, a lipoma, a giant cell tumor and a
hemangioma. All sample specimens, which had originally been
diagnosed by pathologists based on histological data, were
surgically obtained between 2009 and 2015 from the Department of
Orthopaedic Surgery, Kagawa University Hospital in accordance with
institutional guidelines. Informed consent was obtained from all
patients or their parents or guardians before the samples were
collected. All procedures performed in the studies involving human
participants were carried out in accordance with the ethical
standards of the institutional and/or national research committee
and with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards.
Real-time reverse
transcription-polymerase chain reaction (RT-PCR)
All surgically obtained specimens were immediately
stored at −80°C until use. First, we evaluated mRNA expression of
GSK-3 in the samples. Isogen (Nippon Gene, Tokyo, Japan) was used
to extract total RNA from the cells, which was then
reverse-transcribed into cDNA using high-capacity cDNA reverse
transcription kits (Applied Biosystems, Foster City, CA, USA) for
RT-PCR. Real-time quantitative PCR analysis was run on the Eco™
Real-Time PCR system (Illumina Inc., San Diego, CA, USA) using
Power SYBR®-Green PCR Master Mix (Applied Biosystems).
The primers for real-time PCR were synthesized and validated by
Hokkaido System Science Co., Ltd. (Sapporo, Japan). The primers for
GSK-3 were as follows: forward, 5′-ACAGCAGCGTCAGAT GCTAA-3′ and
reverse, 5′-GGGACTGTTCAGGTGGAG-3′.
Immunohistochemical analysis
To determine GSK-3 expression, all surgically
obtained specimens were fixed in 10% formalin, embedded in paraffin
and coded. An osteosarcoma, a giant cell tumor, and a schwannoma
specimen were chosen, and tissue slides were deparaffinized in
xylene for two changes of 5 min each. Sections were hydrated
gradually through a graded-alcohol series with 100, 95, 90, 80 and
70% ethanol solutions for 2 min in each solution, and samples were
reactivated by the pressurization of sections in citric buffer (pH
7.0) for 10 min, and then treated with 3% hydrogen peroxide;
non-specific sites were blocked with 3% bovine serum albumin. Next,
samples were incubated for 60 min with the primary antibody for
GSK-3 (code no. 12456; Cell Signaling Technology, Inc., Beverly,
MA, USA). After rinsing in phosphate-buffered saline (PBS), samples
were incubated with secondary anti-rabbit IgG antibody (code no.
424142; Nichirei Biosciences, Inc., Tokyo, Japan) for 30 min. The
staining reaction was carried out with diaminobenzidine, and the
sections were counterstained with hematoxylin.
In vitro proliferation assay
Cell proliferation was assessed using the CellTiter
96® AQueous One Solution Cell Proliferation Assay (MTS
assay; Promega, Madison, WI, USA). Cells were trypsinized and
seeded at a density of ~1×104 cells/well in 96-well cell
culture plates with 200 µl of culture medium containing 10%
FBS and incubated for 48 h. Following the initial incubation, the
growth medium was replaced with medium containing 10% FBS and
SB216763 at concentrations of 0, 1, 5, 25 or 50 µM. After 24
and 48 h, the medium was removed, cells were washed with PBS and
fresh medium containing MTS reagent was added to each well (100
µl medium plus 20 µl MTS regent/well). After 2 h of
further incubation, an automatic microplate reader
(SpectraMax® Plus 384 microplate reader; Molecular
Devices, Sunnyvale, CA, USA) was used to measure the optical
density of each well at 490 nm (absorbance is directly proportional
to the number of living cells). The proliferation of cells in each
well was calculated as a percent of the control, and a minimum of
three independent experiments were performed for each assay.
Western blot analysis
We used the MG63 cell line in the present study.
First, cells were trypsinized and seeded at a density of
6×105 cells/well in 6-well cell culture plates with 2 ml
of culture medium containing 10% FBS. After 48 h, the cells were
treated with 10% FBS containing SB216763 at concentrations of 0, 1,
5, 10, 25 or 50 µM for 24 h. After treatment, the culture
medium was replaced with lysis buffer (Cell Signaling Technology,
Inc.), and cells were lysed on ice for 20 min. The cell lysates
were then centrifuged at 15,000 × g (Tomy MTX-150; Tomy Seiko Co.,
Ltd., Fukuoka, Japan) at 4°C for 30 min. The supernatant was then
collected as the total cellular protein extract. A BCA protein
assay kit (Pierce, Rockford, IL, USA) was used to determine protein
concentrations, which were then standardized to bovine serum
albumin. The total cellular protein samples were loaded onto sodium
dodecyl sulfate (SDS) polyacrylamide gels (7.5, 10 or 12.5%
commercial precast gel; Wako, Tokyo, Japan), and the proteins were
separated using SDS-polyacrylamide gel electrophoresis (PAGE) under
reducing conditions, and electrophoretically transferred to
nitrocellulose membranes (GE Healthcare Bio-Sciences, Piscataway,
NJ, USA). Next, the membranes were blocked for 90 min in blocking
buffer containing Tris-buffered saline (TBS-T) and EzBlock Chemi
(Atto Co., Tokyo, Japan), and were then incubated overnight at 4°C
with primary antibodies that were diluted in blocking buffer.
Specific horseradish peroxidase (HRP)-conjugated secondary antibody
(all used the same antibody: from rabbits) was used and incubations
were performed overnight at 4°C with gentle agitation. The ECL Plus
Western Blotting Detection System (GE Healthcare Bio-Sciences) and
an LAS-1000 Plus image analyzer (Fuji Film Co., Tokyo, Japan) were
used to detect the bound antibodies. Specific signals were
quantified using densitometric analysis (ImageJ Software; NIH,
Bethesda, MD, USA).
Measurement of single-stranded DNA
DNA in apoptotic cells is sensitive to formamide;
therefore, a monoclonal antibody against single-stranded DNA using
an ApoStrand™ ELISA apoptosis detection kit (Enzo Life Sciences,
Plymouth Meeting, PA, USA) was used according to the manufacturer's
instructions to detect denatured DNA. Cells were seeded into
96-well cell culture plates in culture medium containing 10% FBS.
After 24 h, the medium was replaced with fresh medium containing 1%
FBS and SB216763 at concentrations of 25 or 50 µM and the
cells were then incubated for 24 h. Next, they were fixed, dried
and attached to the plate surface, and then treated with formamide.
Cells were then incubated with an antibody mixture for 30 min after
non-specific binding sites were blocked. Peroxidase substrate was
added to each well after washing, and absorbance was measured at
405 nm using an automatic microplate reader.
Fluorescence microscopic images of the
Annexin V, ethidium homodimer III and Hoechst 33342 triple-staining
assay for detection of apoptosis
Cells from the MG63 cell line were trypsinized and
seeded at a density of ~1×106 cells/well on 25-mm
circular coverslips in 2 ml of culture medium with 10% FBS. After
48 h, the cells were washed with PBS and treated with SB216763 at
concentrations of 0, 5, 25 or 50 µM for 24 h. We then used
Annexin V, ethidium homodimer III and Hoechst 33342 triple-staining
assay to detect the apoptotic cells by using the PromoKine
Apoptotic/Necrotic/Healthy Cells Detection kit (PromoCell GmbH,
Heidelberg, Germany), according to the manufacturer's
recommendations. Stained cells were observed by an epifluorescence
microscope (FSX100; Olympus Optical Co., Tokyo, Japan).
Statistical analysis
One-way or two-way analysis of variance (ANOVA)
followed by post hoc analysis were used for the statistical
evaluation of real-time PCR and measurement of single-stranded DNA.
The significant difference of the cell proliferation assay curves
was analyzed using log IC50 statistically. All values
are expressed as means ± standard deviation, and P<0.05 was
considered to indicate a statistically significanct result using
GraphPad Prism 5 (GraphPad, San Diego, CA, USA). All data were
collected from at least three independent experiments for each
group.
Results
Real-time PCR
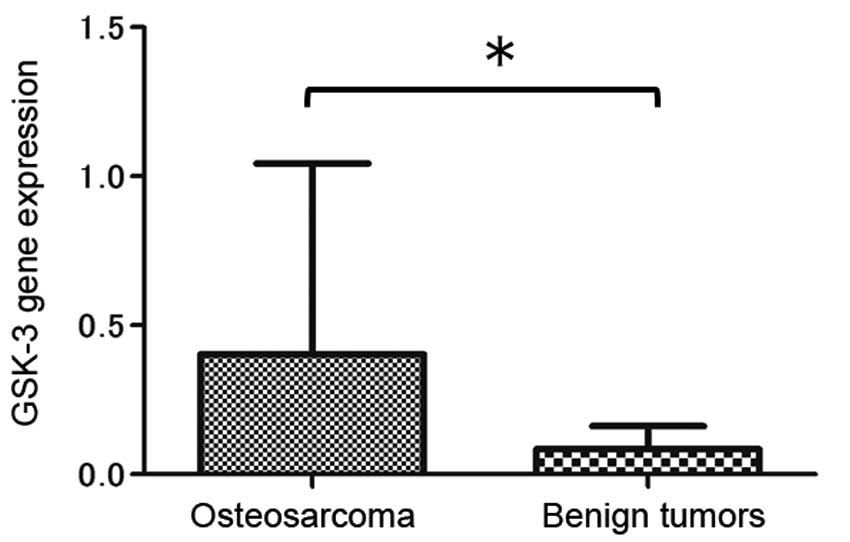
mRNA expression of GSK-3 was evaluated in all
surgically obtained specimens. Compared with that in the benign
bone and soft tissue tumor samples, GSK-3 expression was increased
in the osteosarcoma cells (Fig. 1);
this indicated that a high level of GSK-3 expression is a feature
of osteosarcoma.
Immunohistochemical analysis
Using immunohistochemical staining, we found
aberrant nuclear accumulation of GSK-3 in the human osteosarcoma
cells in comparison with the giant cell tumor and schwannoma
specimens (Fig. 2).
SB216763 inhibits the cell proliferation
of osteosarcoma cells
In our examination of the effect of SB216763 on the
proliferation of osteosarcoma cells (KTHOS, KHOS and MG63), we
found that SB216763 showed a dose- and time-dependent inhibitory
effect on all osteosarcoma cell lines (Fig. 3). The IC50 value at 48 h
of SB216763 treatment was 11.75 µM in the KTHOS cells 36.24
µM in the KHOS cells and 26.68 µM in the MG63
cells.
Western blot analysis
MG63 cells, in which the IC50 value for
SB216763 was between those of KTHOS and KHOS cells, were used for
western blot analysis. We found that SB216763 treatment resulted in
a decrease in GSK-3β, phospho-GSK-3β, NF-κB and Bcl-2 levels in the
MG63 cells (Fig. 4); Bcl-2 plays a
major role in the suppression of apoptosis and is regulated by
NF-κB. Next, to determine whether SB216763 induces
caspase-dependent apoptosis in MG63 cells, we examined the effect
of SB216763 on caspase activity. Western blot analysis indicated
that treatment with SB216763 at concentrations ranging from 1 to 50
µM for 24 h resulted in the cleavage of poly(ADP-ribose)
polymerase (PARP), as well as the activation of caspase-9 and
caspase-3 in the MG63 cells in a dose-dependent manner (Fig. 4). These results suggested that
SB216763 has the ability to induce apoptosis in a caspase-dependent
manner in the MG63 cells.
We also examined the expression levels of Akt/mTOR
signaling pathway-related proteins. Although phospho-Akt expression
was increased in a dose-dependent manner, SB216763 treatment did
not increase Akt, mTOR and phospho-mTOR levels in the MG63 cells
(Fig. 4). These findings indicate
that GSK-3 is involved in MG63 cell proliferation via Akt/mTOR
pathway-independent mechanisms.
Measurement of single-stranded DNA
For the determination of cellular apoptosis, we used
a single-stranded DNA ELISA assay to examine whether SB216763
increases the number of apoptosis-induced cells. SB216763 treatment
resulted in the induction of cellular apoptosis in all osteosarcoma
cell lines. The high-dose treatment increased apoptosis in the
KTHOS cells more than the low-dose treatment. In contrast, no
statistically significant differences were observed between the 25-
and 50-µM doses in the other two cell lines (Fig. 5).
Fluorescence microscopy images
We then used Annexin V, ethidium homodimer III and
Hoechst 33342 triple-staining assay to detect apoptotic cells.
Hoechst 33342-positive cells (blue) were live cells, and Annexin
V-FITC (green) is a marker for early apoptosis and ethidium
homodimer III (red) is a marker for late apoptosis and necrosis. We
observed several Annexin V-FITC-positive cells (early stage of
apoptosis) following SB216763 treatment in a dose-dependent manner
in the MG63 cells (Fig. 6).
Discussion
Osteosarcoma is a tumor characterized by genetic
alterations in the signaling pathways involved in growth and
development. Although a number of anticancer drugs, including
methotrexate, cisplatin, doxorubicin, etoposide and
cyclophosphamide are commonly used in combination to treat patients
with osteosarcoma (24), a
substantial proportion of these patients develop drug resistance
and subsequently die due to disease progression. Therefore, for the
improved management of patients with osteosarcoma, it is extremely
important to identify the molecular mechanisms of this
malignancy.
Although GSK-3, a pluripotent serine/threonine
kinase with a large number of intracellular target proteins, has
traditionally been recognized as a tumor suppressor inactivated in
a variety of tumors, such as oral (25), skin (26) and larynx cancer (27), the functions of GSK-3 in cancer
differ depending on cell type. These differing functions create the
potential for GSK-3 to exert apparently cell- and context-dependent
pro-apoptotic and anti-apoptotic effects (28). In the present study, GSK-3 was
identified as a positive regulator of osteosarcoma cell survival
and proliferation, and SB216763 was shown to inhibit the
proliferation of human osteosarcoma cells via the induction of
apoptosis.
In addition, mRNA expression of GSK-3 was found to
be increased in osteosarcoma cells, and immunohistochemical
analysis revealed aberrant nuclear accumulation of GSK-3 in human
osteosarcoma cells. These findings are supported by those of
previous studies that found nuclear overexpression of GSK-3 in
pancreatic cancer, renal cell carcinoma and leukemia (18,29,30).
SB216763 was also shown to inhibit the proliferation
of human osteosarcoma cells in a time- and dose-dependent manner
using an MTS assay. SB216763 treatment suppressed GSK-3 and
phospho-GSK-3 expression and led to a decrease in NF-κB and Bcl-2
in MG63 cells based on western blot analysis. Bcl-2, the
transcriptional target of NF-κB, participates in the regulation of
cell apoptosis (31). The NF-κB
family of transcription factors is involved in the activation of a
wide range of genes associated with inflammation, differentiation,
tumorigenesis, metastasis, embryonic development and apoptosis
(32–34). These genes are activated in response
to extracellular stimuli such as inflammatory cytokines and growth
factors. In addition, a previous study has shown that Bcl-2 family
proteins are important in the regulation of apoptosis (35). In the present study, SB216763
inhibited osteosarcoma cell proliferation by downregulating the
expression of GSK-3/NF-κB signaling pathway proteins.
Based on results from single-stranded DNA and
fluorescence microscopy analysis, SB216763 treatment induced
apoptosis in all osteosarcoma cell lines. Apoptosis plays an
important role in cell growth and tissue development, and can be
induced by a variety of signals either inside or outside of the
cell (36,37). A key mechanism of anticancer therapy
is the induction of apoptosis, which is regulated by various
factors and signaling pathways, such as the endoplasmic reticulum,
the mitochondrial and the death ligand pathway (38), in target cells. The mitochondrial
pathway is activated in response to the activation of Bcl-2, and in
turn, activates caspase-9 and caspase-3 in the downstream signaling
pathways of Bcl-2 (39). Caspase-9
is a key member of the cysteine-aspartic acid protease family, and
cleaved caspase-9 subsequently processes other caspase members,
including caspase-3, to initiate a caspase cascade, which leads to
apoptosis (40). Caspase-3 is
responsible for the proteolytic cleavage of many key proteins, such
as the nuclear enzyme PARP (41),
and is thus a critical executioner of apoptosis. PARP, which helps
maintain cell viability, is one of the primary cleavage targets of
caspase-3, and cleavage of PARP facilitates cellular disassembly
and serves as a marker for cells undergoing apoptosis (42). Based on the results of the western
blot analysis, which we performed in order to detect the expression
levels of apoptosis-associated proteins, SB216763 treatment was
shown to result in a dose-dependent increase in the expression
levels of cleaved caspase-9, cleaved caspase-3 and cleaved PARP
protein in the MG63 cells. These results suggest that SB216763
treatment suppresses the expression of the anti-apoptotic protein
Bcl-2, and induces apoptosis in osteosarcoma cells via the
mitochondrial pathway.
In the present study, SB216763 treatment was shown
to result in a dose-dependent increase in phospho-Akt based on
western blot analysis. Akt inhibits apoptosis through the
phosphorylation and inactivation of several targets, including
caspase-9, and thus promotes cell survival. These results suggest
that feedback from the induction of apoptosis and GSK-3 inhibition
increases the expression of phospho-Akt; however, SB216763
treatment did not appear to lead to increases in mTOR and
phospho-mTOR in the MG63 cells. Thus, it appears that GSK-3 is
involved in osteosarcoma cell proliferation via Akt/mTOR
pathway-independent mechanisms. Therefore, the control of these
signaling pathways is only one of many GSK-3 functions. These
findings are supported by those in previous studies reporting that
deregulated expression and activity of GSK3 in colorectal and
prostate cancer contribute to cancer cell survival and
proliferation in a manner unrelated to Akt/mTOR signaling
activation (11,17). SB216763 inhibits GSK-3 in an ATP
competitive manner (21). It is
suggested that SB216763 increases phospho-Akt which is a molecule
of the upper reaches of GSK-3 and suppresses the downstream signal
transmission by clearly inhibiting GSK-3.
In conclusion, the findings from the present study
appear to provide evidence of a positive association between GSK-3
activity and tumorigenicity, and that GSK-3 is crucial for the
survival of osteosarcoma cells. In addition, SB216763 appeared to
inhibit the proliferation of osteosarcoma cells in a dose- and
time-dependent manner. The inhibition of GSK-3 resulted in the
induction of caspase-dependent apoptosis through a decrease in
NF-κB and Bcl-2 expression in osteosarcoma cells. Based on these
results, GSK-3 may be a potential novel therapeutic target for the
treatment of human osteosarcoma.
Acknowledgments
The authors thank Mr Toshitaka Nakagawa (Division of
Research Instrument and Equipment, Kagawa University, Faculty of
Medicine, Kagawa, Japan) for technical assistance with the
fluorescence microscopy and the members of the Tokushima Molecular
Pathology Institute (Tokushima, Japan) for technical assistance
with the immunohistochemical analysis.
References
|
1
|
Mueller F, Fuchs B and Kaser-Hotz B:
Comparative biology of human and canine osteosarcoma. Anticancer
Res. 27:155–164. 2007.PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment - where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar
|
|
4
|
Arslan MA, Kutuk O and Basaga H: Protein
kinases as drug targets in cancer. Curr Cancer Drug Targets.
6:623–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doble BW and Woodgett JR: GSK-3: Tricks of
the trade for a multi-tasking kinase. J Cell Sci. 116:1175–1186.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim L and Kimmel AR: GSK3 at the edge:
Regulation of developmental specification and cell polarization.
Curr Drug Targets. 7:1411–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jope RS and Johnson GV: The glamour and
gloom of glycogen synthase kinase-3. Trends Biochem Sci. 29:95–102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen P and Frame S: The renaissance of
GSK3. Nat Rev Mol Cell Biol. 2:769–776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frame S and Cohen P: GSK3 takes centre
stage more than 20 years after its discovery. Biochem J. 359:1–16.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aberle H, Bauer A, Stappert J, Kispert A
and Kemler R: β-catenin is a target for the ubiquitin-proteasome
pathway. EMBO J. 16:3797–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kroon J, in 't Veld LS, Buijs JT, Cheung
H, van der Horst G and van der Pluijm G: Glycogen synthase
kinase-3β inhibition depletes the population of prostate cancer
stem/progenitor-like cells and attenuates metastatic growth.
Oncotarget. 5:8986–8994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shakoori A, Mai W, Miyashita K, Yasumoto
K, Takahashi Y, Ooi A, Kawakami K and Minamoto T: Inhibition of
GSK-3 beta activity attenuates proliferation of human colon cancer
cells in rodents. Cancer Sci. 98:1388–1393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ougolkov AV, Fernandez-Zapico ME, Savoy
DN, Urrutia RA and Billadeau DD: Glycogen synthase kinase-3beta
participates in nuclear factor kappaB-mediated gene transcription
and cell survival in pancreatic cancer cells. Cancer Res.
65:2076–2081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Uddin S, Zimmerman T, Kang JA,
Ulaszek J and Wickrema A: Growth control of multiple myeloma cells
through inhibition of glycogen synthase kinase-3. Leuk Lymphoma.
49:1945–1953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Q, Lu X and Feng YJ: Glycogen synthase
kinase-3beta positively regulates the proliferation of human
ovarian cancer cells. Cell Res. 16:671–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mirlashari MR, Randen I and Kjeldsen-Kragh
J: Glycogen synthase kinase-3 (GSK-3) inhibition induces apoptosis
in leukemic cells through mitochondria-dependent pathway. Leuk Res.
36:499–508. 2012. View Article : Google Scholar
|
|
17
|
Shakoori A, Ougolkov A, Yu ZW, Zhang B,
Modarressi MH, Billadeau DD, Mai M, Takahashi Y and Minamoto T:
Deregulated GSK3beta activity in colorectal cancer: Its association
with tumor cell survival and proliferation. Biochem Biophys Res
Commun. 334:1365–1373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bilim V, Ougolkov A, Yuuki K, Naito S,
Kawazoe H, Muto A, Oya M, Billadeau D, Motoyama T and Tomita Y:
Glycogen synthase kinase-3: A new therapeutic target in renal cell
carcinoma. Br J Cancer. 101:2005–2014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilson W III and Baldwin AS: Maintenance
of constitutive IkappaB kinase activity by glycogen synthase
kinase-3alpha/beta in pancreatic cancer. Cancer Res. 68:8156–8163.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin
O and Woodgett JR: Requirement for glycogen synthase kinase-3beta
in cell survival and NF-kappaB activation. Nature. 406:86–90. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang QL, Xie XB, Wang J, Chen Q, Han AJ,
Zou CY, Yin JQ, Liu DW, Liang Y, Zhao ZQ, et al: Glycogen synthase
kinase-3β, NF-κB signaling, and tumorigenesis of human
osteosarcoma. J Natl Cancer Inst. 104:749–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coghlan MP, Culbert AA, Cross DA, Corcoran
SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee
Cox L, et al: Selective small molecule inhibitors of glycogen
synthase kinase-3 modulate glycogen metabolism and gene
transcription. Chem Biol. 7:793–803. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hitora T, Yamamoto T, Akisue T, Marui T,
Nakatani T, Kawamoto T, Nagira K, Yoshiya S and Kurosaka M:
Establishment and characterization of a KIT-positive and stem cell
factor-producing cell line, KTHOS, derived from human osteosarcoma.
Pathol Int. 55:41–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bacci G and Lari S: Adjuvant and
neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov.
86:253–268. 2001.
|
|
25
|
Mishra R: Glycogen synthase kinase 3 beta:
Can it be a target for oral cancer. Mol Cancer. 9:1442010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leis H, Segrelles C, Ruiz S, Santos M and
Paramio JM: Expression, localization, and activity of glycogen
synthase kinase 3beta during mouse skin tumorigenesis. Mol
Carcinog. 35:180–185. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang T, Wei Y, Honaker Y, Yamaguchi H,
Appella E, Hung MC and Piwnica-Worms H: GSK-3 beta targets Cdc25A
for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation
correlates with Cdc25A overproduction in human cancers. Cancer
Cell. 13:36–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beurel E and Jope RS: The paradoxical pro-
and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic
apoptosis signaling pathways. Prog Neurobiol. 79:173–189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ougolkov AV, Fernandez-Zapico ME, Bilim
VN, Smyrk TC, Chari ST and Billadeau DD: Aberrant nuclear
accumulation of glycogen synthase kinase-3beta in human pancreatic
cancer: Association with kinase activity and tumor
dedifferentiation. Clin Cancer Res. 12:5074–5081. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu Y, Gu X, Li R, Luo Q and Xu Y: Glycogen
synthase kinase-3β inhibition induces nuclear factor-κB-mediated
apoptosis in pediatric acute lymphocyte leukemia cells. J Exp Clin
Cancer Res. 29:1542010. View Article : Google Scholar
|
|
31
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J
Cancer. 105:735–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Van Waes C: Nuclear factor-kappaB in
development, prevention, and therapy of cancer. Clin Cancer Res.
13:1076–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heath-Engel HM, Chang NC and Shore GC: The
endoplasmic reticulum in apoptosis and autophagy: Role of the BCL-2
protein family. Oncogene. 27:6419–6433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mahoney JA and Rosen A: Apoptosis and
autoimmunity. Curr Opin Immunol. 17:583–588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
39
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deveraux QL, Roy N, Stennicke HR, Van
Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS and Reed
JC: IAPs block apoptotic events induced by caspase-8 and cytochrome
c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fernandes-Alnemri T, Litwack G and Alnemri
ES: CPP32, a novel human apoptotic protein with homology to
Caenorhabditis elegans cell death protein Ced-3 and mammalian
interleukin-1 beta-converting enzyme. J Biol Chem. 269:30761–30764.
1994.PubMed/NCBI
|
|
42
|
Oliver FJ, de la Rubia G, Rolli V,
Ruiz-Ruiz MC, de Murcia G and Murcia JM: Importance of
poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson
from an uncleavable mutant. J Biol Chem. 273:33533–33539. 1998.
View Article : Google Scholar : PubMed/NCBI
|