Introduction
In recent years, an increasing consumption of
specific fruit and vegetables containing flavonoids is recognized
as the source of health-benefits through their various biochemical
properties such as potential anti-carcinogenic and cardioprotective
roles (1–3). Ingestion of plant flavonoids is
related with decreased risk of various tumors. Several flavonoids
are investigated as possible mechanisms of anti-proliferation,
anti-MMP secretion, anti-migration, anti-invasion, anti-adhesion,
and anti-angiogenic effects (4–8). Some
flavonoids are also suggested as potential pro-apoptosis inducers
in various cancer cell lines (9,10).
Clearly, plants containing various flavonoids are the source of
potential therapeutic agents for the benefit of human health.
Pancreatic adenocarcinoma and ductal-adenocarcinoma,
the most common, and deadly forms of human pancreatic cancers, are
highly invasive and metastatic and associated with poor survival
rates (11,12). Migratory activity makes pancreatic
cancer therapies difficult with dismal diagnosis and strong
chemo-resistance (13,14). Therefore, new agents inhibiting
migratory activity of pancreatic adenocarcinoma and
ductal-adenocarcinoma are urgently required.
We have previously shown that treatment with
quercetin 3-O-glucoside clearly inhibits the migratory activity of
pancreatic cancer cells even at relatively low dosages by
inhibiting the EGFR signaling pathway (15).
Even though targeting the specific pathway, i.e.,
EGFR signaling has proven effective in human cancers (16,17),
tumors could escape one-direction blockade strategy using
alternative signaling pathways (18–20).
This competence of tumors allowed us to exploit the additive role
of quercetin 3-O-glucoside and its therapeutic potential against
various growth factors.
In the present study, we tried to investigate the
effects of quercetin-O-3 glucoside on the migratory activity
induced by various growth factors in pancreatic cancer cell, and
reveal the mechanisms responsible for anti-migratory effects of
quercetin-O-3 glucoside. In addition, we also tried to verify its
therapeutic potential using combination therapy with gemcitabine
treatment. Our results support that quercetin-3-O glucoside can act
as an inhibitor of local metastasis induced by different growth
factors in pancreatic cancers and could be an effective adjuvant in
chemotherapy.
Materials and methods
Gene expression analysis
Microarray expression profiles were obtained from
the Gene Expression Omnibus (GEO) public microarray database. We
integrated data-sets independently obtained from several research
groups using the absolute normalization method SCAN.UPC (21). The normalization method is dependent
on total number of probes; hence, we restricted the integration to
Affymetrix Human Genome U133 Plus 2.0 Array platform (GPL570) that
has a larger number of probes than GPL96 and GPL97. All data were
normalized by the default option of SCAN.UPC. In total, 8 data-sets
were used i.e., GSE9599, -15471, -16515, -17891, -32676, -39409,
-42952, and -46385.
Cell culture and reagents
CFPAC-1, pancreatic ductal adenocarcinoma cells were
purchased from the American Type Culture Collection (ATCC; Manassa,
VA, USA), and SNU-213, pancreatic adenocarcinoma cells were
obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea). The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM,
CFPAC-1) or RPMI-1640 (SNU-213) medium supplemented with 10% fetal
bovine serum (Gibco-BRL, Gaithersburg, MD, USA), and
1×105 U/l penicillin-100 mg/l streptomycin (Invitrogen,
Carlsbad, CA, USA) at 37°C in a humidified atmosphere containing 5%
CO2. Antibodies against phospho-FAK (Y397), FAK,
phospho-ERK (T202/Y204), ERK, and GAPDH were obtained from Cell
Signaling Technology (Beverly, MA, USA). Suramin sodium, inhibitor
of bFGF signaling pathway was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Quercetin-3-O glucoside and
gemcitabine were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Recombinant bFGF, VEGF-A, and TGF-β1 were purchased from R&D
Systems (Minneapolis, MN, USA).
Migration assay
Cell migration assays were performed as previously
described (15). In brief,
polycarbonate filters were pre-coated with 10 mg/l fibronectin
(Sigma-Aldrich) in phosphate-buffered saline (PBS), for 30 min at
room temperature. The lower chamber was filled with 500 µl
of cultured medium containing 10% serum. After a 24-h starvation in
serum-free medium, cells (5×104 cells/well) were
suspended in 200 µl serum-free medium and loaded into each
upper chamber. Cells were then incubated for 4 h at 37°C. Filters
were fixed with 4% paraformaldehyde and stained with 1% crystal
violet solution. The absorbance of the eluted dye was measured at
560 nm in an ELISA reader (Bio-Rad, Richmond, CA, USA).
Western blotting
Western blotting was performed as previously
described to evaluate phosphorylation of various molecules
(22). The bands were measured by
densitometry using ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Data are presented as means ± standard deviation
(SD). The level of significance for comparisons between two
independent samples was determined using Student's t-tests. Groups
were compared using a one-way analysis of variance (ANOVA) with
Tukey's post-hoc test for significant main effects (SPSS 12.0K for
Windows; SPSS Inc., Chicago, IL, USA).
Results
Expression levels of various growth
factors in human pancreatic cancers
To select manifested-signaling pathways in human
pancreatic cancer cells (HPCCs), we performed mRNA analysis using
Universal Express Codes (UPCs) with the public microarray database
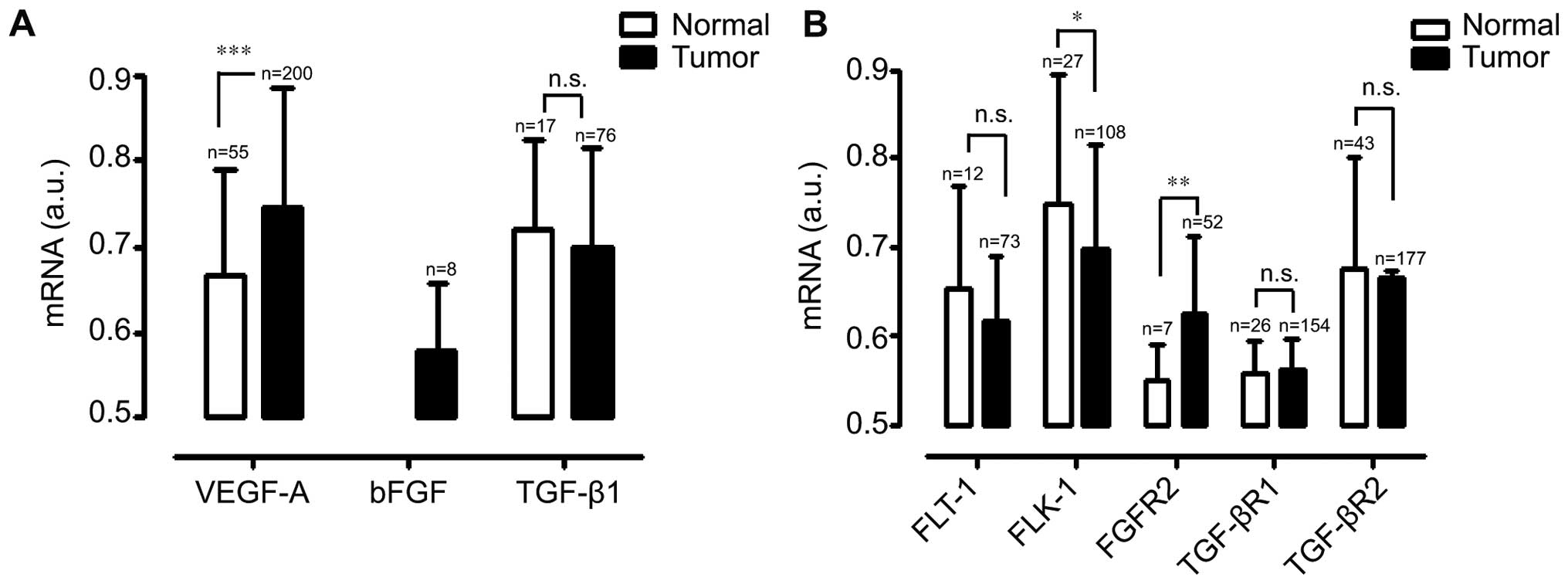
GEO. Pancreatic cancers expressed significantly more bFGF and
VEGF-A mRNAs, as compared with normal pancreatic tissues.
Specifically, pancreatic cancers expressed particularly higher
levels of bFGF, as compared with the normal pancreatic samples. In
contrast, there was no significant difference in TGF-β1 expression
levels between pancreatic cancers and normal pancreatic samples
(Fig. 1A). The receptors of VEGF-A,
bFGF, and TGF-β1 expression profiles were also examined using the
public microarray database GEO. As shown Fig. 1B, FGFR2 expression level was
elevated in pancreatic cancer samples. However, FLT-1, TGF-βR1, and
TGF-βR2 expression levels were similar in both pancreatic cancer
samples and normal pancreatic tissues. Interestingly, the
expression level of FLK-1 was significantly lower in pancreatic
cancer samples, as compared with normal pancreatic tissues. These
results clearly showed that pancreatic cancers has different
expression patterns of growth factors and its receptors including
unique expression patterns of bFGF signaling-related molecules,
bFGF and FGFR2.
Treatment of quercetin-3-O glucoside
decreased the migratory activity induced by different growth
factors in human pancreatic cancer cells
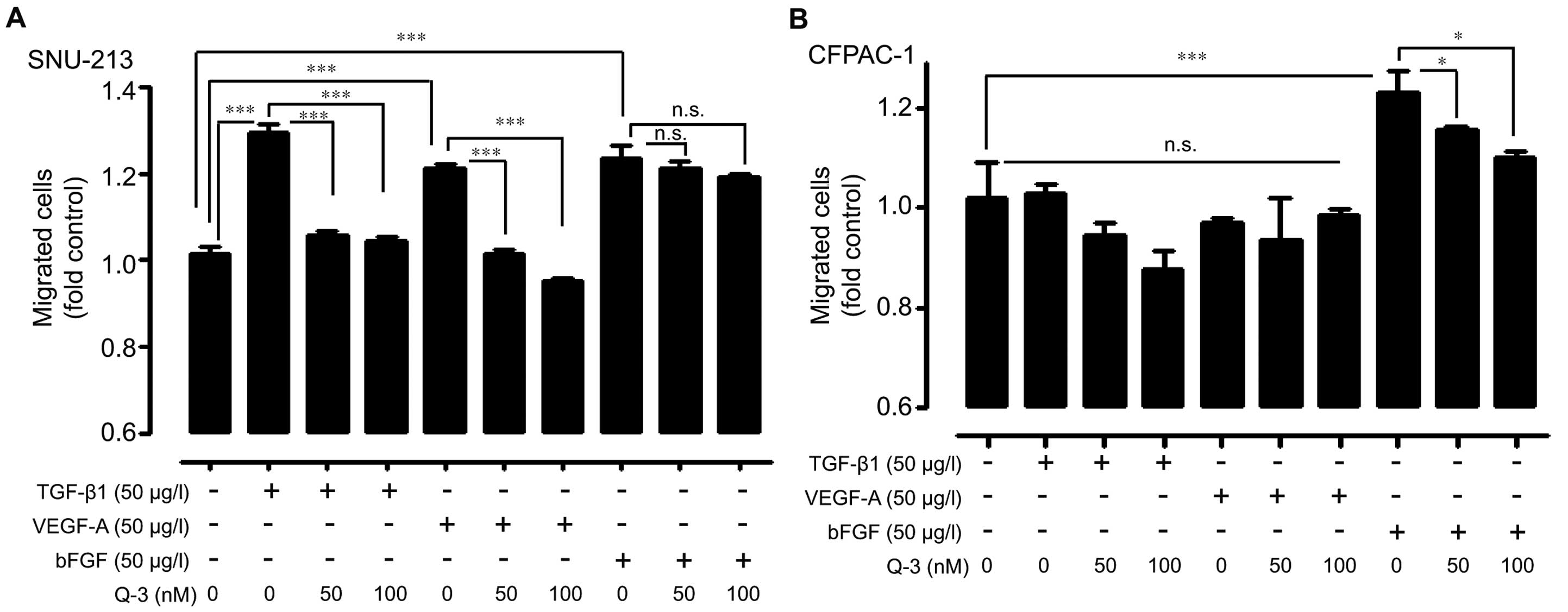
Human pancreatic cancer cells (SNU-213 and CFPAC-1)
were exogenously treated with bFGF, TGF-β1, and VEGF-A under the
absence or presence of relatively low dosages of quercetin-3-O
glucoside to investigate the anti-migratory effects of
quercetin-3-O glucoside against exogenous activations. In SNU-213
cells, TGF-β1 or VEGF-A-induced migration was inhibited by
quercetin 3-O-glucoside even at low dosages (50 and 100 nM); the
same treatment caused a weak effect in bFGF-treated SNU-213 cells
(Fig. 2A). In contrast, there was a
significant inhibitory effect in bFGF-induced migration in CFPAC-1
cells (Fig. 2B). Interestingly,
exogenous TGF-β1 and VEGF-A treatment did not induce migration
activity in CFPAC-1 cells.
We next investigated the involvement of bFGF
signaling pathway using the pharmaceutical bFGF blocker, suramine
sodium, on SNU-213 cell migration. As shown Fig. 3, bFGF treatment-induced cell
migration was dose-dependently inhibited by suramine pre-treatment.
Moreover, co-treatment of suramine and quercetin-3-O glucoside
significantly decreased the bFGF-induced migration (suramine; 100
nM, quercetin-3-O glucoside; 100 nM).
Different modes of quercetin-3-O
glucoside treatment for anti-migratory effect in human pancreatic
cancer cells
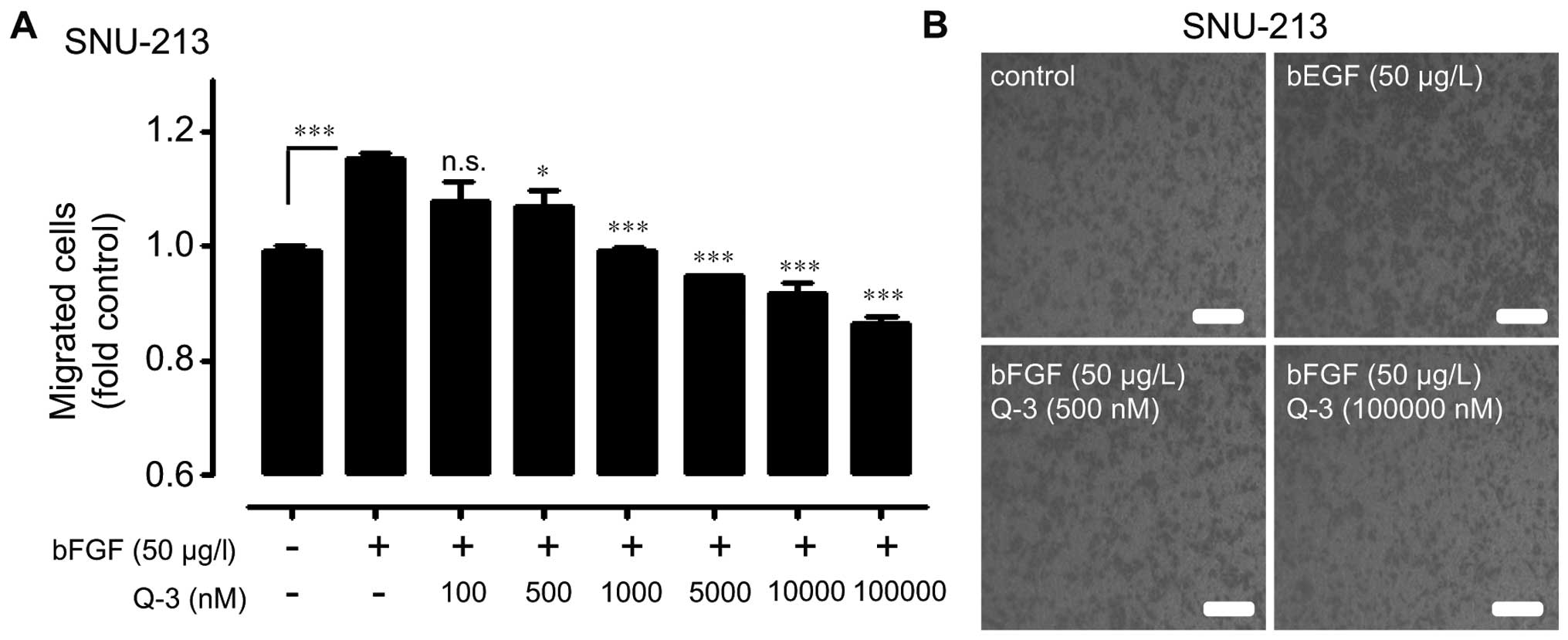
bFGF-treated SNU-213 cells were assayed for
migration activity in the presence of various concentration of
quercetin 3-O-glucoside (0, 100, 500, 1,000, 10,000, and 100,000
nM) to investigate the strategy of the effective blockade of
bFGF-induced migration in SNU-213 cells. Treatment of
quer-cetin-3-O-glucoside significantly decreased bFGF-induced
migration of SNU-213 cells up to 35%, as compared with that in
exogenously bEGF-treated control cells in a dose-dependent manner
(Fig. 4).
We further evaluated whether treatment of quercetin
3-O-glucoside can have synergistic anti-migratory effect when
combined with gemcitabine, a currently used reagent in pancreatic
cancer therapy. At the highest concentration tested (1,000 nM),
gemcitabine alone significantly reduced the cell migrations in
SNU-213 and CFPAC-1 cells, while the low doses of gemcitabine had
little effect (Fig. 5A). We chose
the 10 nM concentration of gemcitabine for a combinatory-strategy.
SNU-213 and CFPAC-1 cells were incubated in the absence or presence
of gemcitabine (10 nM) with different doses of quercetin
3-O-glucoside (0, 50, and 100 nM) and bFGF (50 µg/l). As
shown in Fig. 5B, the SNU-213 cell
migration was significantly reduced up to 22% by 100 nM quercetin
3-O-glucoside treatment. Under the same conditions, CFPAC-1 cell
migration was also reduced up to 15% (Fig. 5C). These results collectively
demonstrated that high dosage treatment of quercetin3-O glucoside
or low dosage co-treatment of quercetin3-O glucoside with
gemcitabine show effective anti-migratory effects in bFGF activated
human pancreatic cancer cells.
Treatment of quercetin 3-O-glucoside
inhibits bFGF-induced migratory activity through the blockade of
ERK1/2 signaling pathway in human pancreatic cancer cells
The migration inhibitory effects induced by
quercetin 3-O-glucoside in bFGF activated human pancreatic cancer
cells were evaluated in the study of bFGF signaling pathway with
effective dose of quercetin 3-O-glucoside. Exogenous bFGF treatment
activated the phosphorylation levels of FAK and ERK1/2; while
pretreatments of quercetin 3-O-glucoside significantly decreased
phosphorylation level of FAK and ERK1/2 (Fig. 6A). In addition, co-treatments of
quercetin3-O glucoside and gemcitabine significantly inhibited FAK
phosphorylation, as compared with quercetin3-O glucoside or
gemcitabine single treatment in bFGF activated human pancreatic
cancer cells (Fig. 6B). These
results suggested that combination therapy of quercetin3-O
glucoside and gemcitabine induces synergistic anti-migratory
effects in bFGF activated human pancreatic cancer cells through the
blockade of FAK and ERK1/2 signaling pathway.
 | Figure 6Intracellular signaling in response to
treatment of quercetin-3-O glucoside and co-treatment with
gemcitabine. (A) Left, SNU-213 cells were pretreated with different
doses of quercetin-3-O-glucoside for 1 h followed by treatment with
exogenous bFGF (50 µg/l) for 30 min and the cell lysates
were subjected to western blot analysis using antibodies specific
to phosphor FAK (Y397), FAK, phosphor-ERK1/2 (T202/Y204), ERK1/2,
and GAPDH. Right, relative pixel intensity for p-FAK and p-ERK was
measured using p-FAK/GAPDH or p-ERK2/GAPDH (B) Left, SNU-213 cells
pre-incubated with quercetin-3-O-glucoside alone or
quercetin-3-O-glucoside and gemcitabine combined, were treated with
50 µg/l of exogenous bFGF for 30 min and the cell lysates
were subjected to western blot analysis using antibodies specific
to phosphor FAK (Y397), FAK, and GAPDH. Right, relative pixel
intensity for p-FAK was measured using p-FAK/GAPDH. |
Discussion
In the present study, we demonstrated that
pancreatic cancers have relatively high mRNA expression patterns of
various growth factors and its receptor such as VEGF-A, bFGF, and
FGFR2, as compared to normal pancreatic samples using the public
microarray database GEO. Furthermore, quercetin-3-O-glucoside can
effectively inhibit human pancreatic cancer cells migration induced
by various growth factors such as VEGF-A, bFGF, and TGF-β. We
further showed that co-treatment of quercetin-3-O-glucoside with
gemcitabine had a synergic effect to suppress the migratory
activity in human pancreatic cancer cells through the inhibition of
FAK and ERK1/2 signaling pathway. These results suggested that the
naturally-occurring antioxidant, quercetin 3-O-glucoside, might be
a cancer cell migratory inhibitor and could be used in adjuvant
therapy.
Multiple phenotypes are caused by reciprocal
interactions of various growth factors and its receptors in most
cancers including pancreatic cancers. We have previously reported
that quercetin 3-O-glucoside effectively inhibits the EGF-induced
migratory activity of human pancreatic cancer cells by inhibiting
the EGFR signaling pathway (15).
Even though targeting the EGFR signaling pathway has been proven
effective in human cancers (16,17),
some tumors could escape the one way blockade strategy using
alternative signaling pathways (18–20).
In the present study, human pancreatic cancer cells thus had a
differential dependency to various growth factor activations such
as VEGF, bFGF, and TGF-β and treatment of quercetin-3-O-glucoside
also had a differential migratory-inhibition effects in SNU-213 and
CFPAC-1 cells (23). Especially,
exogenous bFGF treatment resulted in different responses to
quercetin-3-O-glucoside between SNU-213 and CFPAC-1 cells. Previous
studies have shown that enhanced bFGF expression is correlated with
pancreatic cancer stages in vitro and in vivo,
resistance to chemotherapy, and selection of cancer targeting
candidates (24–28). In agreement with these studies, our
results suggested that elevated bFGF signaling leads to an
increased chemoresistance in SNU-213 cells. As proof-of-concept,
mRNA analysis correctly identified different responses to various
growth factors. Therefore, to overcome the resistance to
quercetin-3-O-glucoside treatment, we demonstrated the strategy for
blockade of bFGF-induced migration using relatively high-dose
treatments within the safe range of quercetin-3-O-glucoside and
co-treatment of quercetin 3-O-glucoside and suramine with reducing
the potential risk of side-effect in SNU-213 cells. Treatment with
low dosages of suramine seems to sensitize the responses to
relatively low dosages of quercetin 3-O-glucoside. Further detailed
experiments are needed to confirm these findings.
Subsequently, quercetin-3-O-glucoside showed a
synergic anti-migratory effect with relatively low dosage of
gemcitabine. To date, gemcitabine is still regarded as the standard
treatment for pancreatic cancer patients despite its controversial
effects. Combinational trials with gemcitabin and cytotoxic
reagents, i.e., 5-FU, cisplatin, oxaloplatin, and capectabine
(29–32) and biological reagents, i.e.,
erlotinib, cetuximab, and bevacizumab (33–35)
have been reported. Some of those treatments had effective
outcomes; however, none of the combination trials were
significantly more effective, as compared with gemcitabin
alone.
Despite the significant improvement in overall
survival rates in other cancers during the last few decades, the
prognosis of pancreatic cancers is unfortunately at a standstill
(36,37), with an almost 100% mortality in
pancreatic cancer patients. The dismal prognosis in pancreatic
cancer is mainly due to its high metastatic potential, the
difficulty of diagnosis, and its high chemo-resistance (38). Clearly, most pancreatic cancer
patients are entirely disadvantaged from the most commonly used
gemcitabine through the chemo-resistance and metastatic behavior
(39).
According to the present study,
quercetin-3-O-glucoside can act as the inhibitor of local
metastasis induced by various growth factors in pancreatic cancers
and is the effective co-treatment with gemcitabine for intractable
pancreatic cancer treatment, despite its phytochemical origin in
intractable pancreatic cancer cells.
Acknowledgments
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (2009-0094059).
References
|
1
|
Formica JV and Regelson W: Review of the
biology of quercetin and related bioflavonoids. Food Chem Toxicol.
33:1061–1080. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed. Diet, nutrition and the
prevention of chronic diseases. World Health Organ Tech Rep Ser.
916:i–viii. 1–149. 2003.PubMed/NCBI
|
|
4
|
Rodriguez J, Yáñez J, Vicente V, Alcaraz
M, Benavente-García O, Castillo J, Lorente J and Lozano JA: Effects
of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma
cell lines: Relationship between structure and activity. Melanoma
Res. 12:99–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vijayababu MR, Arunkumar A, Kanagaraj P,
Venkataraman P, Krishnamoorthy G and Arunakaran J: Quercetin
downregulates matrix metalloproteinases 2 and 9 proteins expression
in prostate cancer cells (PC-3). Mol Cell Biochem. 287:109–116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee WJ, Wu LF, Chen WK, Wang CJ and Tseng
TH: Inhibitory effect of luteolin on hepatocyte growth
factor/scatter factor-induced HepG2 cell invasion involving both
MAPK/ERKs and PI3K-Akt pathways. Chem Biol Interact. 160:123–133.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamaraj S, Anandakumar P, Jagan S,
Ramakrishnan G and Devaki T: Modulatory effect of hesperidin on
benzo(a)pyrene induced experimental lung carcinogenesis with
reference to COX-2, MMP-2 and MMP-9. Eur J Pharmacol. 649:320–327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balakrishnan A and Menon VP: Effect of
hesperidin on matrix metalloproteinases and antioxidant status
during nicotine-induced toxicity. Toxicology. 238:90–98. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sivagami G, Vinothkumar R, Bernini R,
Preethy CP, Riyasdeen A, Akbarsha MA, Menon VP and Nalini N: Role
of hesperetin (a natural flavonoid) and its analogue on apoptosis
in HT-29 human colon adenocarcinoma cell line - a comparative
study. Food Chem Toxicol. 50:660–671. 2012. View Article : Google Scholar
|
|
10
|
Ghorbani A, Nazari M, Jeddi-Tehrani M and
Zand H: The citrus flavonoid hesperidin induces p53 and inhibits
NF-κB activation in order to trigger apoptosis in NALM-6 cells:
Involvement of PPARγ-dependent mechanism. Eur J Nutr. 51:39–46.
2012. View Article : Google Scholar
|
|
11
|
Smith K: Pancreatic cancer: FASCINating
insights into the metastatic nature of pancreatic cancer. Nat Rev
Gastroenterol Hepatol. 11:1392014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar
|
|
13
|
Bardeesy N and DePinho RA: Pancreatic
cancer biology and genetics. Nat Rev Cancer. 2:897–909. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cano CE, Motoo Y and Iovanna JL:
Epithelial-to-mesenchymal transition in pancreatic adenocarcinoma.
Sci World J. 10:1947–1957. 2010. View Article : Google Scholar
|
|
15
|
Lee J, Han SI, Yun JH and Kim JH:
Quercetin 3-O-glucoside suppresses epidermal growth factor-induced
migration by inhibiting EGFR signaling in pancreatic cancer cells.
Tumour Biol. 36:9385–9393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harari PM: Epidermal growth factor
receptor inhibition strategies in oncology. Endocr Relat Cancer.
11:689–708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vokes EE and Chu E: Anti-EGFR therapies:
Clinical experience in colorectal, lung, and head and neck cancers.
Oncology (Williston Park). 20(Suppl 2): 15–25. 2006.
|
|
18
|
Wajed SA, Laird PW and DeMeester TR: DNA
methylation: An alternative pathway to cancer. Ann Surg. 234:10–20.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tabernero J: The role of VEGF and EGFR
inhibition: Implications for combining anti-VEGF and anti-EGFR
agents. Mol Cancer Res. 5:203–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S, Pashtan I, Tsutsumi S, Xu W and
Neckers L: Cancer cells harboring MET gene amplification activate
alternative signaling pathways to escape MET inhibition but remain
sensitive to Hsp90 inhibitors. Cell Cycle. 8:2050–2056. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piccolo SR, Withers MR, Francis OE, Bild
AH and Johnson WE: Multiplatform single-sample estimates of
transcriptional activation. Proc Natl Acad Sci USA.
110:17778–17783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Ku T, Yu H, Chong K, Ryu SW, Choi K
and Choi C: Blockade of VEGF-A suppresses tumor growth via
inhibition of autocrine signaling through FAK and AKT. Cancer Lett.
318:221–225. 2012. View Article : Google Scholar
|
|
23
|
Lee J, Lee J, Yu H, Choi K and Choi C:
Differential dependency of human cancer cells on vascular
endothelial growth factor-mediated autocrine growth and survival.
Cancer Lett. 309:145–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamanaka Y, Friess H, Buchler M, Beger HG,
Uchida E, Onda M, Kobrin MS and Korc M: Overexpression of acidic
and basic fibroblast growth factors in human pancreatic cancer
correlates with advanced tumor stage. Cancer Res. 53:5289–5296.
1993.PubMed/NCBI
|
|
25
|
Nomura S, Yoshitomi H, Takano S, Shida T,
Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A,
et al: FGF10/FGFR2 signal induces cell migration and invasion in
pancreatic cancer. Br J Cancer. 99:305–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lieu C, Heymach J, Overman M, Tran H and
Kopetz S: Beyond VEGF: Inhibition of the fibroblast growth factor
pathway and antiangiogenesis. Clin Cancer Res. 17:6130–6139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wagner M, Lopez ME, Cahn M and Korc M:
Suppression of fibroblast growth factor receptor signaling inhibits
pancreatic cancer growth in vitro and in vivo. Gastroenterology.
114:798–807. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Hylander BL, LeVea C, Repasky EA,
Straubinger RM, Adjei AA and Ma WW: Enhanced FGFR signalling
predisposes pancreatic cancer to the effect of a potent FGFR
inhibitor in preclinical models. Br J Cancer. 110:320–329. 2014.
View Article : Google Scholar :
|
|
29
|
Berlin JD, Catalano P, Thomas JP, Kugler
JW, Haller DG and Benson AB III: Phase III study of gemcitabine in
combination with fluorouracil versus gemcitabine alone in patients
with advanced pancreatic carcinoma: Eastern Cooperative Oncology
Group Trial E2297. J Clin Oncol. 20:3270–3275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heinemann V, Quietzsch D, Gieseler F,
Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M,
Heinrich B, et al: Randomized phase III trial of gemcitabine plus
cisplatin compared with gemcitabine alone in advanced pancreatic
cancer. J Clin Oncol. 24:3946–3952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Louvet C, Labianca R, Hammel P, Lledo G,
Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, et
al GERCOR; GISCAD: Gemcitabine in combination with oxaliplatin
compared with gemcitabine alone in locally advanced or metastatic
pancreatic cancer: Results of a GERCOR and GISCAD phase III trial.
J Clin Oncol. 23:3509–3516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herrmann R, Bodoky G, Ruhstaller T,
Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A,
Pestalozzi B, et al Swiss Group for Clinical Cancer Research;
Central European Cooperative Oncology Group: Gemcitabine plus
capecitabine compared with gemcitabine alone in advanced pancreatic
cancer: A randomized, multicenter, phase III trial of the Swiss
Group for Clinical Cancer Research and the Central European
Cooperative Oncology Group. J Clin Oncol. 25:2212–2217. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al
National Cancer Institute of Canada Clinical Trials Group:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cascinu S, Verdecchia L, Valeri N, Berardi
R and Scartozzi M: New target therapies in advanced pancreatic
cancer. Ann Oncol. 17(Suppl 5): v148–v152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kindler HL, Friberg G, Singh DA, Locker G,
Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM,
et al: Phase II trial of bevacizumab plus gemcitabine in patients
with advanced pancreatic cancer. J Clin Oncol. 23:8033–8040. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koay EJ, Truty MJ, Cristini V, Thomas RM,
Chen R, Chatterjee D, Kang Y, Bhosale PR, Tamm EP, Crane CH, et al:
Transport properties of pancreatic cancer describe gemcitabine
delivery and response. J Clin Invest. 124:1525–1536. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Long J, Zhang Y, Yu X, Yang J, LeBrun DG,
Chen C, Yao Q and Li M: Overcoming drug resistance in pancreatic
cancer. Expert Opin Ther Targets. 15:817–828. 2011. View Article : Google Scholar : PubMed/NCBI
|