Introduction
Colorectal cancer (CRC) is the third most common
cancer, causing as many as 693,900 deaths in 2012 worldwide
(1). As lifestyles have changed
along with socioeconomic development, it is no surprise that risk
factors such as unhealthy diet, obesity, physical inactivity, and
smoking have increased CRC incidence (2). In contrast, CRC screening has led to
improved awareness of self-hygiene and standardized treatments
(3), decreasing the CRC mortality
rate (1). Nevertheless, CRC remains
the fourth and third leading cause of cancer-related death in men
and women, respectively. Furthermore, numerous patients with CRC
still develop recurrence or metastasis, leading to 5-year survival
rates as low as 60–70% (4).
Protein phosphatase 2A (PP2A) is a major
phosphoprotein phosphatase belonging to the superfamily of protein
serine/threonine phosphatases (5).
It plays an important role in tumor suppression by preventing cell
transformation (6). Recently,
investigations based on activating PP2A activity pharmacologically
in cancer have been highlighted (7). FTY720, also known as fingolimod, is an
immunomodulator most widely used in multiple sclerosis and multiple
organ transplantation (8–10). Structurally similar to sphingosine,
it is a PP2A activator (11).
Compared with traditional chemotherapies, FTY720 is less toxic and
has better oral bioavailability, therefore it can be considered an
alternative for cancer therapy (12), and has been widely used in various
cancers. To provide better guidance for cancer treatment, the
cancer inhibitory mechanisms of FTY720 require further
elucidation.
In the present study, we discovered first that
FTY720 promotes autophagy in CRC cell lines and that inhibition of
autophagy by 3-methyladenine (3-MA), a specific autophagy
inhibitor, enhanced FTY720 cytotoxicity, indicating the protective
role of autophagy in FTY720 treatment of CRC. Furthermore,
FTY720-induced autophagy was closely related with cancerous
inhibitor of PP2A (CIP2A), an endogenous PP2A inhibitor. Therefore,
we propose a new strategy for treating CRC through the autophagy
pathway using FTY720.
Materials and methods
Cell lines and cell culture
The human CRC cell lines DLD-1 and LoVo were
maintained in our laboratory. Both cell lines were cultured in
Dulbecco's modified Eagle's medium (DMED; Wisent Inc., St-Bruno,
Quebec, Canada) supplemented with 10% fetal bovine serum (Wisent
Inc.), 100 U/ml penicillin, and 100 µg/ml streptomycin at
37°C in an incubator containing 5% CO2.
Reagents and antibodies
FTY720 and 3-MA were purchased from Sigma-Aldrich
(Sigma, St. Louis, MO, USA). FTY720 was dissolved in dimethyl
sulfoxide (DMSO; Sigma) to a primary concentration of 10 mM, and
3-MA was dissolved in phosphate-buffered saline (PBS; Wisent Inc.)
to a primary concentration of 100 mM. The same concentration of
DMSO was used as vehicle, and did not exceed 1%. The antibodies to
light chain 3 (LC3)-I/II, poly(ADP-ribose) polymerase (PARP),
Bcl-xL and Bcl-2 were purchased from Cell Signaling Technology
(BSN; USA), and CIP2A was from Abcam (Cambridge, MA, USA).
RNA interference (RNAi) and green
fluorescent protein (GFP)-LC3 transfection
Chemically synthesized scrambled RNAi
oligonucleotides and small interfering RNA (siRNA) targeting human
CIP2A were purchased from GenePharma (Shanghai, China). The CIP2A
siRNA sequences are: 5′-GGACCCACGUUUGAUUACUTT-3′ (sense) and
5′-AGUAAUCAAACGUGGGUCCTT-3′ (antisense); the control siRNA
sequences are: 5′-UUCUCCGAACGUGUCACGUDTDT-3′ (sense) and
5′-ACGUGACACGUUCGGAGAADTDT-3′ (antisense). The siRNA transfection
was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instruction. Transfection
efficacy was determined by detecting CIP2A expression levels using
reverse transcription (RT)-PCR and western blotting after 48-h
transfection. The GFP-LC3 was a kind gift from the Department of
Gastric Surgery, The First Affiliated Hospital of Nanjing Medical
University. Transfection was also performed using Lipofectamine
2000, and validation of the transfection was directly observed
under fluorescence microscopy.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was measured using the CCK-8
assay (Beyotime Institute of Biotechnology, Shanghai, China)
according to the manufacturer's protocol. Cells were seeded into
96-well plates at 5000 cells/well. Each group had at least three
replicates. After 24-h incubation, each group was treated with
FTY720 or 3-MA for 24 and 48 h. To assess cell viability, 10
µl CCK-8 mixed with 90 µl complete medium was added
to each well and incubated at 37°C for 2 h. The absorbance was
measured at 450 nm using a microplate reader. Cell viability was
determined as the percentage of absorbance of drug-treated cells to
that of vehicle-treated cells.
Colony formation assay
Cells were seeded into 6-well plates at 500
cells/well in triplicate. After 24-h incubation, cells were
pretreated with 3-MA (5 mM) for 6 h or not treated, and then
treated with FTY720 (2.5 µM) or vehicle for 24 h. Then, they
were incubated in drug-free medium for another two weeks, after
which the colonies that had formed were fixed with 70% methanol and
stained with crystal violet, and could then be observed with the
naked eye. The colony-formation ability was evaluated based on the
proportion of colonies formed in the drug-treated group as compared
to that in the vehicle.
Flow cytometric analysis of
apoptosis
Cells (2×105) were seeded in 12-well
plates for 24 h, and then pretreated with 5 mM 3-MA for 6 h or not
treated, followed by 10 µM FTY720 or vehicle for 24 h. To
detect apoptosis, all cells, including apoptotic, dead, and
adherent cells, were collected and resuspended in cold PBS for
analysis. Apoptosis was detected using an Annexin V-FITC Apoptosis
Detection kit (eBioscience, Vienna, Austria) according to the
manufacturer's instructions. Data were assessed by flow cytometry
(Becton-Dickinson, San Jose, CA, USA).
Cell cycle analysis
Cells (4×105) were seeded in 6-well
plates for 24 h, and then pretreated with 5 mM 3-MA for 6 h or not
treated, followed by 10 µM FTY720 or vehicle for 24 h. After
that, cells were harvested and resuspended in cold PBS, followed by
being fixed with 70% ethanol at 4°C for 30 min then stored at −20°C
overnight. The next day, cells were centrifuged to remove ethanol
and washed with PBS. After another centrifugation, PI/RNase
staining buffer (BD Biosciences) were used according to the
manufacturer's instructions. Data were analyzed by flow cytometry
(Becton-Dickinson).
Transmission electron microscopy
Cells treated with 10 µM FTY720 or vehicle
were collected, washed with warm PBS, and fixed with 2.5%
glutaraldehyde in 0.1 M cacodylate buffer with 1% sucrose for 3 h
at 4°C. Subsequently, cells were washed three times using
cacodylate buffer, then post-fixed in 1% osmium tetroxide in the
same buffer for 3 h. After another three washes in cacodylate
buffer, the cells were dehydrated by an ascending concentration of
ethanol at 4°C. After infiltration with a medium compound
containing epon 812 and Spurr's resin, ultrathin sections were
double-stained with uranyl acetate and lead citrate (13). The results were observed and were
photographed using a JEM-1010 transmission electron microscope
(JEOL, Ltd.); obtainment of the ultrathin sections and observation
were performed by an experienced technologist.
Immunofluorescence assay and GFP-LC3
overexpression
DLD-1 and LoVo cells (2×104) were treated
with 10 µM FTY720 or vehicle for 24 h, and then the cells
were washed and fixed with 4% paraformaldehyde and permeabilized
with 0.5% Triton X-100 in PBS for 10 min. LC3-I and LC3-II (1:200)
were used as primary antibodies, and Alexa Fluor 555-labeled donkey
anti-rabbit immunoglobulin G (Beyotime Institute of Biotechnology)
was used as the secondary anti-body to visualize LC3. To obtain
better magnification, the fluorescence was observed by confocal
microscopy (Zeiss, Jena, Germany) at ×570 magnification. Following
GFP-LC3 transfection, 2×104 cells were treated with 10
µM FTY720 or vehicle for 24 h, fixed with 4%
paraformaldehyde, washed with PBS containing 1% Tween-20 (PBST),
and observed by confocal microscopy.
Quantitative real-time RT-PCR
Total RNA from the cells was extracted using TRIzol
reagent (Invitrogen), and complementary DNA (cDNA) was synthesized
using a PrimeScript RT reagent kit (Takara, Dalian, China)
according to the manufacturer's instructions. The primers used for
quantitative PCR (qPCR) are as follows: Forward,
5′-TGGCAAGATTGACCTGGGATTTGGA-3′ and reverse,
5′-AGGAGTAATCAAACGTGGGTCCTGA-3′ for CIP2A; and forward,
5′-AGAAAATCTGGCACCACACC-3′ and reverse, 5′-TAGCACAGCCTGGATAGCAA-3′
for β-actin. The qPCR was performed using a SYBR Green PCR kit
(Roche, Indianapolis, IN, USA) in a StepOnePlus Real-time PCR
System (Applied Biosystems, Foster City, CA, USA). The PCR cycling
conditions were as follows: 95°C for 30 sec, 40 cycles of 95°C for
5 sec, 60°C for 31 sec; for the dissociation stage: 95°C for 15
sec, 60°C for 1 min, and 95°C for 15 sec. Each sample was analyzed
thrice. ΔΔCt method was used for analysis.
Western blot analysis
Cells were lysed using radioimmunoprecipitation
assay buffer (PARP; Beyotime Institute of Biotechnology), and
equivalent amounts of protein were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
transferred to polyvinylidene fluoride membranes (Millipore,
Bedford, MA, USA). The membranes were blocked in 5% non-fat milk
dissolved in Tris-buffered saline solution containing 0.1% Tween-20
(TBST) for 2–4 h at room temperature, and then incubated with
antibodies specific for LC3, CIP2A, and β-actin (1:1000) at 4°C
overnight. After washing three times with TBST, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:1000; Beijing Biosynthesis Biotechnology) at room temperature
for 2 h. After three TBST washes, bound proteins were visualized
using ECL Plus (Millipore) by a Bio-Imaging System. β-actin was
used as the internal loading control.
Statistical analysis
Statistical analysis was performed using Statistical
Program for Social Sciences (SPSS) 20.0 software (IBM, SPSS Inc.,
Chicago, IL, USA). One-way analysis of variance was used for
comparison between groups. P<0.05 indicated statistically
significant differences.
Results
Effect of FTY720 on CRC cell
viability
To evaluate FTY720 cytotoxicity in vitro, we
treated two randomly selected CRC cell lines: DLD-1 and LoVo, with
or without different concentrations of FTY720. After 24- and 48-h
drug exposure, we detected the cell proliferation capacity using
the CCK-8 assay. As shown in Fig. 1A
and B, the viability of both cell lines decreased as the drug
concentration increased. In the same way, under specific drug
concentrations, 48-h exposure was more effective than 24-h
exposure. The median inhibitory concentrations (IC50)
for each cell line are shown in Table
I. To assess the long-term effect of FTY720, we performed a
colony formation assay. Consistent with the CCK-8 assay, the
results showed that 24-h pretreatment with FTY720 distinctly
decreased the number of colonies formed as compared to the controls
for both cell lines (Fig. 1C and
D).
 | Table IIC50 values of FTY720 in
DLD1 and LoVo cells. |
Table I
IC50 values of FTY720 in
DLD1 and LoVo cells.
| Cell lines | IC50
(µM)
|
|---|
| 24 h | 48 h |
|---|
| DLD1 | 10.05435 | 7.84012 |
| LoVo | 10.23060 | 6.94962 |
FTY720 induces apoptosis in CRC cell
lines
To further evaluate the effect of FTY720 on cell
physiological function, we assessed its apoptotic effect on DLD-1
and LoVo cells, using flow cytometry to analyze the apoptotic
effect. As expected, after 24-h exposure to 10 µM FTY720,
there were markedly elevated percentages of positive-staining DLD-1
and LoVo cells as compared with the vehicle (Fig. 2A–D). In addition, Bcl-xL and Bcl-2
are two anti-apoptotic members of the Bcl-2 family (14). So when apoptosis was induced, both
of them decreased. Furthermore, PARP is a substrate of caspase 3.
Therefore, it is reasonable that the presence or absence of cleaved
PARP would reflect whether FTY720 induced apoptosis. We treated
DLD-1 and LoVo cells with or without 5, 10, or 20 µM FTY720
for 12 h, or with the same concentration (10 µM FTY720) for
different durations (12, 24, 48 h). Western blotting showed that
FTY720-induced Bcl-xL and Bcl-2 decrease (Fig. 3) and PARP cleavage (Fig. 2E) were concentration- and
time-dependent, further confirming the induction of apoptosis and
cell death.
FTY720 arrests CRC cells in the G0/G1
phase
To further elucidate the effect of FTY720 on the
cell cycle phases, we performed flow cytometric analysis of the
cell cycle. As shown in Fig. 4,
both DLD-1 and LoVo cells performed a significantly increased
proportion of G0/G1 phase, followed by a sharp decrease of G2/M
phase proportion after FTY720 treatment. These data suggested that
FTY720 arrests GC cells in the G0/G1 phase and this result was
consistent with a previous study on cholangiocarcinoma (15).
FTY720 induces autophagy in CRC
cells
Besides apoptosis, autophagy is another classic form
of programmed cell death (16).
Autophagy participates in the process of FTY720 cyto toxicity in
ovarian cancer and some hematological malignancies (17,18).
However, in the process of FTY720 treatment in CRC, the involvement
of autophagy requires further elucidation. To this end, we first
used the most traditional method, transmission electron microscopy,
to analyze the ultrastructural morphological changes in DLD-1 and
LoVo cells after 24-h FTY720 (10 µM) treatment. As shown in
Fig. 5, FTY720-treated cells
presented typical autophagosomes with characteristics such as
double-membrane structure containing undigested cytoplasm or
organelles (19). Yet, few
autophagosomes were observed in the vehicle-treated cells. The
ultrastructural morphological changes indicated the participation
of autophagy in FTY720 treatment in CRC.
LC3 is a well-known, reliable indicator of
autophagy. During autophagy, the diffusely distributed endogenous
cytoplasmic LC3 will convert to punctate structures (19). To verify the transformation, we
performed assays observed under confocal microscopy. Using specific
antibodies to cause fluorescence emission, we observed that after
24-h FTY720 (10 µM) treatment, the proportion of cells
forming punctate LC3 was significantly increased as compared with
the vehicle (Fig. 6A and B). To
confirm this effect, we overexpressed GFP-LC3 in both cell lines.
Similar to the expression patterns of endogenous LC3 after FTY720
treatment, there were significantly increased GFP-LC3-transfected
DLD-1 and LoVo cells with punctate fluorescence (Fig. 6C and D).
There are two forms of LC3: LC3-I and LC3-II. When
autophagy is promoted, LC3-I converts to LC3-II (20). This conversion is an indicator of
autophagy (21), and it can be
detected by western blotting. As shown in Fig. 6E, DLD-1 and LoVo cells treated with
or without 5, 10, or 20 µM FTY720 for 12 h or with the same
concentration (10 µM) of FTY720 for different durations (12,
24, 48 h) showed concentration- and time-dependent increased LC3-II
expression levels, indicating that autophagy induced by FTY720 in
CRC is concentration- and time-dependent.
Autophagy plays a protective role in
FTY720-induced cell death in CRC
As stated above, autophagy is also involved in
FTY720 cytotoxicity in ovarian cancer cells. Zhang et al
demonstrated that, other than a cytotoxic effect, autophagy has a
protective effect (18). Yet, the
role of autophagy in FTY720 cytotoxicity in CRC requires further
study. To address this, we used the autophagy inhibitor 3-MA to
perform a series of experiments. We pretreated DLD-1 and LoVo cells
with 3-MA (5 mM) for 6 h, followed by FTY720 (10 µM) or
vehicle treatment for another 24 h. The CCK-8 assay showed that
3-MA enhanced FTY720-induced cytotoxicity (Fig. 7A and B). Similarly, we performed a
colony formation assay using similar treatments (FTY720
concentration was decreased to 2.5 µM). Consistently,
3-MA-pretreated cells had weaker colony-formation capacity
following FTY720 treatment as compared to vehicle-treated cells
(Fig. 1C and D). In the same vein,
we observed apoptosis in a higher percentage of 3-MA-pretreated
cells after FTY720 treatment (Fig.
2A–D). These results all indicate the protective effect of
autophagy on FTY720 cytotoxicity in CRC. Moreover, as to cell
cycle, in 3-MA pretreated cells, the proportion of G0/G1 phase was
not apparently affected. However, different from the results of
cell viability and apoptosis, the proportion of G0/G1 phase in 3-MA
pretreated cells was not markedly increased compared with that with
FTY720 alone (data not shown), indicating that FTY720 induced G0/G1
arrest was not dependent on autophagy.
Inhibition of CIP2A is associated with
FTY720-induced autophagy in CRC
CIP2A is an endogenous inhibitor of PP2A, Cristobal
et al showed that CIP2A is overexpressed in both CRC cell
lines and cancer tissues as compared with normal controls and that
FTY720 suppresses CIP2A expression at protein level (22). Moreover, another PP2A activator,
bortezomib, induces autophagy by the CIP2A-PP2A-Akt-4EBP1 signaling
pathway in hepatocellular carcinoma (23). Consequently, it is reasonable to
assume that CIP2A is associated with FTY720-induced autophagy in
CRC. To validate this assumption, we used siRNA to interfere with
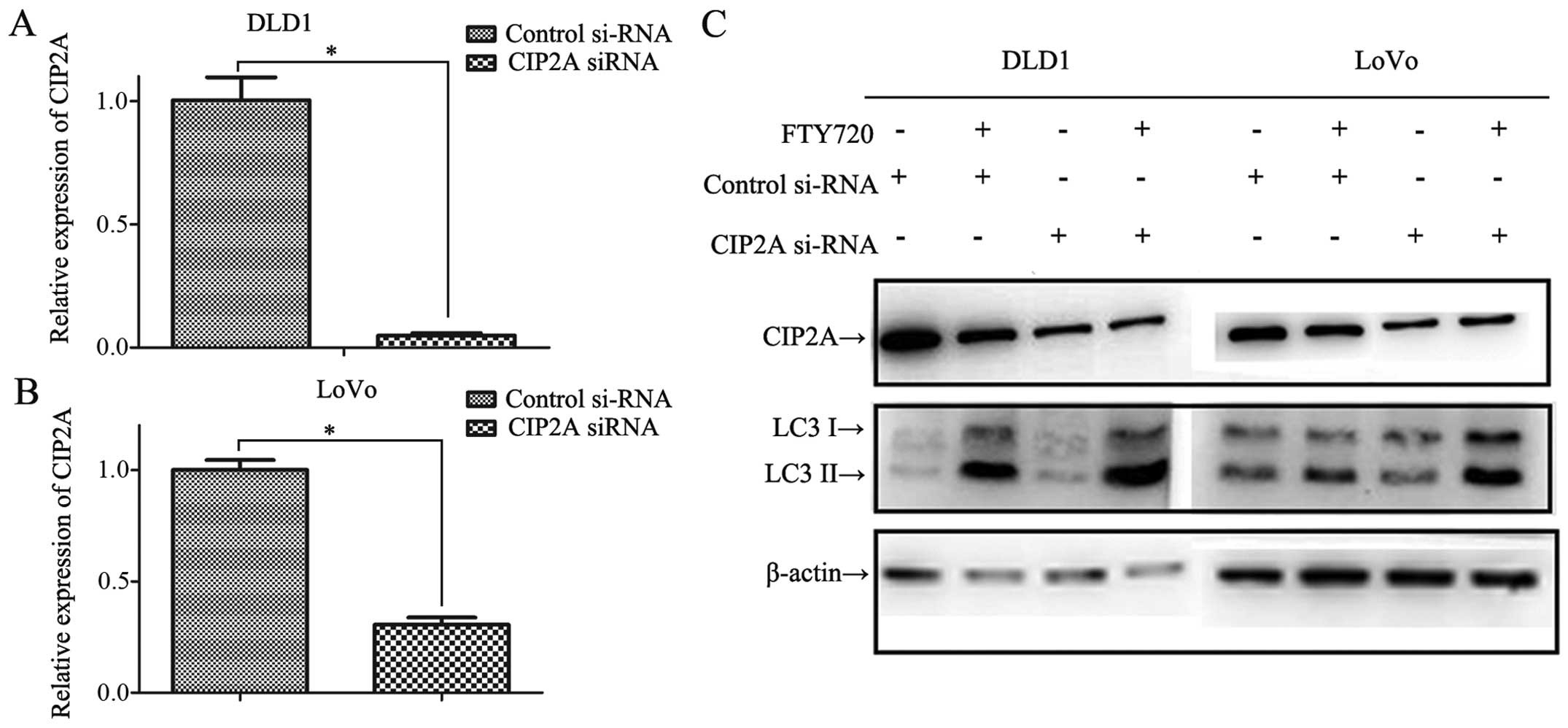
CIP2A expression. The interference efficiency is shown in Fig. 8A–C. Consistent with the previous
study, FTY720 decreased the expression level of CIP2A in
control-RNAi cells (Fig. 8C).
However, CIP2A level of CIP2A-RNAi cells did not change after
FTY720 treatment, possibly because after interference the CIP2A was
at a relative low level. Following FTY720 treatment for the same
concentration and duration, the CIP2A-RNAi cells expressed higher
LC3-II levels compared with that in control-RNAi cells, validating
the participation of CIP2A in FTY720 induction of autophagy in CRC.
Noteworthy, after FTY720 treatment, without a decrease in CIP2A
level in CIP2A-RNAi cells, LC3-II levels still increased,
indicating the participation of CIP2A is only partial.
Discussion
With relatively non-toxic specificity and high oral
bioavailability (12), FTY720 has
been explored in vitro in various cancers, including
hepatocellular carcinoma (24),
acute myeloid leukemia (25),
breast cancer (26), and prostate
cancer (27). In cancer therapy,
the drug is best known as a PP2A activator (6), while its other mechanisms of action
are comparatively less well known. In the present study, we
discovered first that FTY720 promotes autophagy in CRC cell lines
and that inhibiting autophagy using 3-MA, a nonspecific autophagy
inhibitor, enhanced FTY720 efficacy, indicating the protective role
of autophagy in FTY720 cytotoxicity for CRC. Furthermore,
FTY720-induced autophagy was closely related with CIP2A, an
endogenous PP2A inhibitor.
In our study, we first tested the effect of
different drug concentrations and incubation durations on the
viability of DLD-1 and LoVo cells via the CCK-8 assay to determine
the appropriate drug concentration for subsequent experiments. The
results showed that the effect of FTY720 on cells was
concentration- and time-dependent. Moreover, the IC50
data we determined for each cell line were in accordance with that
for ovarian cancer (18) and some
leukemias (28). The significance
of the IC50 of FTY720 not only provides an index for
further investigation but also offers an important reference for
further application to clinical trials. Of note, in the colony
formation experiment, the FTY720 concentration that had partially
inhibited cells in the CCK-8 assay led to whole cell death. The
results are similar to a previous study on ovarian cancer, in which
it was explained that the sensitivity of cells to FTY720 is related
to cell density (18). Noteworthy,
the drug sensitivity also differed when we treated the same number
of cells seeded at different times or treated different numbers of
cells seeded at the same time with FTY720 (data not shown),
validating the influence of cell density on drug sensitivity. Our
IC50 values were tested under 80% cell density.
Similarly, the proportion of apoptotic cells induced by the
indicated drug concentration was not entirely consistent with the
CCK-8 results, indicating that it might also have been affected by
cell density.
There are two classic self-destructive cell
processes: apoptosis and autophagy (16). Herein, we confirm that FTY720
markedly increases the proportion of apoptotic DLD-1 and LoVo
cells, decreases expression levels of anti-apoptotic Bcl-xL and
Bcl-2, and induces the expression of cleaved PARP, the active form
of the caspase-3 substrate. Combined with the findings of a
previous study (22), we note that
FTY720-induced apoptosis is caspase-dependent. Furthermore, we
confirmed first that FTY720 induces time- and dose-dependent
autophagy in DLD-1 and LoVo cells. The validation of autophagy was
performed via several classic experiments. The formation of
autophagosomes, characterized by a double-membrane structure
containing undigested cytoplasm or organelles, is the gold standard
for autophagy; autophagosomes are ultrastructural and should be
observed under transmission electron microscopy. However, observer
subjectivity can influence the decision on the presence of
autophagy. Therefore, we next detected the expression pattern and
level of the microtubule-associated protein LC3, a well-known
indicator of autophagy. There are two forms of LC3: LC3-I and
LC3-II; the initial form of LC3 is LC3-I, which is distributed in
the cytoplasm. Endogenous LC3 or GFP-LC3 visualized under
fluorescence microscopy is observed as diffuse fluorescence.
Otherwise, when autophagy is promoted, LC3-I is converted to
LC3-II, and the latter associates with the autophagosome membranes,
and then endogenous LC3 or GFP-LC3 is visualized as punctate
fluorescence. The conversion of LC3-I to LC3-II can also be
quantitatively and qualitatively detected by western blotting
(19).
In our study, we observed the conversion in both
endogenous LC3 and GFP-LC3, and the ability of FTY720 to promote
autophagy was evaluated based on the proportion of cells that
formed obvious puncta. Likewise, what defines 'obvious' is also
subjective. Then, as a supplement, we performed western blotting to
detect two forms of LC3 expression. However, how to interpret LC3
western blot data is indeed a great controversy (29), our results showed an expression
level of LC3-I that was consistent with the LC3-II expression
level. The reason may be that the relationship between LC3-I and
LC3-II is not always 'precursor and product', because the
conversion of the former to the latter often depends on the cell
line, tissue and methods used to induce autophagy (30). As to 'methods used to induce
autophagy', here we used FTY720. Similarly, in ovarian cancer,
FTY720 also induced a pattern that LC3-I was consistent with LC3-II
(18). Moreover, at present, levels
of LC3-II normalized to actin, but not ratio between LC3-II and
LC3-I, is regarded as the standard to evaluate autophagy in western
blot analysis (31). The above
assays all provided compelling evidence for the participation of
autophagy in FTY720 cytotoxicity on CRC cells.
In apoptosis a cell commits suicide (32), yet autophagy can be cytotoxic or
cytoprotective in cancer (33,34).
In most cases, apoptosis and autophagy are mutually exclusive, and
in some cases the phenotype is mixed (35). Therefore, regarding the effect of
FTY720 on CRC, whether autophagy functions as a killer or a
promoter, or even whether autophagy and apoptosis have a
synergistic or antagonistic effect requires further investigation.
To solve this problem, we pretreated cells with 3-MA, an autophagy
inhibitor (36). Consequently, the
cytotoxicity of FTY720 was enhanced, indicating the protective role
of autophagy, and like in most cases, the interaction between
apoptosis and autophagy is antagonistic. Nevertheless, starkly
different from previous studies, FTY720 induced not only
caspase-dependent apoptosis, but also protective autophagy.
However, FTY720 only promotes either caspase-dependent apoptosis or
autophagy in ovarian cancer, breast cancer, and hepatocellular
carcinoma (18). As for the
protective role of autophagy in FTY720 cytotoxicity in CRC, it is
highly likely that autophagy can be an important factor for FTY720
resistance in CRC treatment. Therefore, eliminating the impact of
this aspect is essential, and we subsequently explored the
mechanisms that may induce autophagy in this process.
FTY720 is a PP2A-activating drug. In this regard, we
used CIP2A, an endogenous PP2A inhibitor, overexpressing it in all
tested CRC cell lines and most cancer tissues as compared with the
normal control. Moreover, FTY720 decreases CIP2A at protein level
(10). Furthermore, bortezomib,
another PP2A activator, induces autophagy through the
CIP2A-PP2A-Akt-4EBP1 signaling pathway in hepatocellular carcinoma
(11). Consequently, we assumed
that FTY720 induces autophagy by acting on CIP2A. As expected,
siRNA interference of CIP2A expression markedly increased the level
of LC3-II. However, without FTY720 treatment, the RNAi cells did
not exhibit LC3 level alteration as compared with the negative
control, suggesting CIP2A per se does not affect autophagy.
The participation of CIP2A in autophagy is established based on the
function of FTY720, in this process, CIP2A functions as an
autophagy inhibitor. Interestingly, CIP2A is frequently reported as
being overexpressed in CRC (37).
Moreover, it indicates poor prognosis and is closely related with
resistance to traditional chemotherapies (38,39).
Yet, as an inhibitor of protective autophagy, high CIP2A expression
can enhance CRC cell sensitivity to FTY720. This holds promise for
patients with CRC who have high CIP2A expression and low
sensitivity to traditional chemotherapies. With regard to these
patients, FTY720 can not only offer satisfactory curative effects,
but also sensitizes them to other chemotherapies by decreasing
CIP2A expression.
In conclusion, FTY720 has a significant cytotoxic
effect on CRC. However, protective autophagy is a risk factor for
FTY720 resistance. Yet, CIP2A, which is highly expressed and
closely related to drug resistance, can block FTY720-induced
autophagy so that it improves FTY720 sensitivity. This provides a
new strategy for treating CRC, especially in cases resistant to
conventional chemotherapies because of high CIP2A levels.
Acknowledgments
This study was supported by a grant from the Fund of
Department of Health of the Jiangsu Province, China.
Abbreviations:
|
PP2A
|
protein phosphatase 2A
|
|
CRC
|
colorectal cancer
|
|
LC3
|
light chain 3
|
|
3-MA
|
3-methyladenine
|
|
CIP2A
|
cancerous inhibitor of PP2A
|
|
PARP
|
poly (ADP-ribose) polymerase
|
|
RNAi
|
RNA interference
|
|
GFP
|
green fluorescent protein
|
|
siRNA
|
small interfering RNA
|
|
RT
|
reverse transcription
|
|
qPCR
|
quantitative PCR
|
|
CCK-8
|
Cell Counting Kit-8
|
|
FITC
|
fluroescein isothiocyanate
|
|
PBST
|
PBS containing 1% Tween-20
|
|
cDNA
|
complementary DNA
|
|
TBST
|
Tris-buffered saline solution
containing 0.1% Tween-20
|
|
IC50
|
The median inhibitory
concentrations
|
|
SD
|
standard deviation
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar
|
|
4
|
Fang YJ, Wu XJ, Zhao Q, Li LR, Lu ZH, Ding
PR, Zhang RX, Kong LH, Wang FL, Lin JZ, et al: Hospital-based
colorectal cancer survival trend of different tumor locations from
1960s to 2000s. PLoS One. 8:e735282013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmen Figueroa-Aldariz M,
Castañeda-Patlán MC, Santoyo-Ramos P, Zentella A and Robles-Flores
M: Protein phosphatase 2A is essential to maintain active Wnt
signaling and its Abeta tumor suppressor subunit is not expressed
in colon cancer cells. Mol Carcinog. 54:1430–1441. 2015. View Article : Google Scholar
|
|
6
|
Perrotti D and Neviani P: Protein
phosphatase 2A: A target for anticancer therapy. Lancet Oncol.
14:e229–e238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohama T: Targeting PP2A inhibitors as a
novel anti-cancer strategy] Nihon Yakurigaku Zasshi. 145:293–298.
2015.In Japanese.
|
|
8
|
Coles A: Newer therapies for multiple
sclerosis. Ann Indian Acad Neurol. 18(Suppl 1): S30–S34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao M, Liu Y, Xiao Y, Han G, Jia L, Wang
L, Lei T and Huang Y: Prolonging survival of corneal
transplantation by selective sphingosine-1-phosphate receptor 1
agonist. PLoS One. 9:e1056932014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin J, Zhang HJ, Wang XJ, Zhou WQ, Yin DL
and Chen XG: Effect of a novel selective S1P1 agonist, Syl948, on
mouse skin transplantation. Yao Xue Xue Bao. 49:627–631. 2014.In
Chinese. PubMed/NCBI
|
|
11
|
Brinkmann V: FTY720: Mechanism of action
and potential benefit in organ transplantation. Yonsei Med J.
45:991–997. 2004. View Article : Google Scholar
|
|
12
|
Kovarik JM, Schmouder R, Barilla D,
Riviere GJ, Wang Y and Hunt T: Multiple-dose FTY720: Tolerability,
pharmacokinetics, and lymphocyte responses in healthy subjects. J
Clin Pharmacol. 44:532–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rybaczek D, Musiałek MW and Balcerczyk A:
Caffeine-induced premature chromosome condensation results in the
apoptosis-like programmed cell death in root meristems of Vicia
faba. PLoS One. 10:e01423072015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Hoof C and Goris J: Phosphatases in
apoptosis: To be or not to be, PP2A is in the heart of the
question. Biochim Biophys Acta. 1640:97–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Z, Wang J, Zheng T, Liang Y, Yin D,
Song R, Pei T, Pan S, Jiang H and Liu L: FTY720 inhibits
proliferation and epithelial-mesenchymal transition in
cholangiocarcinoma by inactivating STAT3 signaling. BMC Cancer.
14:7832014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Guo Q, Chen J, Hu J, Wang S and
Sun Y: Role of long non-coding RNA HULC in cell proliferation,
apoptosis and tumor metastasis of gastric cancer: A clinical and in
vitro investigation. Oncol Rep. 31:358–364. 2014.
|
|
17
|
Alinari L, Baiocchi RA and Praetorius-Ibba
M: FTY720-induced blockage of autophagy enhances anticancer
efficacy of milatuzumab in mantle cell lymphoma: Is FTY720 the next
autophagy-blocking agent in lymphoma treatment? Autophagy.
8:416–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang N, Qi Y, Wadham C, Wang L, Warren A,
Di W and Xia P: FTY720 induces necrotic cell death and autophagy in
ovarian cancer cells: A protective role of autophagy. Autophagy.
6:1157–1167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kabeya Y, Mizushima N, Yamamoto A,
Oshitani-Okamoto S, Ohsumi Y and Yoshimori T: LC3, GABARAP and
GATE16 localize to autophagosomal membrane depending on form-II
formation. J Cell Sci. 117:2805–2812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cristóbal I, Manso R, Rincón R, Caramés C,
Senin C, Borrero A, Martínez-useros J, Rodriguez M, Zazo S,
Aguilera O, et al: PP2A inhibition is a common event in colorectal
cancer and its restoration using FTY720 shows promising therapeutic
potential. Mol Cancer Ther. 13:938–947. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu HC, Hou DR, Liu CY, Lin CS, Shiau CW,
Cheng AL and Chen KF: Cancerous inhibitor of protein phosphatase 2A
mediates bortezomib-induced autophagy in hepatocellular carcinoma
independent of proteasome. PLoS One. 8:e557052013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmed D, de Verdier PJ, Ryk C, Lunqe O,
Stål P and Flygare J: FTY720 (Fingolimod) sensitizes hepatocellular
carcinoma cells to sorafenib-mediated cytotoxicity. Pharmacol Res
Perspect. 3:e001712015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Estella-Hermoso de Mendoza A,
Castello-Cros R, Imbuluzqueta E, Cirauqui C, Pippa R, Odero MD and
Blanco-Prieto MJ: Lipid nanosystems enhance the bioavailability and
the therapeutic efficacy of FTY720 in acute myeloid leukemia. J
Biomed Nanotechnol. 11:691–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hait NC, Avni D, Yamada A, Nagahashi M,
Aoyagi T, Aoki H, Dumur CI, Zelenko Z, Gallagher EJ, Leroith D, et
al: The phosphorylated prodrug FTY720 is a histone deacetylase
inhibitor that reactivates ERα expression and enhances hormonal
therapy for breast cancer. Oncogenesis. 4:e1562015. View Article : Google Scholar
|
|
27
|
Cristóbal I, González-Alonso P, Daoud L,
Solano E, Torrejón B, Manso R, Madoz-gúrpide J, Rojo F and
García-Foncillas J: Activation of the tumor suppressor PP2A emerges
as a potential therapeutic strategy for treating prostate cancer.
Mar Drugs. 13:3276–3286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Q, Zhao X, Frissora F, Ma Y, Santhanam
R, Jarjoura D, Lehman A, Perrotti D, Chen CS, Dalton JT, et al:
FTY720 demonstrates promising preclinical activity for chronic
lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood.
111:275–284. 2008. View Article : Google Scholar
|
|
29
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barth S, Glick D and Macleod KF:
Autophagy: Assays and artifacts. J Pathol. 221:117–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al Nomenclature Committee on Cell Death
2009: Classification of cell death: Recommendations of the
Nomenclature Committee on Cell Death 2009. Cell Death Differ.
16:3–11. 2009. View Article : Google Scholar :
|
|
33
|
Gómez VE, Giovannetti E and Peters GJ:
Unraveling the complexity of autophagy: Potential therapeutic
applications in Pancreatic Ductal Adenocarcinoma. Semin Cancer
Biol. 35:11–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ozpolat B and Benbrook DM: Targeting
autophagy in cancer management - strategies and developments.
Cancer Manag Res. 7:291–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seglen PO and Gordon PB: 3-Methyladenine:
Specific inhibitor of autophagic/lysosomal protein degradation in
isolated rat hepatocytes. Proc Natl Acad Sci USA. 79:1889–1892.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Böckelman C, Koskensalo S, Hagström J,
Lundin M, Ristimäki A and Haglund C: CIP2A overexpression is
associated with c-Myc expression in colorectal cancer. Cancer Biol
Ther. 13:289–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Teng HW, Yang SH, Lin JK, Chen WS, Lin TC,
Jiang JK, Yen CC, Li AF, Chen PC, Lan YT, et al: CIP2A is a
predictor of poor prognosis in colon cancer. Journal of
gastrointestinal surgery. 16:1037–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding Y, Wang Y, Ju S, Wu X, Zhu W, Shi F
and Mao L: Role of CIP2A in the antitumor effect of bortezomib in
colon cancer. Mol Med Rep. 10:387–392. 2014.PubMed/NCBI
|
|
40
|
Zhang N, Wu ZM, Mcgowan E, Shi J, Hong ZB,
Ding CW, Xia P and Di W: Arsenic trioxide and cisplatin synergism
increase cytotoxicity in human ovarian cancer cells: therapeutic
potential for ovarian cancer. Cancer Sci. 100:2459–2464. 2009.
View Article : Google Scholar : PubMed/NCBI
|