Introduction
Lung cancer is a leading cause of cancer-related
death world-wide, and more than 85% of cases are non-small cell
lung cancers (NSCLCs) (1).
Adenocarcinoma is the most common pathological type of NSCLC.
Despite the availability of treatment, including surgery,
radiotherapy, chemotherapy and targeted therapy, the prognosis of
these patients is dismal, and the predicted 5-year survival rate of
advanced NSCLC is still less than 15% (2). Similar to other solid cancers,
metastatic spread of NSCLC is the primary cause of death for these
patients (3). Thus, elucidation of
the molecular mechanisms that control NSCLC metastasis is urgently
needed.
Several studies have demonstrated that the level of
reactive oxygen species (ROS) is increased in malignant tumors,
including cervical, pancreatic, breast and lung cancer (4–8). These
findings show that ROS play an important role in tumorigenesis,
progression and metastasis. Reduction of the ROS level exhibits
anticancer effects (9). However,
how lung adenocarcinoma cells control the generation of ROS in
order to mediate a malignant function remains unclear.
MicroRNAs (miRNAs) are a class of endogenous, small
(~22-nt) non-coding single-stranded RNA molecules that negatively
regulate the expression of their target genes. Growing evidence
indicates that aberrant expression of miRNAs plays an important
role in the development of lung cancer (10–15).
Recent studies have shown that deregulation of miR-99a is
frequently found in lung cancer (16–18).
Furthermore, Chen et al reported that miR-99a inhibited
proliferation, migration and invasion of non-small cell lung cancer
cells by targeting insulin-like growth factor 1 receptor (IGF-1R)
(19).
In the present study, we demonstrated that miR-99a
plays an important role in the regulation of cellular invasion and
migration by directly targeting NOX4 ex vivo and in
vivo. By targeting NOX4, overexpression of miR-99a decreased
the capabilities of cellular invasion and migration in a
ROS-dependent manner. Moreover, miR-99a directly interacts with the
3′UTR of NOX4 to repress NOX4 expression. These findings indicate
that NOX4 is a bona fide target of miR-99a and suggest that
overexpression of miR-99a could be a critical therapeutic strategy
for lung adenocarcinoma.
Materials and methods
Patients and tissue specimens
A total of 90 paired samples of human lung
adenocarcinoma and their matched adjacent non-cancerous tissues
were collected at the time of surgery between March 2007 and April
2013 at the Department of Surgery in Tianjin Medical University
Cancer Institute and Hospital. Matched normal tissues were obtained
2-cm distant from the tumor margin, and were further confirmed by
pathologists. Upon resection, all specimens were immediately frozen
in liquid nitrogen and stored at −80°C. All patients were initially
treated patients. The use of the tissue samples for all experiments
was approved by the Ethics Committee of Tianjin Medical University
Cancer Institute and Hospital.
Cell lines and cell culture
Human lung adenocarcinoma cell lines A549 and Calu3
were obtained from the China Academia Sinica Cell Repository
(Shanghai, China). The cells were cultured according to a routine
cultivation method (20).
Reagents, plasmids, oligonucleotides and
transfection
N-acetyl-L-cysteine (NAC) was purchased from
Sigma-Aldrich. Plasmid containing NOX4 lacking the 3′UTR was
purchased from GenePharma Co., Ltd. (Shanghai, China). The
pGL3-NOX4-3′UTR-Luc reporter was created by ligation of the
PCR-amplified 3′UTR of NOX4 into the EcoRI/EcoRV
sites of the modified pGL3 vector (Promega, Madison, WI, USA). The
primers for PCR amplification of the NOX4-3′UTR were: NOX4-3′UTR
forward, 5′-CGCCTCCCGGGTTTGCACCACTCTCCTGCCTCAGCCTCCTG-3′ and
reverse, 5′-ATCATTTTTATTGTCTCAAGAAGAACTTAATAGCAATTAG-3′. The
oligonucleotides for miR-99a and anti-miR-99a were purchased from
GenePharma Co., Ltd. The sequences were 2′-OMe-anti-miR-99a
(anti-miR-99a) 5′-CACAAGAUCGGAUCUACGGGUU-3′ and miR-99a mimics
(miR-99a), 5′-AACCCGUAGAUCCGAUCUUGUG-3′. miR-99a or anti-miR-99a
(200 pmol) was transfected using Lipofectamine 2000 (Invitrogen)
according to the manufacturer's protocol. Scrambled
oligonucleotides were used as control. The scrambled sequence was
5′-UUCUUCGAACGUGUCACGUTT-3′.
RNA isolation and quantitative real-time
polymerase chain reaction
As described in detail previously (21), by using TRIzol reagent (Invitrogen)
to isolate total RNA, total RNA (0.05 mg) was reverse-transcribed
using the TaqMan MicroRNA Reverse Transcription kit (Applied
Biosystems). Comparative real-time polymerase chain reaction (PCR)
was performed as previously reported (21). Normalization was performed by
simultaneous quantification of small endogenous nuclear RNAU6
(RNU6B), and relative expression was calculated by the comparative
CT method.
Invasion assay
As described in detail previously (22), the invasive potential of the
transfected cells was evaluated by measuring the number of cells
invading the Matrigel-coated Transwell chambers (Becton
Dickinson).
Wound-healing assay
Transfected cells were seeded into 6-well culture
plates. As described in detail previously (22), by using a sterile plastic
100-µl micropipette tip, an artificial homogenous wound was
created in the monolayer. Cells that migrated into the wounded area
or cells with extended protrusion from the border of the wound were
photographed under an inverted microscope (×40 magnification;
Olympus, Japan). Three areas were selected from each well at
random, and the cells in three wells of each group were quantified
in each experiment.
Measurement of the intracellular ROS
concentration
Intracellular ROS concentrations were quantified
with 2′,7′,-dichlorofluorescein diacetate (DCFH-DA). After
transfection, the culture medium was removed from the cells in a
96-well black plate, and 100 ml of 10 mM DCFH-DA in PBS was added.
After incubation at 37°C in 5% CO2 for 30 min, the cells
were washed with warmed PBS. Then, 100 ml of PBS was added into
each well. Fluorescence was measured on a microplate reader using
488 nm excitation and 525 nm emission wavelengths.
Luciferase reporter assay
Cells were cultured in 96-well plates and
co-transfected with pGL3-NOX4-3′UTR or pGL3-NOX4-3′UTR mutant
vector, and anti-miR-99a, miR-99a or scramble sequences. Following
a 72-h incubation, luciferase activity was measured using a
Dual-Luciferase reporter system (Promega).
Western blot analysis
The protocol for the western blot-ting was
previously described in detail (22). Membranes were incubated with primary
antibodies against NOX4, Akt, pAkt, MMP2, or MMP9 (1:1,000
dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed
by incubation with an HRP-conjugated secondary antibody (1:1,000
dilution; Zymed, USA). The membrane was stripped and reprobed with
a primary antibody against GAPDH or β-actin (1:1,000 dilution;
Santa Cruz Biotechnology).
Nude mouse tumor xenograft model
A549 cells were subcutaneously injected into
5-week-old female nude mice (Cancer Institute of The Chinese
Academy of Medical Science). The survival of the mice was monitored
every day. The animals received sterile food and water. As tumor
implantation was conducted into the right leg, all mice maintained
their health until the research was halted. When the tumor volume
reached 50 mm3, the mice were randomly divided into two
groups (8 mice/group). The two groups of mice were treated with
scrambled oligo or miR-99a by local injection of xenograft tumors
in multiple sites. The treatment was performed once every 3 days
for 15 days. The tumor volume was measured with a caliper every 2
days, using the formula: Volume = length × width2/2.
Twenty-eight days after initiation of the treatment, the mice were
sacrificed by CO2 inhalation followed by cervical
dislocation, and paraffin-embedded tissue sections were used for
immunohistochemistry. Animal experiments were approved by the
Ethics Committee of the Tianjin Huanhu Hospital.
Statistical analysis
Survival models were used to evaluate the clinical
parameters related to overall survival (OS). Survival curves were
estimated by the Kaplan-Meier method. Differences with a p-value
<0.01 were considered statistically significant. All statistical
analyses were performed using GraphPad Prism 5 software.
Results
Downregulation of miR-99a expression is
related with advanced stage and metastasis of lung
adenocarcinoma
Several studies have shown that miR-99a expression
is downregulated in various types of tumors (16–18,23–25).
We used RT-PCR to evaluate miR-99a expression in tumor and adjacent
normal tissues of lung adenocarcinoma patients. The
clinicopathological characteristics of the 90 lung adenocarcinoma
patients are summarized in Table I.
Among the total 90 lung adenocarcinoma patients, miR-99a was
downregulated in tumor tissues of 61 patients. We found a
statistically significant association between the expression of
miR-99a and lung adenocarcinoma clinical stage as well as
metastasis. Notably, lower levels of miR-99a expression were
associated with late-stage tumors (stage III and IV) compared with
early-stage tumors (stage I and II) (0.7154±0.0441 vs.
2.020±0.1795, respectively; P<0.0001) (Fig. 1A and Table I). In addition, lower levels of
miR-99a expression were associated with metastatic tumors compared
with the level in non-metastatic tumors (0.8042±0.0652 vs.
2.4617±0.1792, respectively; P<0. 0001) (Fig. 1B and Table I). By analyzing the clinical data
from all patients, overall survival of the patients in the high
miR-99a expression group was longer than that in the patients with
low miR-99a expression (84.49±3.195 vs. 19.01±0.7722, P<0.0001).
Kaplan-Meier analysis showed that downregulation of miR-99a
expression was associated with poor overall survival (P<0.0001)
(Fig. 1C). These data suggest that
downregulation of miR-99a expression is a frequent event and a poor
prognostic factor in human lung adenocarcinoma.
 | Table ImiR-99a expression and the
clinicopathological parameters of the 90 lung adenocarcinoma
patients. |
Table I
miR-99a expression and the
clinicopathological parameters of the 90 lung adenocarcinoma
patients.
| Patient
characteristics | No. of
patients | Expression of
miR-99a | P-value |
|---|
| Age (years) |
| ≥60 | 61 | 1.2397±0.1783 | 0.7921 |
| <60 | 29 | 1.1851±0.0987 | |
| Gender |
| Male | 51 | 1.0017±0.0872 | 0.2319 |
| Female | 39 | 1.4766±0.0927 | |
| TNM stage |
| Stage I+II | 31 | 2.0200±0.1795 | <0.0001 |
| Stage III+IV | 59 | 0.7154±0.0441 | |
| Metastasisa |
| No | 22 | 2.4617±0.1797 | <0.0001 |
| Yes | 68 | 0.8042±0.0652 | |
miR-99a inhibits proliferation, invasion
and migration of lung adenocarcinoma
Our clinical data demonstrated that miR-99a was
downregulated in the lung adenocarcinoma tissues. Next, we aimed to
ascertain the mechanism of how miR-99a regulates the lung
adenocarcinoma cell phenotype. Previous studies have reported that
miR-99a regulates cell cycle progression and cell proliferation by
targeting mTOR, AKT1 and FGFR3 (16,24,25).
However, its role in invasion and migration has not been fully
elucidated. To examine whether miR-99a affects lung adenocarcinoma
cell migration and invasion, we performed gain-of-function and
loss-of-function analyses by overexpressing or suppressing miR-99a
with the miR-99a mimic or anti-miR-99a (Fig. 2A). MTT assays showed that miR-99a
overexpression significantly inhibited the proliferation of lung
adenocarcinoma cells (Fig. 2B).
Interestingly, the Transwell assay revealed that overexpression of
miR-99a significantly decreased cell invasion potential of the A549
and Calu3 cells compared with the control cells. Moreover,
suppression of miR-99a increased cell invasion (Fig. 2C). Consistent with the Transwell
assay results, overexpression of miR-99a significantly inhibited
A549 and Calu3 cell migration, whereas suppression of miR-99a
increased cell migration as shown in the wound healing assays
(Fig. 2D). These findings indicate
that miR-99a plays an important role in the invasion and migration
of lung adenocarcinoma cells.
NOX4 is a target of miR-99a
To determine the mechanism involved in the
regulation of invasion and migration by miR-99a, we performed an
miRNA target search using TargetScan (www.targetscan.org) and identified a 'seed sequence'
of miR-99a that matches the 3′UTR of the NOX4 gene (Fig. 3A). To determine whether NOX4 is a
target gene of miR-99a, we generated a pGL3-NOX4-3′UTR luciferase
reporter vector and pGL3-NOX4-3′UTR mutant plasmid, in which the
seed sequence of miR-99a was mutated. We transfected the luciferase
vectors into miR-99a-knockdown or ectopically miR-99a-expressing
A549 cells. Luciferase results showed that knockdown of miR-99a
significantly enhanced the activity of the wild-type reporter
vector, whereas overexpression of miR-99a decreased its luciferase
activity (Fig. 3B). No effects were
observed with co-expression of the mutant reporter. Furthermore,
western blot analysis showed that NOX4 expression was upregulated
in the cells with suppressed miR-99a and downregulated in the cells
with overexpression of miR-99a compared with the controls (Fig. 3C). These data demonstrated that NOX4
is a target gene of miR-99a.
Overexpression of NOX4 overrides
miR-99a-mediated invasion and migration effects
Having demonstrated that NOX4 is a direct target
gene of miR-99a, we next examined the role of NOX4 in the
miR-99a-mediated lung adenocarcinoma cell invasion and migration.
We co-transfected miR-99a and NOX4 lacking the 3′UTR into the A549
or Calu3 cells. Western blotting determined that miR-99a decreased
NOX4 expression and NOX4 lacking the 3′UTR reversed NOX4 expression
after transfection of miR-99a (Fig.
4A). Transwell and wound healing assays showed that the cells
transfected with NOX4 lacking the 3′UTR significantly reversed cell
invasion and migration after transfection of miR-99a (Fig. 4B and C). Thus, these results
indicate that NOX4 is a bona fide target gene of miR-99a and
mediates miR-99a to regulate the invasion and migration of lung
adenocarcinoma cells.
miR-99a regulates lung adenocarcinoma
invasion and migration by targeting NOX4-mediated ROS
production
Furthermore, we explored the molecular mechanism of
miR-99a-mediated invasion and migration by targeting NOX4. NOX4 is
a member of the NADPH oxidases, and its primary function is to
produce superoxide or hydrogen peroxide, including ROS. Thus, we
evaluated ROS levels after overexpression or repression of miR-99a
by using DCFH-DA. The results showed that miR-99a significantly
inhibited ROS levels, whereas knockdown of miR-99a increased ROS
levels (Fig. 5A). Several studies
have shown that ROS mediate invasion and migration. Then, western
blot results showed that knockdown of miR-99a resulted in
significantly increased pAkt, MMP2 and MMP9, while overexpression
of miR-99a had the opposite effects (Fig. 5B). Previous studies have shown that
MMP2 and MMP9 mediate cell invasion and migration (26,27).
To further show that miR-99a modulates cell invasion and migration
dependent on ROS levels, the cells were treated with NAC as a ROS
scavenger (Fig. 5C). The results
showed that NAC significantly attenuated anti-miR-99a-induced cell
invasion and migration (Fig. 5D and
E). Western blotting showed that NAC also decreased
anti-miR-99a-induced pAkt, MMP2 and MMP9 expression (Fig. 5F). Thus, these data revealed that
miR-99a regulated invasion and migration by targeting the NOX4-ROS
pathway.
miR-99a inhibits lung adenocarcinoma
xenograft growth by targeting NOX4
Since miR-99a is frequently downregulated in lung
adenocarcinoma and plays an important role in cell survival, we
further examined the effects of the overexpression of miR-99a on
tumor growth. The results showed that overexpression of miR-99a
significantly inhibited tumor growth in vivo (Fig. 6A). RT-PCR showed increased
expression of miR-99a in the miR-99a treatment group (Fig. 6B). Immunohistochemical staining
analysis revealed that NOX4 levels were downregulated in the
miR-99a treatment group (Fig. 6C),
confirming the in vitro data of NOX4 as a direct target gene
of miR-99a. Additionally, pAkt, MMP2 and MMP9 expression levels
were decreased in sections from the xenograft tumors treated with
miR-99a. No change in Akt expression was observed (Fig. 6C). These findings further indicate
that miR-99a targets NOX4 and that miR-99a mimics could be a
therapeutic means for lung adenocarcinoma intervention.
Discussion
As well as in other solid cancers, metastatic spread
is the primary cause of death for NSCLC patients (3). Cancer metastasis is a complex
regulated process in which invasion and migration of cancer cells
play an important role in determining the capability of cancer
cells to metastasize. Aberrant expression of miRNAs in cancer cells
plays an important role in mediating cell invasion and migration by
targeting specific target genes (28,29).
In our study, we identified that miR-99a was significantly
downregulated in lung adenocarcinoma tissues compared with adjacent
normal tissues. Moreover, we found a statistically significant
association between downregulation of miR-99a and clinical late
stage as well as metastasis in lung adenocarcinoma patients.
Kaplan-Meier survival analyses showed that downregulation of
miR-99a was a poor prognostic factor for lung adenocarcinoma
patients. Next, we identified whether miR-99a regulated the
invasion and migration of lung adenocarcinoma. Transwell and wound
healing assays showed that miR-99a negatively regulated invasion
and migration of lung adenocarcinoma cells. MTT assay confirmed
that miR-99a significantly inhibited the proliferation of lung
adenocarcinoma cells. Taken together, miR-99a regulated
proliferation, invasion and migration of lung adenocarcinoma cells.
Downregulation of miR-99a may act as an independent poor prognostic
factor for lung adenocarcinoma patients.
To determine the mechanism by which miR-99a
regulates invasion and migration in lung adenocarcinoma cells, we
performed an miRNA target search using TargetScan and found the
'seed sequence' of miR-99a that matched with the 3′UTR of the NOX4
gene, which was found to regulate the invasion and migration of
NSCLC cells (30). Luciferase
reporter assay and western blotting showed that NOX4 is a target
gene of miR-99a. Furthermore, by co-transfection of miR-99a and
NOX4 lacking its 3′UTR, we identified that NOX4 is a direct target
gene of miR-99a and modulated miR-99a-mediated invasion and
migration of lung adenocarcinoma cells by targeting NOX4.
How does miR-99a regulate the invasion and migration
of lung adenocarcinoma cells by targeting NOX4? Some studies have
shown that NOX4 regulates invasion and migration of cells by
producing ROS (31,32). Thus, we used DCFH-DA to quantify
intercellular ROS. The results showed that miR-99a significantly
decreased the production of ROS. In addition, repression of miR-99a
significantly increased production of ROS. Zha et al
reported that ROS activates the PI3K/Akt pathway (33). In our study, by western blotting, we
demonstrated that miR-99a downregulated the NOX4/ROS/Akt pathway to
inhibit MMP2 and MMP9 expression. MMPs are a family of
zinc-dependent endopeptidases that degrade all extracellular matrix
components, including collagens, fibronectin, laminin and basement
membrane proteoglycans. Among the MMPs, much attention has focused
on MMP2 and MMP9 in NSCLC (34).
MMPs are key proteases modulating invasion and migration of NSCLC.
In our study, overexpression of miR-99a downregulated expression of
MMP2 and MMP9, and vice versa. In a mouse subcutaneous xenograft
model, we identified that miR-99a inhibited the growth of lung
adenocarcinoma cells. Moreover, miR-99a downregulated NOX4, pAkt,
MMP2 and MMP9 expression. These results were the same as that in
vivo. Taken together, our data demonstrated that miR-99a
inhibited the invasion and migration of lung adenocarcinoma cells
by targeting the NOX4-mediated ROS/Akt pathway.
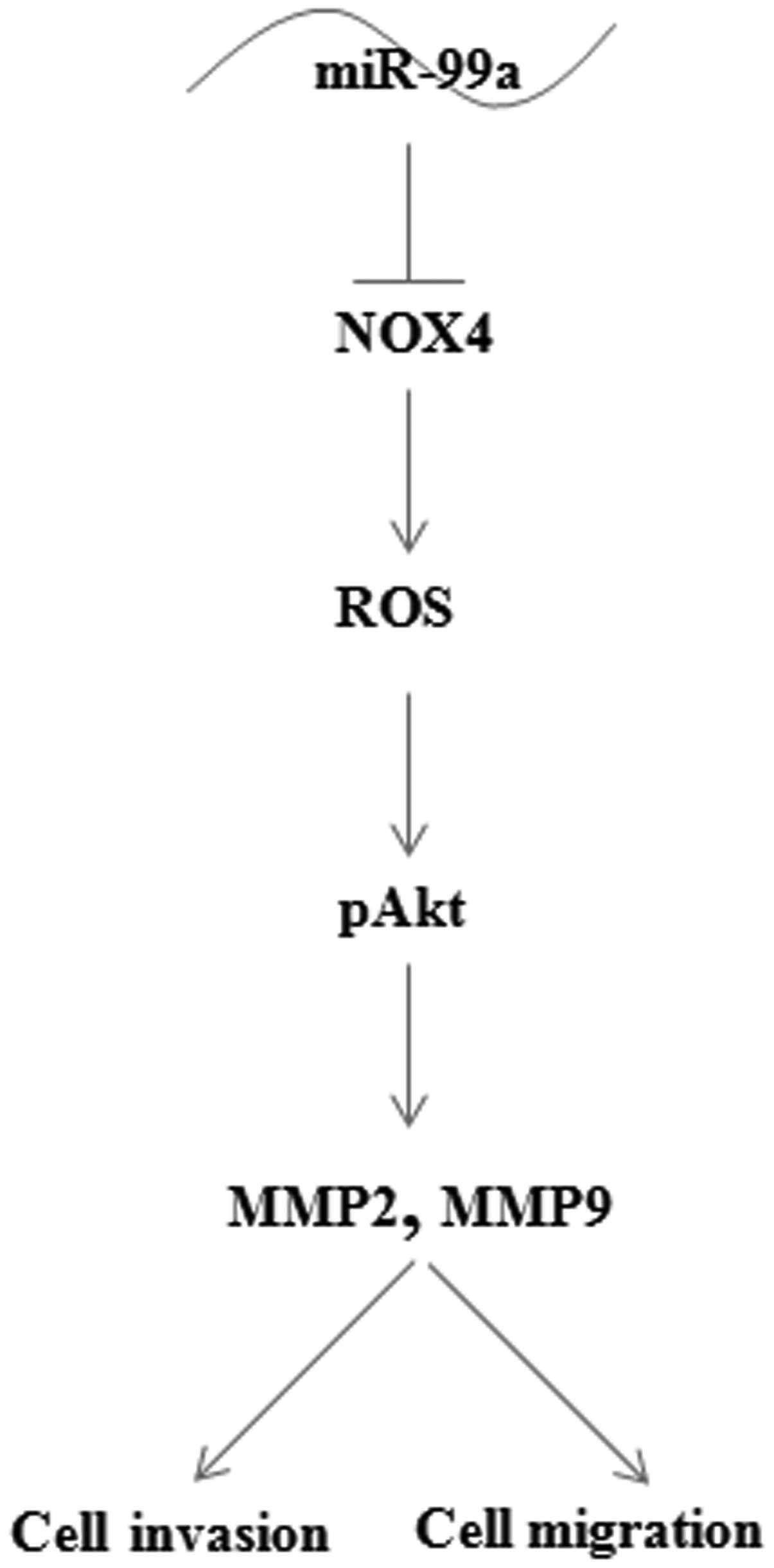
In summary, our data demonstrated that miR-99a was
frequently downregulated in lung adenocarcinoma. As Fig. 7 shows, downregulation of miR-99a
resulted in an increase in expression of its target gene NOX4. NOX4
increased ROS production to activated Akt, MMP2 and MMP9 expression
which mediated the invasion and migration of lung adenocarcinoma
cells. Thus, our data suggest that overexpression of miR-99a is a
potential therapeutic strategy for preventing lung adenocarcinoma
cell invasion and migration.
Acknowledgments
This study was supported by the China National
Natural Scientific Fund (81372518, to P. W.), Tianjin Science and
Technology Committee (13JCYBJC21700, to C. Z.), and by Tianjin
Health Bureau (12KG113, to C. Z.).
References
|
1
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou X, Li D, Wang X, Zhang B, Zhu H and
Zhao J: Galectin-1 is overexpressed in CD133+ human lung
adenocarcinoma cells and promotes their growth and invasiveness.
Oncotarget. 6:3111–3122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goyette MA and Côté JF: NSCLC metastasis:
Going with ELMO3. Oncotarget. 5:5850–5851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Filippova M, Filippov V, Williams VM,
Zhang K, Kokoza A, Bashkirova S and Duerksen-Hughes P: Cellular
levels of oxidative stress affect the response of cervical cancer
cells to chemotherapeutic agents. Biomed Res Int. 2014:5746592014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki S, Okada M, Shibuya K, Seino M,
Sato A, Takeda H, Seino S, Yoshioka T and Kitanaka C: JNK
suppression of chemotherapeutic agents-induced ROS confers
chemoresistance on pancreatic cancer stem cells. Oncotarget.
6:458–470. 2015.
|
|
6
|
Khojastehfar A, Aghaei M, Gharagozloo M
and Panjehpour M: Cadmium induces reactive oxygen species-dependent
apoptosis in MCF-7 human breast cancer cell line. Toxicol Mech
Methods. 25:48–55. 2015. View Article : Google Scholar
|
|
7
|
Huang H, Shah K, Bradbury NA, Li C and
White C: Mcl-1 promotes lung cancer cell migration by directly
interacting with VDAC to increase mitochondrial Ca2+
uptake and reactive oxygen species generation. Cell Death Dis.
5:e14822014. View Article : Google Scholar
|
|
8
|
Joh HM, Choi JY, Kim SJ, Chung TH and Kang
TH: Effect of additive oxygen gas on cellular response of lung
cancer cells induced by atmospheric pressure helium plasma jet. Sci
Rep. 4:66382014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung KH, Lee JH, Thien Quach CH, Paik JY,
Oh H, Park JW, Lee EJ, Moon SH and Lee KH: Resveratrol suppresses
cancer cell glucose uptake by targeting reactive oxygen
species-mediated hypoxia-inducible factor-1α activation. J Nucl
Med. 54:2161–2167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fortunato O, Boeri M, Verri C, Moro M and
Sozzi G: Therapeutic use of microRNAs in lung cancer. Biomed Res
Int. 2014:7569752014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L and Jin H: MicroRNAs as novel
biomarkers in the diagnosis of non-small cell lung cancer: A
meta-analysis based on 20 studies. Tumour Biol. 35:9119–9129. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortinovis D, Monica V, Pietrantonio F,
Ceresoli GL, La Spina CM and Wannesson L: MicroRNAs in non-small
cell lung cancer: Current status and future therapeutic promises.
Curr Pharm Des. 20:3982–3990. 2014. View Article : Google Scholar
|
|
13
|
Del Vescovo V, Grasso M, Barbareschi M and
Denti MA: MicroRNAs as lung cancer biomarkers. World J Clin Oncol.
5:604–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fortunato O, Boeri M, Verri C, Conte D,
Mensah M, Suatoni P, Pastorino U and Sozzi G: Assessment of
circulating microRNAs in plasma of lung cancer patients. Molecules.
19:3038–3054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Y, Gao F, Ma JL, Sun WZ and Song LP:
The potential clinical applications and prospects of microRNAs in
lung cancer. Onco Targets Ther. 7:901–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu SH, Zhang CL, Dong FS and Zhang YM:
miR-99a suppresses the metastasis of human non-small cell lung
cancer cells by targeting AKT1 signaling pathway. J Cell Biochem.
116:268–276. 2015. View Article : Google Scholar
|
|
17
|
Wu D, Zhou Y, Pan H, Zhou J, Fan Y and Qu
P: microRNA-99a inhibiting cell proliferation, migration and
invasion by targeting fibroblast growth factor receptor 3 in
bladder cancer. Oncol Lett. 7:1219–1224. 2014.PubMed/NCBI
|
|
18
|
Gu W, Fang S, Gao L, Tan Y and Yang Z:
Clinic significance of microRNA-99a expression in human lung
adenocarcinoma. J Surg Oncol. 108:248–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Zhao Z, Liu Y and Mu D:
microRNA-99a is downregulated and promotes proliferation, migration
and invasion in non-small cell lung cancer A549 and H1299 cells.
Oncol Lett. 9:1128–1134. 2015.PubMed/NCBI
|
|
20
|
Zhang C, Kang C, Wang P, Cao Y, Lv Z, Yu
S, Wang G, Zhang A, Jia Z, Han L, et al: MicroRNA-221 and -222
regulate radiation sensitivity by targeting the PTEN pathway. Int J
Radiat Oncol Biol Phys. 80:240–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F,
Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z and Chun-Sheng
K: MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell
proliferation and radioresistance by targeting PTEN. BMC Cancer.
10:3672010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang C, Zhang J, Hao J, Shi Z, Wang Y,
Han L, Yu S, You Y, Jiang T, Wang J, et al: High level of
miR-221/222 confers increased cell invasion and poor prognosis in
glioma. J Transl Med. 10:1192012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song Y, Dou H, Wang P, Zhao S, Wang T,
Gong W, Zhao J, Li E, Tan R and Hou Y: A novel small-molecule
compound diaporine A inhibits non-small cell lung cancer growth by
regulating miR-99a/mTOR signaling. Cancer Biol Ther. 15:1423–1430.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Han Y, Cheng K, Zhang G and Wang
X: miR-99a directly targets the mTOR signalling pathway in breast
cancer side population cells. Cell Prolif. 47:587–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang H, Qu L, Wang Y, Cong J, Wang W and
Yang X: miR-99a promotes proliferation targeting FGFR3 in human
epithelial ovarian cancer cells. Biomed Pharmacother. 68:163–169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang
L, Zhang H, Chen X, Yang Y and Liu G: miRNA-29c suppresses lung
cancer cell adhesion to extracellular matrix and metastasis by
targeting integrin β1 and matrix metalloproteinase2 (MMP2). PLoS
One. 8:e701922013. View Article : Google Scholar
|
|
27
|
Chen PM, Wu TC, Shieh SH, Wu YH, Li MC,
Sheu GT, Cheng YW, Chen CY and Lee H: MnSOD promotes tumor invasion
via upregulation of FoxM1-MMP2 axis and related with poor survival
and relapse in lung adenocarcinomas. Mol Cancer Res. 11:261–271.
2013. View Article : Google Scholar
|
|
28
|
Kogo R, How C, Chaudary N, Bruce J, Shi W,
Hill RP, Zahedi P, Yip KW and Liu FF: The microRNA-218~Survivin
axis regulates migration, invasion, and lymph node metastasis in
cervical cancer. Oncotarget. 6:1090–1100. 2015. View Article : Google Scholar :
|
|
29
|
Zhang Y, Zhao FJ, Chen LL, Wang LQ, Nephew
KP, Wu YL and Zhang S: miR-373 targeting of the Rab22a oncogene
suppresses tumor invasion and metastasis in ovarian cancer.
Oncotarget. 5:12291–12303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang C, Lan T, Hou J, Li J, Fang R, Yang
Z, Zhang M, Liu J and Liu B: NOX4 promotes non-small cell lung
cancer cell proliferation and metastasis through positive feedback
regulation of PI3K/Akt signaling. Oncotarget. 5:4392–4405. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Han N, Yin T, Huang L, Liu S, Liu D,
Xie C and Zhang M: Lentivirus-mediated Nox4 shRNA invasion and
angiogenesis and enhances radiosensitivity in human glioblastoma.
Oxid Med Cell Longev. 2014:5817322014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boudreau HE, Casterline BW, Rada B,
Korzeniowska A and Leto TL: Nox4 involvement in TGF-beta and
SMAD3-driven induction of the epithelial-to-mesenchymal transition
and migration of breast epithelial cells. Free Radic Biol Med.
53:1489–1499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zha L, Chen J, Sun S, Mao L, Chu X, Deng
H, Cai J, Li X, Liu Z and Cao W: Soyasaponins can blunt
inflammation by inhibiting the reactive oxygen species-mediated
activation of PI3K/Akt/NF-κB pathway. PLoS One. 9:e1076552014.
View Article : Google Scholar
|
|
34
|
Lee KR, Lee JS, Song JE, Ha SJ and Hong
EK: Inonotus obliquus-derived polysaccharide inhibits the migration
and invasion of human non-small cell lung carcinoma cells via
suppression of MMP-2 and MMP-9. Int J Oncol. 45:2533–2540.
2014.PubMed/NCBI
|