Introduction
The Ras-ERK signaling pathway mediates numerous
cellular functions such as cell proliferation, differentiation,
transformation and survival in different tissues and cell types
(1–4). Epidermal growth factor
(EGF)-stimulated Ras activation is mediated by the adaptor protein
Grb2, which can associate with activated EGFR directly via Y1068
and Y1086 sites, or indirectly through tyrosine phosphorylated Shc
(5,6). Through binding to Grb2, the protein
Sos, a guanine nucleotide exchange factor (GEF) of Ras, relocates
to EGFR at the plasma membrane and activates membrane-associated
Ras, which in turn activates the subsequent Raf-MEK-ERK signaling
cascade (7,8). Finally, ERK translocates to the
nucleus and activates several transcriptional factors such as c-Fos
and c-Jun, driving cell proliferation and other processes (9,10).
In eukaryotes, the standard cell cycle which is
regulated by a series of molecular events is often divided into two
periods: the interphase period and mitosis. At the cellular
interphase, signaling from the Ras-ERK pathway facilitates
progression through G1/S phase and through processes involved in
nuclear transcription factor phosphorylation, immediate-early gene
induction, expression of cell cycle genes that direct DNA synthesis
and regulation of translational initiation (11,12).
Nevertheless, one of the marked changes in mitosis is general
inhibition of membrane traffic. Accumulated studies have shown that
membrane traffic is inhibited during mitosis and mitotic cells
likely fail to respond to transmembrane signaling (13–17).
The events during mitosis possibly preserve the high energy
requirements needed for the dynamic structural changes that are
occurring at this time of the cell cycle.
Previous research has found that some signaling
pathways such as ERK signaling, are affected in mitosis (18,19).
Mitotic cells are less responsive to extracellular growth factor
stimulation as compared with interphase cells. Due to reduced EGF
and inhibition of EGF receptor dimerization, EGFR activity by EGF
is reduced in mitosis (20,21) and in turn affects the ERK signal
transduction pathway. In addition, some Ras-ERK pathway proteins
may undergo unique regulation during mitosis. Phosphorylated MEK-1
regulates partial proteolysis at the N terminus in mitosis, which
results in the inability for MEK-1 to interact with and activate
ERK1/2 proteins. However, it is unlikely that MEK1 is completely
uncoupled from ERK1/2 during mitosis since activation of protein
kinase C by treatment with phorbol esters can still activate the
Raf-1/MEK/ERK pathway in mitotic cells (22). Notably, Raf-1 was activated in cells
arrested in mitosis with nocodazole (23). Although the research demonstrated
that Raf-1 activity is related to nocodazole stimulation, the
regulatory mechanism is unclear at present, and the Raf function in
mitosis remains unknown. As a Raf upstream protein, Ras activity in
mitosis has not been investigated in detail.
The present study aimed to investigate the Ras-ERK
signaling pathway in mitotic COS7 cells. Our findings indicate that
the activities of Ras-ERK pathway proteins are almost blocked in
mitosis, except for Raf protein. In addition, the ability of
Grb2/Shc binding to activate EGFR was reduced. Thus, the inhibition
of the Ras-ERK pathway in mitotic COS7 cells may be the dual
results of the difficulty in the the transduction of EGF signaling
by EGFR or Raf to downstream proteins.
Materials and methods
Cell culture
COS7 cells were grown at 37°C in Dulbecco's modified
Eagle's medium (DMEM) containing 5% fetal bovine serum (FBS),
penicillin and streptomycin (100 U/ml) and were maintained in a 5%
CO2 atmosphere.
Antibodies and chemicals
Antibodies specific for phospho-ERK1/2 (E-4),
tubulin, phospho-MEK1/2 (ser218/ser222), pY1068-EGFR, pY1086-EGFR,
Grb2 (C-23), Shc (PG-797), GST (B-14) and phospho-Raf (Tyr340/341)
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
An antibody specific for Ras was purchased from Upstate
Biotechnology Inc. (Lake Placid, NY, USA). HRP-conjugated secondary
antibodies were purchased from Bio-Rad (Hercules, CA, USA).
Nocodazole and AG1478 were purchased from Calbiochem (La Jolla, CA,
USA). EGF and phorbol 12-myristate 13-acetate (PMA) were purchased
from Sigma (St. Louis, MO, USA). Unless otherwise specified, all of
the chemicals were purchased from Sigma.
Cell treatment
To detect protein activity in mitosis, COS7 cells
were treated with 200 ng/ml nocodazole for 24 h, and then
stimulated with or without 50 ng/ml EGF or 1 µM PMA for the
indicated time (5, 15 and 30 min).
To further investigate Raf activity in response to
EGF, COS7 cells were pretreated with 0.5 µmol AG1478 for 30
min, and then stimulated with or without 50 ng/ml EGF for 15 min in
the continuous presence of the inhibitor.
Fluorescence microscopy
COS7 cells grown on glass cover-slips were treated
with or without 200 ng/ml nocodazole for 24 h and then treated with
50 ng/ml Texas Red (TR)-EGF for the indicated time (5, 15 and 30
min). After that, COS7 cells were fixed with icy methanol for 10
min followed by analysis and imaging with a Zeiss Axiovert 200
microscope (Carl Zeiss, Thornwood, NY, USA) and an AttoArc2 HBO
100W light source (Atto Instruments, Rockville, MD, USA).
Immunoprecipitation
COS7 cells were lysed with immuno-precipitation
buffer overnight at 4°C. COS7 cell lysates were then centrifuged at
21,000 × g for 30 min to remove debris. The supernatants,
containing 1 mg of total protein, were incubated with 1 µg
of mouse anti-EGFR antibody to immunoprecipitate EGFR from the COS7
cells. For the control experiments, primary antibodies were
replaced with normal mouse or sheep IgG (Sigma), and no EGFR was
precipitated by normal IgG.
Ras activation assay
Ras activation was assayed by a method described by
Herrmann et al (24).
Briefly, COS7 cells which had been treated as required were lysed
and scraped into 0.5 ml of BOS buffer [50 mM Tris-HCl (pH 7.4), 200
mM NaCl, 1% NP-40, 10% glycerol, 10 mM NaF, 2.5 mM
MgCl2, 1 mM EDTA), and then centrifuged at 21,000 × g
and 4°C for 30 min. Glutathione S-transferase (GST) fused to
the Raf binding domain (GST-RBD), pre-coupled to
glutathione-agarose beads in BOS buffer, was added, and the lysates
were incubated at 4°C for 1 h. The beads were collected by
centrifugation and washed three times with BOS buffer, and then
loading buffer was added. Ras was detected with the monoclonal
anti-Ras antibody, followed by a horseradish peroxidase
(HRP)-coupled anti-mouse antibody.
Immunoblotting
For the detection of phospho-EGFR, phospho-Ras,
phospho-Raf, phospho-MEK, phospho-ERK, clathrin and caveolin in
total lysates of COS7 cells, aliquots containing 20 µg of
protein from each cell lysate were used. For the detection of EGFR,
Shc and Grb2 in the anti-EGFR immunoprecipitates, 1/10 of the
immunoprecipitate from each lysate was used. Protein samples were
separated by electrophoresis through sodium dodecyl sulfate 10–7.5%
polyacrylamide-containing gels and electrophoretically transferred
onto nitrocellulose filter paper. Filters were then probed with the
respective primary antibody. The primary antibodies were detected
with a polyclonal goat anti-rabbit IgG coupled to HRP or a
polyclonal goat anti-mouse IgG coupled to HRP followed by enhanced
chemiluminescence development (Pierce Chemical, Rockford, IL, USA)
and light detection with Bioshine Chemi Q 2550.
Statistical analysis
In all cases, the data from three independent
experiments were evaluated. The data of the western blot analyses
are expressed as means ± SE of triplicate measurements. All data
were analyzed with the software package SPSS 19.0. Significance was
declared at P<0.05.
Results
Endocytosis of EGF-EGFR during
mitosis
It has been shown that EGF induces dimerization of
EGF receptor protein in intact cells (25,26).
After dimerization of EGFR, they form complexes and the latter in
turn are endocytosed. In the present study, we used TR-EGF to treat
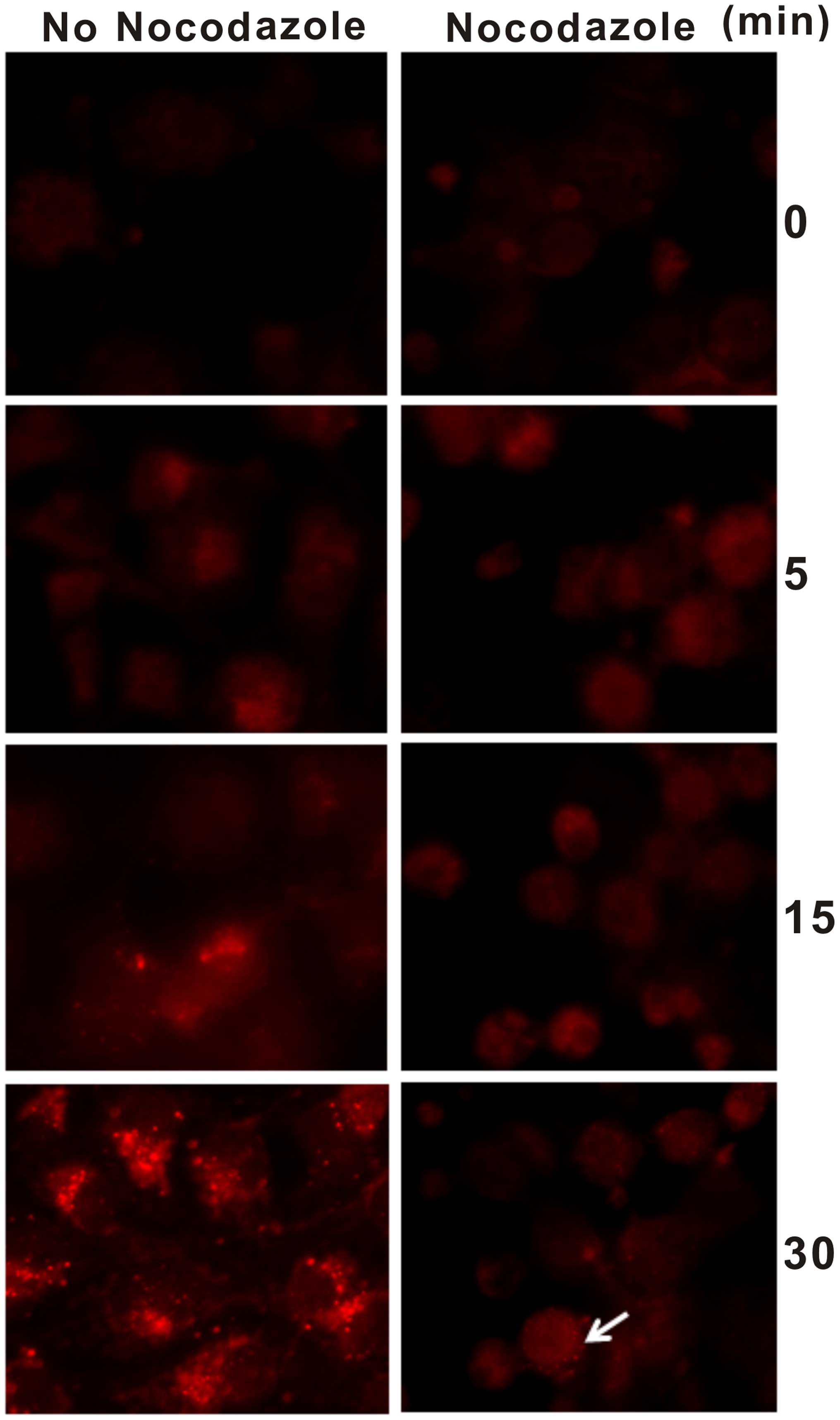
COS7 cells in response to nocodazole and observed the endosomes in
the cytoplasm. We found that the endosomes in the cytoplasm were
observed after 5 min of TR-EGF stimulation and more with extension
of time in the nocodazole-untreated COS7 cells. Nevertheless, only
a few endosomes were detected after 15 min of TR-EGF stimulation in
the nocodazole-treated COS7 cells and others were blocked in the
plasma membrane (Fig. 1). In
addition, the endosomes in the nocodazole-untreated COS7 cells were
obviously more than that in the nocodazole-treated COS7 cells.
Receptor-mediated endocytosis consists of two
pathways, clathrin-dependent and clathrin-independent endocytosis.
The two pathways are detected by analyzing marker proteins,
clathrin and caveolin. We found that clathrin expression levels
were almost not upregulated after mitotic COS7 cells were
stimulated for 30 min by EGF, although the levels of clathrin in
asynchronous COS7 cells were significantly increased in the
presence of EGF (Fig. 2A). Caveolin
expression levels in both the mitotic COS7 and asynchronous COS7
cells were unaffected compared with these levels in the control
(Fig. 2B).
EGFR signaling during mitosis
EGFR activation is inhibited during mitosis
(21,22). Inhibition of EGFR activity may be
beneficial for preventing the activation of signal transduction
pathways that promote gene expression to preserve energy needs
which are required for mitotic structural changes. After EGFR was
stimulated, five important residues (including Y992, Y1048, Y1068,
Y1086 and Y1173) were previously found to be phosphorylated; and
two residues of which, Y1068 and Y1086, are directly related with
Grb2/Shc (5,6). Thus, we detected the two
phosphorylation sites. The 1068 and 1086 sites in mitosis were
obviously phosphorylated and moreover, their phosphorylation levels
were higher than those in the asynchronous COS7 cells (Fig. 3A and B). After EGFR phosphorylation,
activated EGFR directly or indirectly recruits various signaling
proteins, such as Shc and Grb2 to initiate the signal transduction
pathways. Our investigation certified that the expression levels of
Grb2 and Shc in the mitotic COS7 cells were unaffected compared
with levels in the asynchronous COS7 cells (Fig. 4A–a). However, Grb2 and Shc hardly
bound to EGFR in the mitotic COS7 cells (Fig. 4A–b), which directly blocked the
downstream protein Ras activity. Since activated Ras mediates
numerous cellular functions in different tissues and cell types
(1–4), we employed the pull-down assay to
detect Ras activity. The results demonstrated that the
phosphorylation level of Ras in the mitotic COS7 cells was lower
than that in the asynchronous COS7 cells in response to EGF
(Fig. 4B), demonstrating the
downregulation of Ras activity.
Raf phosphorylation during mitosis
Raf is a downstream protein of Ras. Earlier studies
that suggested a potential involvement of ERK in mitotic events
reported that Raf-1 was phosphorylated in cells arrested in mitosis
with nocodazole (23). In the
present study, we investigated Raf phosphorylation. The results
demonstrated that Raf phosphorylation by EGF was increased in the
mitotic COS7 and asynchronous COS7 cells (Fig. 5A). Except for Ras signaling, PKC
activity by PMA can also activate Raf (27). In the present study, we found that
PMA increased the phosphorylation levels of Raf in the mitotic COS7
cells (Fig. 5B), particularly a
significant difference was noted in the PMA-treated mitotic COS7
cells for 5 min compared with the PMA-untreated mitotic COS7 cells
(P<0.05). After EGFR activity was inhibited by AG1478, a potent
and specific inhibitor of EGF receptor tyrosine kinase activity,
EGF-induced phosphorylation of Raf-1 in the asynchronous and
mitotic COS7 cells was significantly blocked (Fig. 5C). These results suggest that Raf
phosphorylation is closely associated with EGFR signaling.
Inhibition of MEK and ERK activity during
mitosis
Activated Raf phosphorylates and activates MEK and
the latter in turn activates ERK. Due to the changes in signaling
proteins in the Ras signaling pathway, the expression levels of
downstream proteins are possibly affected. To explore the
mechanisms involved in Ras signaling in mitosis, the activation of
MEK and ERK in mitosis and interphase was compared. As expected,
the phosphorylation of MEK and ERK in the mitotic COS7 cells in
response to EGF was almost completely inhibited; the
phosphorylation levels of which were less than that in the
asynchronous COS7 cells in the presence of EGF (Fig. 6A and B).
Discussion
In the present study, we described reduced endosomes
of EGF-EGFR during mitosis. The activities of Ras, MEK and ERK by
EGF were blocked in mitosis. In addition, we found that the Raf
activity in nocodazole-treated COS7 cells was inhibited by AG1478.
Grb2 and Shc hardly bound activated EGFR in mitosis. The above
results suggest that the molecular mechanism of the Ras-ERK pathway
is inhibited in mitotic COS7 cells.
Previous findings indicated that the majority of
EGFR without EGF stimulation is concentrated in caveolae via some
localization signals (28–31). In response to EGF stimulation, EGFR
quickly exits from caveolae/rafts and undergoes signal transduction
and endocytosis (29). In the
present study, after COS7 cells were treated for 15 min by TR-EGF,
many endosomes were found in the asynchronous COS7 cells, which
suggests that COS7 cells permit the endocytosis of EGF-EGFR
complexes and do not quickly degrade these endosomes since
internalized EGF and EGFR complexes are targeted to lysosomes for
degrading them (32). However, we
found that there were few endosomes in the nocodazole-treated COS7
cells, which is possibly related to the physiology of mitotic
cells. In mitosis, the membrane traffic is inhibited and EGFR are
not dimerized (20), blocking the
endocytosis of EGF-EGFR complexes. Another possible reason is that
endosomes in mitotic cells are rapidly degraded. To further analyze
the endocytosis of EGF-EGFR complexes, we detected clathrin and
caveolin which are two important proteins involved in the
endocytosis pathway. Generally, the plasma membrane receptor
tyrosine kinases (RTKs) are endocytosed through clathrin-dependent
and clathrin-independent pathways. We found that clathrin
expression in the mitotic COS7 cells was inhibited whereas caveolin
expression in mitosis was no different compared with that in
asynchronous COS7 cells, demonstrating that the clathrin-dependent
pathway is blocked in mitosis (33). Previous research demonstrated that
EGFR endocytosed by the clathrin-independent/caveolin pathway is
targeted to lysosomes while the EGFR endocytosed by the
clathrin-dependent pathway is possibly associated with increased
signaling (34). Therefore,
inhibition of the clathrin-dependent pathway in mitosis is
beneficial to mitotic COS7 cells which will preserve the high
energy requirements needed for the dynamic structural changes that
are occurring at this time of the cell cycle.
Accumulated evidence suggests that EGFR kinase
activation causes auto-phosphorylation of several tyrosine residues
within the C-terminal domain. These phosphorylated residues serve
as docking sites to recruit downstream signaling proteins and
adaptor/accessory proteins containing SH2 or PTB domains (35,36).
EGF-stimulated Ras activation is subjected to two phosphorylated
residues such as Y1068 and Y1086 sites (5,6). We
investigated that Y1068 and Y1086 residues were obviously
phosphorylated in mitosis. It is shown that these residues are
unaffected by the inhibition of endocytosis in mitosis. Due to the
important roles of the two sites to the Ras-ERK pathway, their
activation possibly initiates the Ras-ERK pathway.
Grb2 is an adaptor protein that contains one SH2 and
two SH3 domains. Grb2 SH2 domain binds to RTKs either directly or
indirectly through another adaptor protein, Shc. Grb2 SH3 domains
interact with son-of sevenless (Sos). Recruitment of Grb2/Sos to
the plasma membrane results in the activation of Ras and the
subsequent activation of the Raf-MEK-ERK signaling pathway
(7,8). After COS7 cells were collected, we
analyzed the interaction of Grb2 and Shc with activated EGFR. The
results demonstrated that Grb2 and Shc in the mitotic COS7 cells
could not powerfully bind to EGFR compared with that in the
asynchronous COS7 cells. It is indicated that EGFR signal
transduction possibly is blocked. Due to Grb2 and/or Shc unbinding
activated EGFR, Grb2 could not carry Sos to reach the plasma
membrane, inhibiting Ras phosphorylation. Then, our results
demonstrated that Ras activation was blocked in mitosis, enclosing
the Ras-ERK signaling pathway.
Next, Raf, an important downstream protein of Ras,
was detected. Raf is composed of three conservation regions, CR1,
CR2 and CR3 (37). The initial
process of Raf activation involves the interaction of active
GTP-bound Ras with the Ras binding domain (RBD) and the cysteine
rich domain of CR1, and subsequent recruitment of Raf to the
membrane for further activation (37,38). A
previous study demonstrated that Raf in nocodazole-treated COS7
cells could be activated (39).
Since Ras in mitosis was inhibited, why was Raf still activated? To
answer the question, we collected the mitotic COS7 cells and
detected Raf phosphorylation. Treatment with EGF activated Raf in
mitosis, and PMA also increased the phosphorylation level of Raf in
mitosis. These results suggest that Raf is possibly affected by
more than one signaling pathway. Except for Ras, there are other
signaling proteins affecting Raf activity in mitotic cells
(27). Therefore, although Ras is
inhibited in mitosis, Raf possibly is activated by other signaling
proteins. Yet, Raf activity is greatly related to EGF stimulation.
To confirm the event, we employed AG1478, a special inhibitor of
EGFR activity, to treat COS7 cells. The results demonstrated that
Raf phosphorylation was blocked by AG1478 in the asynchronous and
mitotic COS7 cells, which supports our hypothesis. However, the
function of Raf activity in mitosis needs to be further
studied.
Activated Raf phosphorylates and activates
mitogen-activated protein kinase kinase (MEK) (40–42).
MEK in turn phosphorylates ERK via phosphorylation of a Thr-Glu-Tyr
motif in the activation loop (43,44).
Since Raf in mitosis is activated and MEK/ERK are its downstream
proteins, we collected and analyzed the phosphorylation of MEK/ERK.
Our finding demonstrated that MEK/ERK phosphorylation by EGF in
mitosis was inhibited compared with that in the asynchronous COS7
cells. These results suggest that activated Raf in mitosis possibly
loses the ability to stimulate downstream signaling proteins,
consistent with the results of Laird et al (45).
In conclusion, the alteration of EGFR endocytosis in
mitotic COS7 cells affects cell signaling. The activities of
signaling proteins including Ras, MEK and ERK were significantly
decreased in the mitotic COS7 cells. Due to the difficulty in the
transduction of EGF signaling by EGFR or Raf to downstream
proteins, Ras-ERK pathway in mitotic COS7 cells is blocked in dual
pressures of signal transduction inhibition.
Acknowledgments
The present study was supported by the ‛National
Natural Science Foundation of China (31272409)' and the ‛Science
Foundation of Shaanxi Province of China (2013KTZB02-02-03)'.
References
|
1
|
Cha DS, Datla US, Hollis SE, Kimble J and
Lee MH: The Ras-ERK MAPK regulatory network controls
dedifferen-tiation in Caenorhabditis elegans germline. Biochim
Biophys Acta. 1823:1847–1855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi C and Helfman DM: The Ras-ERK pathway
modulates cytoskeleton organization, cell motility and lung
metastasis signature genes in MDA-MB-231 LM2. Oncogene.
33:3668–3676. 2014. View Article : Google Scholar
|
|
3
|
Nishida Y: Function of Raf/MAP kinase
cascade in the regulation of cellular proliferation and
differentiation. Tanpakushitsu Kakusan Koso. 41(Suppl 12):
S1673–S1679. 1996.
|
|
4
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batzer AG, Rotin D, Ureña JM, Skolnik EY
and Schlessinger J: Hierarchy of binding sites for Grb2 and Shc on
the epidermal growth factor receptor. Mol Cell Biol. 14:5192–5201.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaoka T, Langlois WJ, Leitner JW,
Draznin B and Olefsky JM: The signaling pathway coupling epidermal
growth factor receptors to activation of p21ras. J Biol
Chem. 269:32621–32625. 1994.PubMed/NCBI
|
|
7
|
Kolch W: Meaningful relationships: The
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351:289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higashi N, Kunimoto H, Kaneko S, Sasaki T,
Ishii M, Kojima H and Nakajima K: Cytoplasmic c-Fos induced by the
YXXQ-derived STAT3 signal requires the co-operative MEK/ERK signal
for its nuclear translocation. Genes Cells. 9:233–242. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manimala NJ, Frost CD, Lane ML, Higuera M,
Beg R and Vesely DL: Cardiac hormones target nuclear oncogenes
c-Fos and c-Jun in carcinoma cells. Eur J Clin Invest.
43:1156–1162. 2013.PubMed/NCBI
|
|
11
|
Lavoie JN, L'Allemain G, Brunet A, Müller
R and Pouysségur J: Cyclin D1 expression is regulated positively by
the p42/p44MAPK and negatively by the
p38/HOGMAPK pathway. J Biol Chem. 271:20608–20616. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weber JD, Raben DM, Phillips PJ and
Baldassare JJ: Sustained activation of
extracellular-signal-regulated kinase 1 (ERK1) is required for the
continued expression of cyclin D1 in G1 phase. Biochem J.
326:61–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berlin RD, Oliver JM and Walter RJ:
Surface functions during Mitosis I: Phagocytosis, pinocytosis and
mobility of surface-bound Con A. Cell. 15:327–341. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berlin RD and Oliver JM: Surface functions
during mitosis. II. Quantitation of pinocytosis and kinetic
characterization of the mitotic cycle with a new fluorescence
technique. J Cell Biol. 85:660–671. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kreiner T and Moore HP: Membrane traffic
between secretory compartments is differentially affected during
mitosis. Cell Regul. 1:415–424. 1990.PubMed/NCBI
|
|
16
|
Sager PR, Brown PA and Berlin RD: Analysis
of transferrin recycling in mitotic and interphase HeLa cells by
quantitative fluorescence microscopy. Cell. 39:275–282. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Warren G, Featherstone C, Griffiths G and
Burke B: Newly synthesized G protein of vesicular stomatitis virus
is not transported to the cell surface during mitosis. J Cell Biol.
97:1623–1628. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayne C, Xiang X and Luo Z: MEK inhibition
and phosphorylation of serine 4 on B23 are two coincident events in
mitosis. Biochem Biophys Res Commun. 321:675–680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Margadant C, Cremers L, Sonnenberg A and
Boonstra J: MAPK uncouples cell cycle progression from cell
spreading and cyto-skeletal organization in cycling cells. Cell Mol
Life Sci. 70:293–307. 2013. View Article : Google Scholar :
|
|
20
|
Kiyokawa N, Lee EK, Karunagaran D, Lin SY
and Hung MC: Mitosis-specific negative regulation of epidermal
growth factor receptor, triggered by a decrease in ligand binding
and dimerization, can be overcome by overexpression of receptor. J
Biol Chem. 272:18656–18665. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newberry EP and Pike LJ:
Cell-cycle-dependent modulation of EGF-receptor-mediated signaling.
Biochem Biophys Res Commun. 208:253–259. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klein S, Kaszkin M, Barth H and Kinzel V:
Signal transduction through epidermal growth factor receptor is
altered in HeLa monolayer cells during mitosis. Biochem J.
322:937–946. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laird AD, Taylor SJ, Oberst M and
Shalloway D: Raf-1 is activated during mitosis. J Biol Chem.
270:26742–26745. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herrmann C, Martin GA and Wittinghofer A:
Quantitative analysis of the complex between p21ras and
the Ras-binding domain of the human Raf-1 protein kinase. J Biol
Chem. 270:2901–2905. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cochet C, Kashles O, Chambaz EM, Borrello
I, King CR and Schlessinger J: Demonstration of epidermal growth
factor-induced receptor dimerization in living cells using a
chemical covalent cross-linking agent. J Biol Chem. 263:3290–3295.
1988.PubMed/NCBI
|
|
26
|
Gamett DC, Pearson G, Cerione RA and
Friedberg I: Secondary dimerization between members of the
epidermal growth factor receptor family. J Biol Chem.
272:12052–12056. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hyde R, Corkins ME, Somers GA and Hart AC:
PKC-1 acts with the ERK MAPK signaling pathway to regulate
Caenorhabditis elegans mechanosensory response. Genes Brain Behav.
10:286–298. 2011. View Article : Google Scholar
|
|
28
|
Couet J, Sargiacomo M and Lisanti MP:
Interaction of a receptor tyrosine kinase, EGF-R, with caveolins.
Caveolin binding negatively regulates tyrosine and serine/threonine
kinase activities. J Biol Chem. 272:30429–30438. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mineo C, Gill GN and Anderson RG:
Regulated migration of epidermal growth factor receptor from
caveolae. J Biol Chem. 274:30636–30643. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smart EJ, Ying YS, Mineo C and Anderson
RG: A detergent-free method for purifying caveolae membrane from
tissue culture cells. Proc Natl Acad Sci USA. 92:10104–10108. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamabhai M and Anderson RG: Second
cysteine-rich region of epidermal growth factor receptor contains
targeting information for caveolae/rafts. J Biol Chem.
277:24843–24846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carpenter G: Receptors for epidermal
growth factor and other polypeptide mitogens. Annu Rev Biochem.
56:881–914. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fielding AB, Willox AK, Okeke E and Royle
SJ: Clathrin-mediated endocytosis is inhibited during mitosis. Proc
Natl Acad Sci USA. 109:6572–6577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aguilar RC and Wendland B: Endocytosis of
membrane receptors: Two pathways are better than one. Proc Natl
Acad Sci USA. 102:2679–2680. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jorissen RN, Walker F, Pouliot N, Garrett
TP, Ward CW and Burgess AW: Epidermal growth factor receptor:
Mechanisms of activation and signalling. Exp Cell Res. 284:31–53.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pawson T: Specificity in signal
transduction: From phosphot-yrosine-SH2 domain interactions to
complex cellular systems. Cell. 116:191–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morrison DK and Cutler RE Jr: The
complexity of Raf-1 regulation. Curr Opin Cell Biol. 9:174–179.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vojtek AB, Hollenberg SM and Cooper JA:
Mammalian Ras interacts directly with the serine/threonine kinase
Raf. Cell. 74:205–214. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dangi S and Shapiro P: Cdc2-mediated
inhibition of epidermal growth factor activation of the
extracellular signal-regulated kinase pathway during mitosis. J
Biol Chem. 280:24524–24531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dent P, Haser W, Haystead TA, Vincent LA,
Roberts TM and Sturgill TW: Activation of mitogen-activated protein
kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science.
257:1404–1407. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Howe LR, Leevers SJ, Gómez N, Nakielny S,
Cohen P and Marshall CJ: Activation of the MAP kinase pathway by
the protein kinase raf. Cell. 71:335–342. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kyriakis JM, App H, Zhang XF, Banerjee P,
Brautigan DL, Rapp UR and Avruch J: Raf-1 activates MAP
kinase-kinase. Nature. 358:417–421. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lange-Carter CA, Pleiman CM, Gardner AM,
Blumer KJ and Johnson GL: A divergence in the MAP kinase regulatory
network defined by MEK kinase and Raf. Science. 260:315–319. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moodie SA, Willumsen BM, Weber MJ and
Wolfman A: Complexes of Ras. GTP with Raf-1 and mitogen-activated
protein kinase kinase. Science. 260:1658–1661. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laird AD, Morrison DK and Shalloway D:
Characterization of Raf-1 activation in mitosis. J Biol Chem.
274:4430–4439. 1999. View Article : Google Scholar : PubMed/NCBI
|