Introduction
Breast cancer is a common primary tumor type that
can metastasize to the brain (1).
Triple-negative (TN) breast cancer is especially aggressive, and
approximately 6% of patients with TN breast cancer develop brain
metastases (2,3). The prognosis of patients with TN brain
metastases is a dismal 3 months.
Recent studies demonstrate a beneficial relationship
between β-adrenergic receptor antagonists and breast cancer
recurrence and metastases (4–6).
Clinical data suggest that select β2-receptor antagonist intake by
patients for cardiac indications was associated with higher
recurrence-free and overall survival in the TN breast cancer
subgroup (4,5). In select studies, no significant
difference was observed in patients with estrogen receptor-positive
(ER+) breast cancers or those who took β1-adrenergic
receptor antagonist (4,5). Similarly, select β-receptor
antagonists reduced formation of tumor recurrence and distant
metastases in breast cancer patients (6).
Neurotransmitters have been implicated in the
increasing metastatic potential of brain metastatic cells (7,8).
Modern forms of Paget's 'seed and soil' hypothesis suggest that
cells that carry or evolve adaptations to new microenvironments
will successfully metastasize (9).
One potential advantage for brain metastastic cells is the
expression of functional neurotransmitter receptors, such as the
β-adrenergic receptors. Epinephrine and norepinephrine bind to
β-adrenergic receptors (10). As
early as 1989, there was evidence suggesting a relevant role for
β-adrenergic receptors in tumor progression, where administration
of isoproterenol (β-adrenergic receptor agonist) to lung tumor
cells increased cell proliferation, an effect abrogated by
propranolol (β-adrenergic receptor antagonist) (11).
A standard treatment for patients with breast cancer
is surgical tumor resection. Stress and the corresponding increased
β-adrenergic receptor activation from surgeries increased the tumor
growth and metastasis, in in vivo mouse experiments which
were abrogated by propranolol (12,13).
Stress signaling can be activated by norepinephrine. Norepinephrine
administration to cancer cells can increase migration and invasion,
two characteristics of metastasis, in addition to endothelium
adhesion (14,15). It has been suggested that
perioperative β-blockade may play a role in reducing cancer
recurrence and metastases (16),
which has been demonstrated in animal models (17). Propranolol inhibits
norepinephrine-induced invasion and migration of cancer cells
(18,19). The possible benefit of β-adrenergic
blockade in human breast cancer patients during perioperative
surgery-induced stress has not yet been investigated.
We performed a retrospective cohort study to test
for an association between perioperative β-blocker use and
postoperative breast cancer recurrence and metastases.
Subsequently, we utilized the established MDA-MB-231 (231) primary
TN breast cancer cells and its brain-trophic derivative,
MDA-MB-231Br (231Br), in in vitro and in vivo studies
to investigate the effects of β2-adrenergic receptor agonists and
antagonists on the metastatic potential of TN breast cancer
cells.
Materials and methods
Bioinformatic review and analysis
After IRB approval, female breast cancer patients
diagnosed with stage II or III primary breast cancer who underwent
mastectomy or segmentectomy at the City of Hope between the years
2000 and 2010 were identified through the City of Hope's Cancer
Registry. A total of 1,029 subjects were included in the study. To
determine the subjects taking perioperative β-blockers, a
large-scale string-parsing query across electronic records of
physician dictations and pharmacy data was conducted. Anesthesia
records were neither computerized nor included in this study. All
brand and generic names of β-blockers not specifically intended for
glaucoma treatment and available in the United States were included
in the study. Patients were considered to have been on
perioperative β-blockers if the computational query of medical
records, limited to 45 days from the patient's date of surgery,
indicated β-blocker use. The time to recurrence was the primary
endpoint during the data collection. Recurrence-free survival was
measured from the date of surgery to the first recurrence (local or
metastasis), death from oncological cause, or the date of the last
follow-up, whichever occurred first. Kaplan-Meier estimates and Cox
regression were performed to determine the effect that the
perioperative administration of β-blockers had on cancer recurrence
in our cohort.
Cell culture
The MDA-MB-231 (231) cell line, its brain-trophic
derivative MDA-MB-231Br (231Br), a low passage TN brain metastasis
cell line COH-BBM3 (BBM3), primary Her2+ breast cancer
SkBr3 cell line, and low passage Her2-amplified brain metastasis
cell lines COH-BBM1 (BBM1), COH-BBM2 (BBM2) were cultured in
Dulbecco's modified Eagle's medium (DMEM)-F12 media (Life
Technologies) supplemented with 10% fetal bovine serum (FBS;
Hyclone), 1% glutamax and 1% antibiotic-antimycotic (both from Life
Technologies) (7).
Immunohistochemistry of paraffin-embedded
tissue
Patient breast-to-brain metastasis (BBM) tissue
specimens were obtained with IRB approval, formalin-fixed, and
embedded. Paraffin blocks were sectioned onto slides (10 µm)
and baked for 3 h. Wax was removed from the patient BBM tissue
specimens and primary breast cancer array slides (US Biomax) by
xylene, and the tissue was hydrated with a gradient of ethanol
washes and PBS wash. For antigen retrieval, slides were boiled in
10 mM sodium-citrate buffer [Tris sodium citrate (J.T. Baker), pH
6.0] for 1 h. Slides were permeabilized in 0.3% Tween-20
(Sigma-Aldrich) at 37°C for 45 min and blocked with 1% bovine serum
albumin (BSA; Sigma-Aldrich), 10% normal goat serum (NGS;
Invitrogen), and 0.3 M glycine (Fisher Scientific) at room
temperature for 1 h. Primary antibodies in a primary carrier of
1.5% NGS and 1% BSA in PBS were applied overnight at 4°C.
Fluorescent secondary antibodies (Jackson ImmunoResearch
Laboratories) were applied the following day for 1 h at room
temperature protected from light. Coverslips (Warner Instruments)
were placed on slides mounted with Immunogold with DAPI (Life
Technologies). Slides were imaged on a Carl Zeiss LSM confocal
microscope. Antibodies used were the following: ADRB1 (Cell
Signaling Technology) and ADRB2 (Thermo Scientific).
qRT-PCR
mRNA was extracted from 1.0×106 231,
231Br, BBM1, BBM2, BBM3 or SkBr3 cells using an mRNA extraction kit
(Qiagen). qPCR was performed on a Neurotransmitters and Receptors
qPCR Array plate (SaBiosciences). Data for ADRB2 in the
metastasis cell lines were quantified relative to their primary
breast cancer subtype counterparts after normalization to actin,
i.e., BBM3 and 231Br were compared to 231, and BBM1 and BBM2 were
compared to SkBr3.
Western blot analysis
Cells were trypsinized and then centrifuged at 1,500
rpm for 5 min. Collected cells were homogenized by lysis buffer
[glycerol, 3 M KCl and 10% NP-40 (all from Sigma-Aldrich), 1 M Tris
(Bio-Rad) and 1 M DTT (Sigma-Aldrich)] in the presence of
phosphatase inhibitor, EDTA, and protease inhibitor (all from
Thermo Scientific) for 15 min. The tubes were spun down at 12,000
rpm for 10 min and the protein in the supernatant was collected.
Forty micrograms of protein boiled for 5 min with loading buffer
[95% laemmli, and 5% β-mercaptoethanol (both from Bio-Rad)] were
loaded onto Mini-PROTEAN TGX gels (Bio-Rad). The gel was run at 200
V and transferred onto a membrane at 100 V for 1 h. After
confirming protein transfer on the membrane with Ponceau staining,
the membrane was blocked for 1 h with blocking buffer (Thermo
Scientific). Primary antibody diluted in blocking buffer was
applied overnight at 4°C. The following day, horseradish peroxidase
(HRP)-conjugated secondary antibody (Thermo Scientific) was applied
for 1 h at room temperature. Membranes were exposed to SuperSignal
West Pico Chemiluminescent Substrate (Thermo Scientific) for 5 min.
Membranes were scanned by Li-COR c-DiGit membrane scanner (Li-COR).
Antibodies used were as follows: ADRB1 (Cell Signaling Technology),
ADRB2 (Thermo Scientific) and β-actin (Cell Signaling
Technology).
Cell proliferation
231 or 231Br cells (3.0×103/well) were
seeded on 24-well plates. After 24 h, they were treated with one of
the following treatments (day 0): propranolol (33.3 µM),
terbutaline sulfate (16.67 µM), or a combination of
propranolol and terbutaline sulfate. Cells were counted daily for 5
days.
Migration assays
231 or 231Br cells (7.0×104/well) were
plated into a migration assay plate (Ibidi). Twenty-four hours
after plating, a silicon separator was removed, mimicking scratch
conditions, and one of the following treatments was applied:
propranolol (100 µM), terbutaline sulfate (50 µM), or
a combination of propranolol and terbutaline sulfate. Tiled images
(2×5) were captured at 0 and 9 h under bright field microscopy and
phase contrast microscopy at a ×5 magnification. Quantification at
9 h of migration was carried out through ImageJ relative to time 0
under bright field microscopic images. Representative qualitative
images of the quantified areas were displayed by phase contrast
images.
Invasion assays
Matrigel (3 mg/ml) was coated onto 0.8-µm
invasion inserts (Millipore), which were placed each in a well of a
24-well plate in a 37°C incubator and allowed to solidify for 1 h.
Cells (1.5×105) were placed in the insert in serum-free
media. Serum-free media with or without drugs were placed in the
well in the following concentrations: propranolol (100 µM),
terbutaline sulfate (50 µM), or a combination of propranolol
and terbutaline sulfate. After 24 h, the inserts were washed in
PBS, cells were fixed with 4% paraformaldehyde for 10 min at room
temperature and stained with crystal violet (0.2% in 25% methanol)
for 15 min at room temperature. Remaining cells and Matrigel were
swabbed out with Q-tips. Five sections on each insert were imaged
by bright-field at a ×5 magnification under a Zeiss Observer Z1
Live Cell microscope and quantified. Calculations were carried out
relative to the invasion of the control cells.
In vivo animal models
231Br cells (5.0×104) labeled with
firefly-luciferase remained untreated or were pre-treated with
propranolol (33.3 µM) for 12 h. Cells were injected into
NOD-SCID mice in the intracardial space under IACUC approval (City
of Hope IACUC #10044). Tumors were monitored weekly by
bioluminescence imaging (BLI). Upon euthanasia, the brains were
dissected, fixed in formalin, and sectioned onto slides in
10-µm sections for hematoxylin and eosin staining to confirm
tumor location. Images were acquired and tiled at ×5
magnification.
Results
Patients receiving perioperative
β-blockers have a lower rate of postoperative cancer recurrence and
metastases
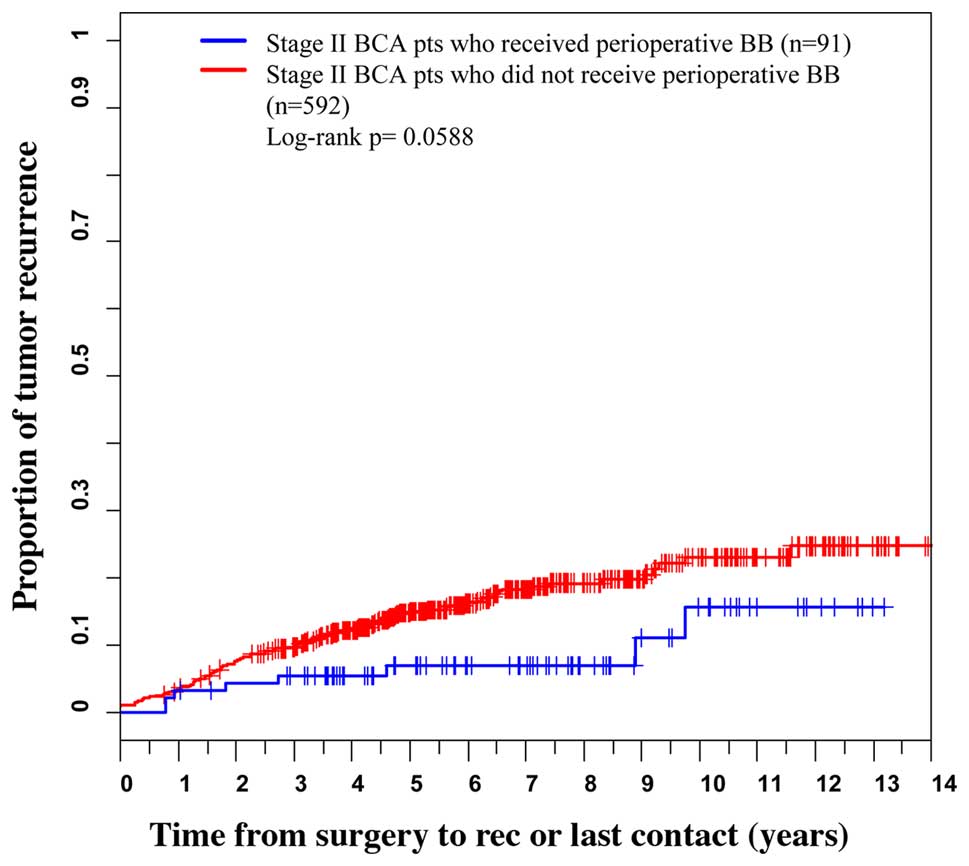
The median age (range) of the patients at diagnosis
was 53 years (21–92). Sixty-eight percent of our subjects were
stage II patients and 91 of these subjects received perioperative
β-blockers. We found that for stage II patients who received
perioperative β-blockers the hazard ratio for recurrence and
metastases calculated with the Cox regression was 0.51. (95% CI:
0.23–0.97; p=0.041). The log-rank test for this stage II subgroup
did not achieve statistical significance at the α=0.05 level
(p=0.0588). The Kaplan-Meier curves appear in Fig. 1. There was no statistical difference
in post-operative recurrence associated with β-blocker usage for
stage III breast cancer patients. In addition, there was no
difference in overall survival with β-blocker use for either
group.
Triple-negative primary breast cancer and
brain metastatic tissues express ADRB2
We then investigated the effects of β-adrenergic
receptor activation and blockade on both primary breast cancer and
brain metastatic cells. Since β1- and β2-adrenergic receptor
subtypes are frequently targeted by β-blockers, we initially
analyzed expression of these subtypes in formalin-fixed patient
brain metastatic specimens. ADRB1 expression was low in the
Her2+ and TN brain metastatic tissues (Fig. 2). There was low β2-adrenergic
receptor (ADRB2) expression in the Her2+ brain
metastatic tumors compared to the primary Her2+ breast
tumors and consistently high β2-adrenergic receptor expression in
TN primary and brain metastatic tumors (Fig. 3A). For this reason, we decided to
focus our experiments on the β2-adrenergic receptor in the TN tumor
subtype. For the purposes of our study, we utilized the TN primary
breast cancer cell line 231 and its brain-derivative 231Br. The
high expression of β2-adrenergic receptor protein (ADRB2) in TN
primary and metastatic cells was observed by western blot analyses
(Fig. 3B). Finally, to confirm
decreased ADRB2 in Her2 brain metastases and increased ADRB2
in TN brain metastases compared to their primary breast cancer
subtypes, we analyzed ADRB2 expression at the mRNA level
with qRT-PCR. We analyzed 2 separate Her2+ brain
metastasis cell lines (COH-BBM1, and COH-BBM2) with an
Her2+ primary breast cancer cell line (SkBr3) and 2
separate TN brain metastatic cell lines (COH-BBM3 and 231Br) with a
TN primary breast cancer cell line (231). Her2+ brain
metastatic cells expressed a 16-fold decrease in ADRB2
compared to primary Her2+ cells and TN brain metastatic
cells with a 2-fold increase compared to primary TN cells (Fig. 3C).
β2-adrenergic receptor agonists promote
proliferation of brain metastatic cells
We investigated the effects of β2-adrenergic
receptor activation on characteristics of metastasis, namely
proliferation, migration and invasion (20,21).
Studies have shown that terbutaline sulfate, a β2-adrenergic
receptor agonist, increases tumor weights similar to those under
stress (22,23). Hence we used terbutaline sulfate to
mimic stress conditions in vitro. Clinical data suggest a β1
to β2 ratio activity of propranolol to be 1:2, and its clinical
effects may be duration-dependent (5). Propranolol also antagonized
noradrenaline-induced β2-adrenergic receptor activation 2 to 3
times more than β1-adrenergic receptors (24), thus we utilized this commonly used
antagonist to inhibit β2-adrenergic receptors.
We performed a proliferation assay over a 5-day
period. Terbutaline sulfate significantly increased proliferation
in brain metastatic cells (p<0.0001). Propranolol decreased the
rate of cell proliferation compared to the control cells and also
eliminated the effects of terbutaline sulfate when co-administered
to cells in vitro (p<0.0001) (Fig. 4A). 231 cells were not sensitive to
terbutaline sulfate. They were, however, sensitive to propranolol
and displayed decreased cell proliferation when compared to the
control cells (p<0.0001), including when combined with
terbutaline sulfate (Fig. 4B).
β2-adrenergic receptor agonists promote
migration of brain metastatic cells
We proceeded to observe another characteristic of
metastasis, migration. After creating a scratch in the plate, we
imaged the scratch after 9 h. At the concentrations utilized for
this migration assay, we observed significant migration
(p<0.0001) in 231Br cells treated with terbutaline sulfate.
Approximately 50% of the scratch was filled after 9 h with
terbutaline sulfate, and propranolol lessened these effects to ~80%
compared to the control migration (p<0.01) (Fig. 5A). At 9 h, there was no significant
difference in migration between the control and the
propranolol-treated 231Br cells, as expected from data in a
previous study (25). In 231 cells,
no drug treatments at the utilized concentration made any
significant difference in the migration into the scratch (Fig. 5B).
Propranolol decreases β2 agonist-promoted
invasion in brain metastatic cells
The effects of the β2-adrenergic receptor agonists
and antagonists on the third characteristic of metastasis,
invasion, was analyzed through an invasion assay. Cells were placed
on an invasion insert coated with Matrigel and allowed to invade
for 24 h. There was a significant difference (p<0.05) in
invasion between 231Br cells treated with propranolol and 231Br
cells treated with terbutaline sulfate (Fig. 6A). There was also a significant
decrease in 231Br cell invasion following treatment with
propranolol + terbutaline sulfate (p<0.05) compared to cells
treated with terbutaline sulfate. In the primary 231 breast cancer
cells, there was no significant difference in cells treated with
propranolol and/or terbutaline sulfate compared to the control
cells at the concentrations used for 24 h (Fig. 6B).
Effects of propranolol on brain
metastasis development in vivo
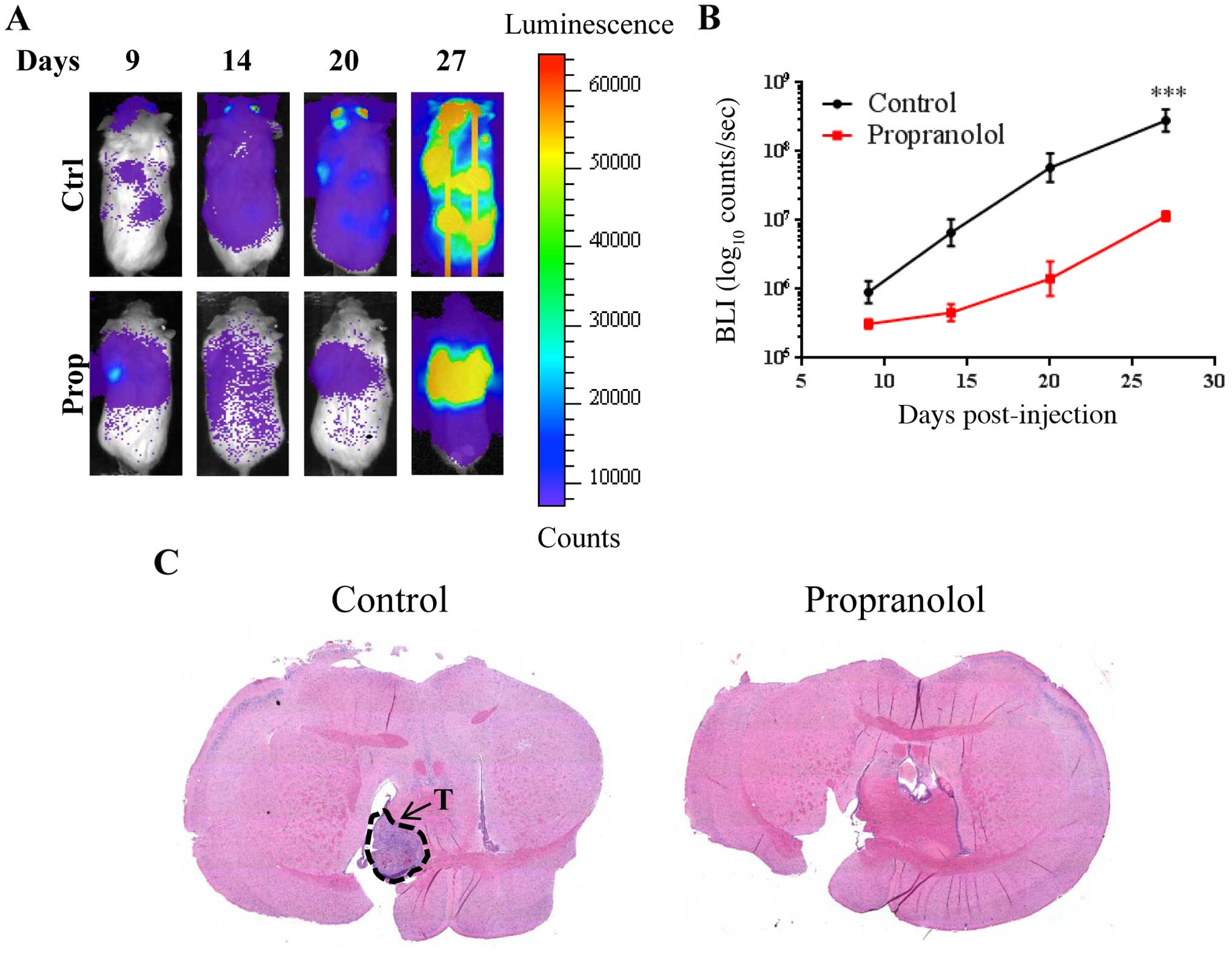
Finally, we investigated the effects of propranolol
in vivo. 231Br cells labeled with firefly luciferase
(231Br-FF) with or without a 12-h non-cytotoxic pre-treatment of
propranolol were injected into female NOD/SCID mice and monitored
for brain metastases by bioluminescence imaging (BLI). 231Br cells
pretreated with propranolol established brain metastasis at
significantly decreased rates (p<0.001) compared to the control
cells (Fig. 7A and B). By day 27,
the BLI showed increased brain metastases in the control cells when
compared with the cells pre-treated with propranolol. In fact, the
cells pre-treated with propranolol had higher qualitative BLI
intensity in the lung, indicating increased lung metastasis. Tumor
formation in the brain for mice injected with control 231Br-FF
cells was confirmed by staining with hematoxylin and eosin
(H&E) (Fig. 7C, left). Lack of
tumor formation in the brain for mice injected with 231Br-FF cells
pre-treated with propranolol was also confirmed by H&E staining
(Fig. 7C, right).
Discussion
We found that stage II breast cancer patients who
were administered β-blockers at the time of their tumor resection
surgery had decreased postoperative cancer recurrence and
metastases using Cox regression, and we suspect that statistical
significance with the Kaplan-Meier estimate was limited by sample
size. We found no difference in overall survival associated with
perioperative β-blocker use. It should be noted that the patients
were considered to be on perioperative β-blockers if computer-based
review of electronic medical records indicated β-blocker use within
45 days of surgery. The review did not include anesthesia records.
Overall, our results showed a potential beneficial association
between β-blocker use and breast cancer recurrence and metastases
in accordance with previous studies (4–6). Based
on these findings, we explored cellular and biological
mechanisms.
We investigated the effects of β2-adrenergic
receptor agonists and antagonists on TN primary breast cancer and
breast cancer brain metastatic cells. Regulation of metastasis
development may stem from the body's own signaling substances, for
example, the physiological levels of norepinephrine (26). Our previous research indicates that
there is a bi-directional interaction between tumor cells and the
physiological brain microenvironment. We continued our
investigation into the effects of β2-adrenergic receptor targeting
on three characteristics of metastasis: proliferation, migration
and invasion. The data generated in this study further support the
hypothesis that the brain microenvironment has the potential to aid
the establishment of brain metastases.
Our initial studies showed high protein expression
of the β2-adrenergic receptor in both TN primary and brain
metastasis cells. Upon further investigation, at the concentrations
of β2-adrenergic receptor agonists and antagonists utilized, brain
metastatic cells responded to propranolol (β1 and β2-adrenergic
receptor antagonist) by decreasing metastatic potential and to
terbutaline sulfate (β2-adrenergic receptor agonist) by increasing
metastatic potential. The primary breast cancer cells failed to
show any response. External stress, such as surgery can stimulate
epinephrine and norepinephrine release, which can increase
metastatic ability (22). To mimic
the response noted in surgical stress in vitro, we used
terbutaline sulfate to activate the β2-adrenergic receptor
(22,27). Treatment with terbutaline sulfate
significantly increased cell proliferation and migration compared
to the control brain metastatic cells. Terbutaline sulfate
significantly increased cell invasion in the brain metastatic cells
compared to the cells treated with propranolol. Combination
treatment with propranolol and terbutaline sulfate abrogated the
increased effects of terbutaline in regards to all three
characteristics of metastasis in the brain metastatic cells, but
not its primary breast cancer counterpart. In vivo, there
was a significant decrease in brain metastasis in mice injected
with cells pretreated with propranolol at a non-cytotoxic
concentration and time of treatment.
There are discrepancies regarding the effects of
β2-adrenergic receptor agonism or antagonism on cancer cells.
Although other studies, including this study, have shown that
β2-adrenergic receptor agonism increases metastatic potential
(28–30), other studies indicate that
β2-agonism increases apoptosis and tumor regression when using
pirbuterol, a β2-adrenergic receptor agonist (31). This could be attributed to pathway
specificity, whereas some agonists can block the
Ras/Raf-1/Mek-1/Erk1/2 pathway (31), and some may inhibit the cAMP/PKA
pathway (29).
Our results indicate that β2-adrenergic receptor
treatment affects brain metastatic cells, or at the least brain
metastatic cells have increased sensitivity to these treatments,
potentially due to the adaptations metastatic cells have for the
brain micro-environment. One reason for the differences in
sensitivity of a primary breast cancer cell line and its brain
metastatic cell line counterpart could be attributed to a pathway
switch between the cells in a primary vs. a secondary
microenvironment (13,22). Changes in the expression levels of
β-adrenergic receptor subtypes were also noted in more aggressive
derivatives of breast cancer cells, of which metastasis is one
(32). Supporting our theory that
brain metastatic cells have increased sensitivity to β2-adrenergic
receptor targeting, propranolol alone in previous research
inhibited norepinephrine-induced lymph node metastases in prostate
cancer cells, but there were no effects on primary tumors (33). These could explain the decrease in
the sensitivity of primary breast cancer cells to treatments
targeting a neurotransmitter receptor.
We demonstrated a potential beneficial association
between perioperative β-blockade and breast cancer recurrence and
metastases. Such an association has been demonstrated for other
cancer types in animal models (17). In addition, we showed that
β2-adrenergic receptor activation of TN breast-to-brain metastatic
cells increased in vitro proliferation, migration and
invasion. These effects were blocked by propranolol. Propranolol
also decreased establishment of brain metastases in vivo.
Our findings add to a growing body of evidence that suggests a
complex relationship between breast cancer metastases and
β-adrenergic activation and inhibition. Further studies into this
relationship and to the possible therapeutic applications of
selective β2 antagonists are warranted. Eventually it may be
possible to identify a subset of TN breast cancer patients who may
benefit from therapeutic blockade of stress-induced catecholamine
release during the perioperative period.
Abbreviations:
|
231Br
|
MDA-MB-231Br
|
|
ADRB2
|
β2-adrenergic receptor
|
|
TN
|
triple-negative
|
|
BBM
|
breast-to-brain metastasis
|
|
231
|
MDA-MB-231
|
References
|
1
|
Schouten LJ, Rutten J, Huveneers HA and
Twijnstra A: Incidence of brain metastases in a cohort of patients
with carcinoma of the breast, colon, kidney, and lung and melanoma.
Cancer. 94:2698–2705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pestalozzi BC: Brain metastases and
subtypes of breast cancer. Ann Oncol. 20:803–805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tseng LM, Hsu NC, Chen SC, Lu YS, Lin CH,
Chang DY, Li H, Lin YC, Chang HK, Chao TC, et al: Distant
metastasis in triple-negative breast cancer. Neoplasma. 60:290–294.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Melhem-Bertrandt A, Chavez-Macgregor M,
Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD,
Hortobagyi GN and Gonzalez-Angulo AM: Beta-blocker use is
associated with improved relapse-free survival in patients with
triple-negative breast cancer. J Clin Oncol. 29:2645–2652. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barron TI, Connolly RM, Sharp L, Bennett K
and Visvanathan K: Beta blockers and breast cancer mortality: A
population-based study. J Clin Oncol. 29:2635–2644. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Powe DG, Voss MJ, Zänker KS, Habashy HO,
Green AR, Ellis IO and Entschladen F: Beta-blocker drug therapy
reduces secondary cancer formation in breast cancer and improves
cancer specific survival. Oncotarget. 1:628–638. 2010. View Article : Google Scholar
|
|
7
|
Neman J, Termini J, Wilczynski S, Vaidehi
N, Choy C, Kowolik CM, Li H, Hambrecht AC, Roberts E and Jandial R:
Human breast cancer metastases to the brain display GABAergic
properties in the neural niche. Proc Natl Acad Sci USA.
111:984–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Termini J, Neman J and Jandial R: Role of
the neural niche in brain metastatic cancer. Cancer Res.
74:4011–4015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
10
|
Purves D, Augustine GJ, Fitzpatrick D,
Katz LC, LaMantia AS, McNamara JO and Williams SM: Neuroscience.
2nd edition. Sinauer Associates; Sunderland, MA: 2001
|
|
11
|
Schuller HM and Cole B: Regulation of cell
proliferation by beta-adrenergic receptors in a human lung
adenocarcinoma cell line. Carcinogenesis. 10:1753–1755. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G,
Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, et al:
Surgical stress promotes tumor growth in ovarian carcinoma. Clin
Cancer Res. 15:2695–2702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sloan EK, Priceman SJ, Cox BF, Yu S,
Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas
BD, Wu L, et al: The sympathetic nervous system induces a
metastatic switch in primary breast cancer. Cancer Res.
70:7042–7052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang XY, Wang HC, Yuan Z, Huang J and
Zheng Q: Norepinephrine stimulates pancreatic cancer cell
proliferation, migration and invasion via β-adrenergic
receptor-dependent activation of P38/MAPK pathway.
Hepatogastroenterology. 59:889–893. 2012.
|
|
15
|
Strell C, Niggemann B, Voss MJ, Powe DG,
Zanker KS and Entschladen F: Norepinephrine promotes the
β1-integrin-mediated adhesion of MDA-MB-231 cells to vascular
endothelium by the induction of a GROα release. Mol Cancer Res.
10:197–207. 2012. View Article : Google Scholar
|
|
16
|
Gottschalk A, Sharma S, Ford J, Durieux ME
and Tiouririne M: Review article: The role of the perioperative
period in recurrence after cancer surgery. Anesth Analg.
110:1636–1643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benish M, Bartal I, Goldfarb Y, Levi B,
Avraham R, Raz A and Ben-Eliyahu S: Perioperative use of
beta-blockers and COX-2 inhibitors may improve immune competence
and reduce the risk of tumor metastasis. Ann Surg Oncol.
15:2042–2052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sood AK, Bhatty R, Kamat AA, Landen CN,
Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S and Cole SW:
Stress hormone-mediated invasion of ovarian cancer cells. Clin
Cancer Res. 12:369–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D
and Zhang M: Norepinephrine-induced invasion by pancreatic cancer
cells is inhibited by propranolol. Oncol Rep. 22:825–830.
2009.PubMed/NCBI
|
|
20
|
Marchesi F, Monti P, Leone BE, Zerbi A,
Vecchi A, Piemonti L, Mantovani A and Allavena P: Increased
survival, proliferation, and migration in metastatic human
pancreatic tumor cells expressing functional CXCR4. Cancer Res.
64:8420–8427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Entschladen F, Drell TL IV, Lang K, Joseph
J and Zaenker KS: Tumour-cell migration, invasion, and metastasis:
Navigation by neurotransmitters. Lancet Oncol. 5:254–258. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang J, Li Z, Lu L and Cho CH:
β-adrenergic system, a backstage manipulator regulating tumour
progression and drug target in cancer therapy. Semin Cancer Biol.
23:533–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thaker PH, Han LY, Kamat AA, Arevalo JM,
Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori
M, et al: Chronic stress promotes tumor growth and angiogenesis in
a mouse model of ovarian carcinoma. Nat Med. 12:939–944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gille E, Lemoine H, Ehle B and Kaumann AJ:
The affinity of (-)-propranolol for beta 1- and beta
2-adrenoceptors of human heart. Differential antagonism of the
positive inotropic effects and adenylate cyclase stimulation by
(-)-noradrenaline and (-)-adrenaline. Naunyn Schmiedebergs Arch
Pharmacol. 331:60–70. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masur K, Niggemann B, Zanker KS and
Entschladen F: Norepinephrine-induced migration of SW 480 colon
carcinoma cells is inhibited by beta-blockers. Cancer Res.
61:2866–2869. 2001.PubMed/NCBI
|
|
26
|
Drell TL IV, Joseph J, Lang K, Niggemann
B, Zaenker KS and Entschladen F: Effects of neurotransmitters on
the chemokinesis and chemotaxis of MDA-MB-468 human breast
carcinoma cells. Breast Cancer Res Treat. 80:63–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lucin KM, Sanders VM and Popovich PG:
Stress hormones collaborate to induce lymphocyte apoptosis after
high level spinal cord injury. J Neurochem. 110:1409–1421. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pérez Piñero C, Bruzzone A, Sarappa MG,
Castillo LF and Lüthy IA: Involvement of α2- and β2-adrenoceptors
on breast cancer cell proliferation and tumour growth regulation.
Br J Pharmacol. 166:721–736. 2012. View Article : Google Scholar
|
|
29
|
Zhang D, Ma QY, Hu HT and Zhang M:
β2-adrenergic antagonists suppress pancreatic cancer cell invasion
by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 10:19–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lang K and Bastian P: Neurotransmitter
effects on tumor cells and leukocytes. Prog Exp Tumor Res.
39:99–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carie AE and Sebti SM: A chemical biology
approach identifies a beta-2 adrenergic receptor agonist that
causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2
pathway. Oncogene. 26:3777–3788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Badino GR, Novelli A, Girardi C and Di
Carlo F: Evidence for functional beta-adrenoceptor subtypes in CG-5
breast cancer cell. Pharmacol Res. 33:255–260. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Palm D, Lang K, Niggemann B, Drell TL IV,
Masur K, Zaenker KS and Entschladen F: The norepinephrine-driven
metastasis development of PC-3 human prostate cancer cells in
BALB/c nude mice is inhibited by beta-blockers. Int J Cancer.
118:2744–2749. 2006. View Article : Google Scholar
|