Introduction
Pancreatic cancer is a common malignant tumor of the
digestive system. Most pancreatic cancers are pancreatic ductal
adenocarcinomas. Pancreatic cancer is among the deadliest cancer in
the world, its 5-year survival rate is only 4% and after surgical
radical resection is less than 20% (1). The poor survival of pancreatic cancer
is due to its late diagnosis, early metastasis, and insufficient
therapy. Unfortunately, the survival rate of patients with
pancreatic cancer have not been increased in recent years. Deeper
understanding of pancreatic cancer based on the molecular
mechanisms, has revealed that pancreatic cancer is due to genetic
and epigenetic alterations (2). New
tumor makers which could help detect pancreatic cancer in the early
stage and provide potential therapeutic targets are necessary and
valuable.
Epigenetics modification is defined as the
modification influencing gene transcription and translation
inherited without changing the DNA sequence, including DNA
methylation, histone modifications, chromatin remodeling and RNA
interference (3). DNA methylation
is a common epigenetic modification reported to affect chromatin
structure and gene stability (4,5). The
aberrant DNA promoter methylation of tumor suppressor genes (TSGs)
play an important role in mostly cancer including esophageal,
gastric and colorectal cancer. In addition, several TSGs including
p16, UCHL1 and ROR2 have been confirmed with promoter aberrant
methylation resulting in silence (6–8).
Protocadherin-10 (PCDH10) belongs to the
protocadherin family, locating in human chromosome 4q28.3.
Protocadherins are predominantly expressed in the nervous system,
playing an important role in signaling pathways and cell-cell
adhesion (9,10). Accumulating evidence indicates that
protocadherins including PCDH8, PCDH17 and PCDH20 can suppress
tumorigenesis and tumor progress in multiple carcinomas (11–13).
Early studies have demonstrated that PCDH10 is frequently
downregulated by promoter methylation and functions as a tumor
suppressor gene in gastric, colorectal cancer, lung cancer and in
many other carcinomas (14–16). However, the research on PCDH10 in
pancreatic cancer is rare, its expression, methylation status and
biological function in pancreatic cancer are not know yet. We
examined PCDH10 mRNA expression and promoter methylation status in
pancreatic cancer cells, and the overexpression of PCDH10 to detect
its biological function in pancreatic cancer cells. In addition, we
preliminarily explored the mechanism of PCDH10 in pancreatic cancer
cells.
Materials and methods
Cell lines
Human pancreatic cancer cell lines (Capan-1, Panc-1,
AsPC-1 and BxPC-3) and human normal pancreatic ductal epithelial
cell line (HPDE6-C7) were from Chongqing Key Laboratory of
Molecular Oncology and Epigenetics. The cells were maintained at
37°C in Dulbecco's modified Eagle's medium (DMEM) or RPMI-1640
medium (HyClone Laboratories, Inc., Logan, UT, USA) with 10% fetal
bovine serum (FBS; Gibco, Grand Island, NY, USA).
Semi-quantitative reverse transcription
PCR (RT-PCR) analysis
Total RNA was isolated from cells by TRIzol
(Invitrogen), RNA concentration and purity were tested, the value
of OD(260)/OD(280) should be at the range of 1.8–2.0. Total RNA was
reverse transcribed into cDNA according to the reverse
transcription kit (Promega, Madison, WI, USA). RT-PCR amplification
primer synthesis by Takara Bio (Shiga, Japan), the RT-PCR primer
sequences are listed in Table I.
RT-PCR steps according to the GoTaq polymerase (Promega)
instructions, and the amplification conditions were: 95°C for 2
min; 95°C for 30 sec, 55°C for 30 sec, 70°C for 30 sec (PCDH10
using 32 cycles, β-actin using 23 cycles); and 70°C for 3 min. PCR
products were analyzed on 2% agarose gel electrophoresis, and
Bio-Rad gel imaging system was used for analysis of exposure and
quantity.
 | Table IRT-PCR and MSP primers. |
Table I
RT-PCR and MSP primers.
| PCR | Primer | Sequence (5′-3′) | Product size
(bp) | PCR cycles | Annealing temperature
(°C) |
|---|
| RT-PCR | PCDH10-sense |
ACTGCTATCAGGTATGCCTG | 219 | 32 | 55 |
| PCDH10-antisense |
GTCTGTCAACTAGATAGCTG | | | |
| β-actin-sense |
CTCCATCCTGGCCTCGCTGT | 268 | 23 | 55 |
|
β-actin-antisense |
GCTGTCACCTTCACCGTTCC | | | |
| MSP | PCDH10-bm1 |
TCGTTAAATAGATACGTTACGC | 153 | 40 | 60 |
| PCDH10-bm2 |
TAAAAACTAAAAACTTTCCGCG | | | |
| PCDH10-bu1 |
GTTGTTAAATAGATATGTTATGT | 155 | 40 | 58 |
| PCDH10-bu2 |
CTAAAAACTAAAAACTTTCCACA | | | |
DNA bisulfite treatment and
methylation-specific PCR (MSP) assays
Genomic DNA was extracted from cells with using DNA
Extraction kits (Tiangen, Co., Ltd., Beijing, China), DNA bisulfite
modification and methylation status of PCDH10 in pancreatic cancer
cells and normal pancreatic ductal epithelial cells were detected
by using the EZ DNA Methylation-Gold™ kit; Zymo Research Corp.,
Irvine, CA, USA). The methylated and unmethylated MSP primers are
shown in Table I (17). Methylation specific PCR (MSP) was
performed using AmpliTaq Gold (Applied Biosystems). PCR products
were analyzed on 2% agarose gel electrophoresis.
5-aza-2′-deoxycytidine (Aza) and
trichostain A (TSA) treatment
Following overnight incubation, Capan-1 and BxPC-3
cells were treated with 5-aza-2′-deoxycytidine (Sigma-Aldrich; 10
µM) for 3 days, and then trichostain A (100 ng/ml; Cayman
Chemical, Ann Arbor, MI, USA) was added to cell medium. After 24 h,
the total RNA and DNA were extracted.
Transfection and G418 selection
The cells (Capan-1 and BxPC-3) were plated in 6-well
plates, transfection was performed at 75% confluence. Discarding
the medium, the cells were washed with serum-free medium 2 times,
then each well was added with 1 ml serum-free medium. A total of 4
µg plasmid (pcDNA3.1-PCDH10 or pcDNA3.1-vector) (GeneChem)
and 5 µl Lipofectamine™ 2000 (Invitrogen) were diluted by
500 µl serum-free medium, respectively, and allowed to
incubate for 5 min at room temperature. Plasmid and Lipofectamine™
2000 diluent were mixed and incubated for 20 min at room
temperature, and each well was added with 1 ml the above mixture.
After culturing for 4–6 h at 37°C, the medium was discarded adding
2 ml medium with 10% FBS. after 48 h, G418 (Amresco, Solon, OH,
USA) was added into the medium (G418 concentration was tested
before, Capan-1 800 µg/ml and BxPC-3 700 µg/ml) for
~2 weeks until single clones formed, then maintaining the
concentration of G418 by half the culture continued. The medium
containing G418 was changed each 3 days.
The expression of PCDH10 in transfected
cells
Cells (Capan-1 and BxPC-3) were transfected with
plasmid pcDNA3.1-PCDH10 or pcDNA3.1-vector, after 48 h, the total
RNA and proteins were extracted. RT-PCR was used to examine the
expression of PCDH10 in cells at mRNA level. Western blot analysis
was performed to test the protein level: total proteins were
extracted with PIPA lysate buffer (Beyotime Institute of
Biotechnology, Shanghai, China), and concentration was measured
with BCA kit (Beyotime Institute of Biotechnology). After
polyacrylamide gel electrophoresis, proteins were transfered onto
polyvinylidene fluoride (PVDF) membranes (Beyotime Institute of
Biotechnology). The membranes were left in 5% skimmed milk for 2 h
at room temperature, then incubated with primary antibodies
overnight [PCDH10 mouse anti-human monoclonal antibody (Abnova,
Taipei, Taiwan); β-actin mouse anti-human monoclonal antibody (Cell
Signaling Technology, Inc., Danvers, MA, USA)] at 4°C, washed with
TBST buffer, incubated with the second antibodies (goat anti-mouse;
Cell Signaling Technology) with horseradish peroxidase (HRP) for 2
h at room temperature. The membranes were washed again, then the
proteins were visualized with BeyoECL Plus kits (Beyotime Institute
of Biotechnology). Results were analyzed with fusion software.
Cell proliferation colony formation
assays
The cells (Capan-1 and BxPC-3) were seeded in a
96-well plate at a density of 5×103 cells/well, each
sample set in three wells and allowed to culture overnight. Cells
were transfected with plasmid pcDNA3.1-PCDH10 or pcDNA3.1-vector
Cell Counting kit-8 (Dojindo Molecular Technologies, Inc.,
Rockville, MD, USA) was used to to evaluated cells proliferation
ability, the detection points were 24, 48 and 72 h after
transfection.
The cells (Capan-1 and BxPC-3) stably transfected
with pcDNA3.1-vector or pcDNA3.1-PCDH10 were seeded in a 6-well
plate at a density of 1×103 cells/well. Each sample set
in three wells. For two weeks, the clones (≥50 cells) were counted
after fixing with paraformaldehyde and staining with Gentian
violet.
Wound-healing and Transwell invasion
assays
Cell (BxPC-3) migration was assessed with scratch
wound-healing assay. Stably transfected cells were seeded in a
6-well plate until confluence, cell layer was scratched with a 2
µl pipette tip and washed with PBS, maintained in serum-free
DMEM. Cells were photographed under a phase-contrast microscope
after incubation for 0, 24 and 48 h.
The invasion ability of cells (BxPC-3) was measured
by 24-well Transwell™ (Corning, Inc., Corning, NY, USA) with
Matrigel (BD Biosciences, San Diego, CA, USA). The Matrigel was
diluted with serum-free medium according to the instructions, added
into the upper chamber, incubated at 37°C until the Matrigel
solidified. A total of 700 µl medium containing 10% FBS was
added into the lower chamber, 100 µl serum-free medium with
2×104 cells was added to the upper chamber. After
incubation for 48 h, cells that migrated were fixed and stained,
then counted under a microscope in three fields.
Cell apoptosis assays
The apoptosis of Capan-1 cells was detected by
combining flow cytometry and Annexin V-FITC/PI staining. After
transfection for 48 h, the cells were digested with EDTA-free
trypsin enzyme and collected, washed with PBS once gently and
collected again. Cells were resuspended with 1 ml PBS, counted and
transfered to 1.5 ml EP tube. Taking 5–10×104 cells,
1,000 g/5 min, discarding the clear liquid, 195 µl Annexin
V-FITC was added to suspend the cells, subsequently adding 5
µl Annexin V-FITC. Adding 10 µl organism iodide
dyeing liquid, incubated for 10–20 min at room temperature, then,
evaluated by flow cytometry.
Expression of the key cell apoptosis
regulators examined by western blot analysis
After polyacrylamide gel electrophoresis, proteins
were transfered onto PVDF membranes. The membranes were left in 5%
skimmed milk for 2 h at room temperature then incubated with
primary antibodies overnight (AKT, p-AKT, PARP, caspase-3,
cytochrome-9 and Bcl-2 are rabbit anti-human polyclonal antibodies;
Cell Signaling Technology), at 4°C, washed with TBST buffer,
incubated in the second antibody (goat anti-rabbit; Cell Signaling
Technology) with HRP mark, for 2 h at room temperature. The
membranes were washed again, then the proteins were visualized with
BeyoECL Plus kits. Data were analyzed with fusion software.
Statistical analysis
Experiments were performed in triplicate. Data are
presented as the mean ± SD and analyzed with Student's t-test, and
the analyses were performed with the SPSS version 21. P<0.05 was
considered to indicate a statistically significant result.
Results
PCDH10 is downregulated in pancreatic
cancer cells
We used RT-PCR to examine the expression of PCDH10
in pancreatic cancer cells and the normal pancreatic ductal
epithelial cells. The result demonstrated that PCDH10 was
downregulated in pancreatic cancer cells (Capan-1, Panc-1 and
BxPC-3) compared to the normal pancreatic ductal epithelial cells
(Fig. 1A).
PCDH10 is frequently downregulated with
promoter methylation in pancreatic cancer cells and restored by
methylation inhibitor histone deacetylase inhibitor
MSP was performed to analyze the methylation status
in the CpG island of PCDH10 promoter in pancreatic cancer cells as
well as the normal pancreatic ductal epithelial cells. The cells
(Capan-1 and BxPC-3) in which PCDH10 was silenced displayed
promoter methylation, while was not found in normal cells (Fig. 1B). Then, the cells were treated with
methylation inhibitor Aza and histone deacetylase inhibitor TSA. As
expected, the expression of PCDH10 was recovered (Fig. 1C). The results revealed that DNA
promoter methylation contributes to PCDH10 silence in pancreatic
cancer cells.
The expression of PCDH10 significantly
increased after transfection
RT-PCR results showed that the expression of PCDH10
mRNA in cells transfected with pcDNA3.1-PCDH10 was significantly
higher than those in cells transfected with pcDNA3.1-vector.
Western blot analysis showed that the expression of PCDH10 protein
in cells transfected with PCDH10 was increased compared with cells
transfected with plasmid vector (P<0.01; Fig. 2).
Re-expression of PCDH10 inhibits the
proliferation and colony formation of pancreatic cancer cells
Several studies have found that PCDH10 can inhibit
cancer growth, whether this applies to pancreatic cancer is
unclear. To ascertain this question, cell proliferation and colony
formation assays were performed in the study. Cell Counting kit-8
assay demonstrated that the cells transfected with PCDH10 grew
significant slower than cells transfected with vector at 24, 48 and
72 h (P<0.01; Fig. 3).
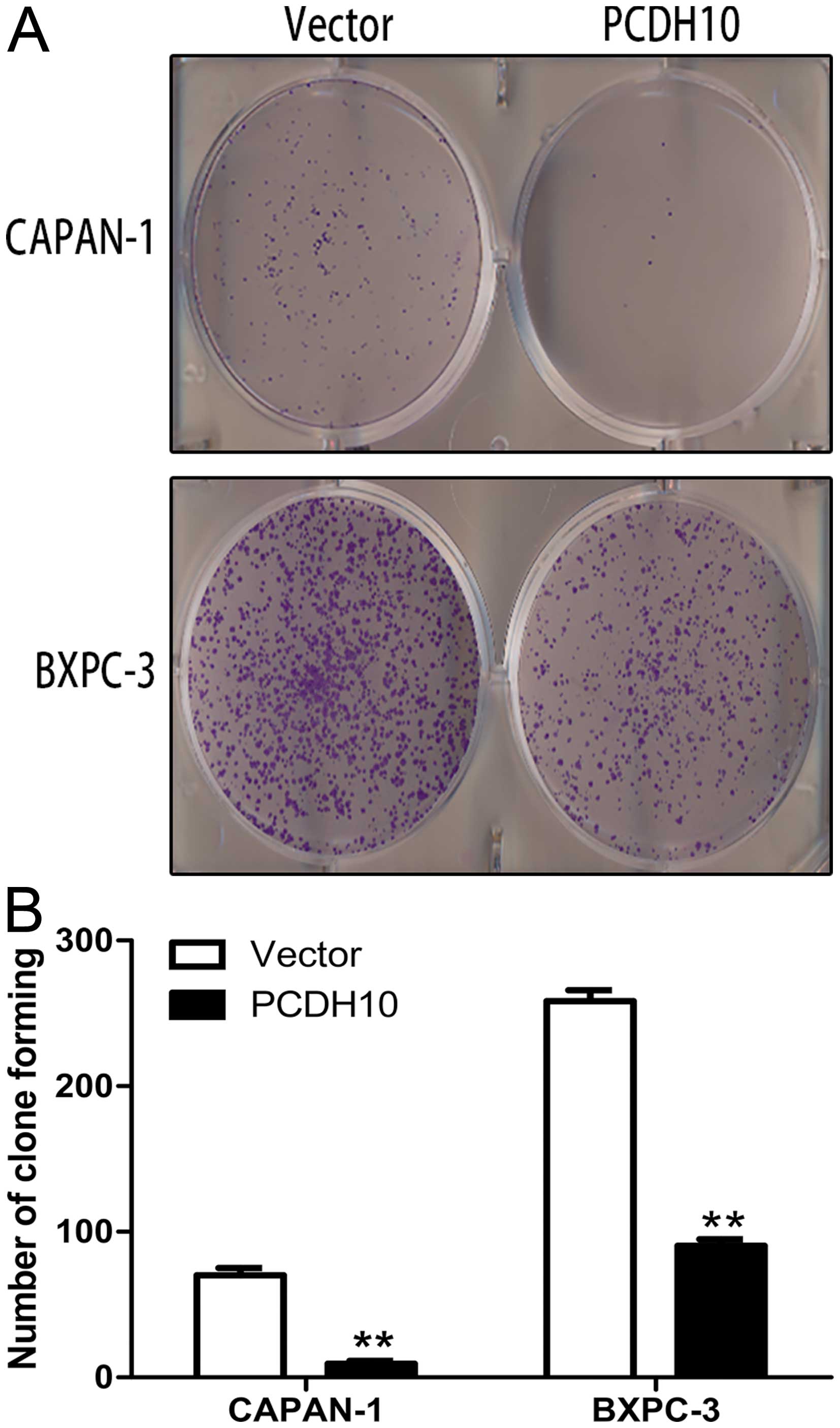
Furthermore, the colony formation assay showed the
PCDH10-transfected cells formed markedly fewer and smaller colonies
than the vector-transfected cells (P<0.01; Fig. 4). These results revel that PCDH10
can inhibit pancreatic cancer cell growth.
PCDH10 inhibits the migration and
invasion ability of pancreatic cancer cells
Wound-healing and Transwell™ invasion assays were
performed to test the effect of PCDH10 on the migration and
invasion ability of pancreatic cancer cells BxPC-3. Cells
transfected with PCDH10 spread significantly more slowly than cells
transfected with vector in the wound-healing test (P<0.01;
Fig. 5). The Transwell™ invasion
assay showed a marked suppression of invasion in the
PCDH10-transfected cells (P<0.01; Fig. 6). The above results suggested that
PCDH10 may play an important role in metastatic diffusion of
pancreatic cancer cells.
Ectopic PCDH10 expression induces
apoptosis of pancreatic cancer cells
We performed flow cytometry combined with Annexin
V-FITC/PI staining to explore the effect of PCDH10 on apoptosis in
Capan-1. Apoptosis of cells transfected with PCDH10 was obviously
increased compared with the cells transfected with vector
(P<0.05; Fig. 7). This evidence
suggests that PCDH10 can induce apoptosis of pancreatic cancer
cells.
The effect of PCDH10 on the key cell
apoptosis regulators
The flow cytometry results confirmed that PCDH10 can
induce apoptosis in pancreatic cancer cells, thus, we performed
western blot analysis to detect the level of apoptosis-related
proteins. Fig. 4 shows the
pro-apoptosis proteins (PARP, caspase-3 and caspase-9) were
increased and anti-apoptosis protein (bcl-2) was decreased. In
addition, we detected the AKT and p-AKT levels upstream of the
apoptosis pathway, the proteins (AKT and p-AKT) were decreased
(P<0.05; Fig. 8). These results
indicate that PCDH10 induced pancreatic cancer cell apoptosis may
be via regulating the AKT pathway.
Discussion
The generation of most cancers involving pancreatic
cancer are due to the genetic and epigenetic alterations of tumor
suppressor genes (TSGs). Genetic alterations often accompany gene
mutations which can change the DNA sequence. For instance, the
tumor suppressor gene TP53 has frequent mutations resulting in
inactivation in pancreatic cancer (18). Several studies have suggested that
epigenetic alterations are important mechanism for tumor suppressor
genes silencing, and aberrant DNA methylation could be a novel
tumor marker for early diagnose and prognosis (19–21).
DNA methylation as a tumor marker has some advantages: first,
aberrant DNA methylation is more common as an early event in
cancers; second, the techniques of detecting aberrant DNA
methylation are simple, rapid and sensitive; finally, DNA is more
stable than any other molecular markers similar to proteins. In DNA
methylation, unlike genetic alteration, the silence of gene
expression resulted by methylation alteration can be reversed by
pharmacologic demethylation, this indicates that aberrant DNA
methylation is a process recovering the expression of TSGs which
changed by epigenetics and restoring their tumor suppression
functions in cancer. Many TSGs and increasing number of genes with
functional cancer suppression are silenced in pancreatic cancer by
promoter methylation. Specifically, the inactivation of p16 tumor
suppressor gene by promoter methylation in pancreatic cancer is
well known (22). This indicated
that abnormal methylation of TSGs contributes to pancreatic cancer
pathogenesis.
In the present study, we explored PCDH10 in the
expression, epigenetic alteration, biological function in
pancreatic cancer cells. Our results demonstrate that the PCDH10
expression is downregulated in pancreatic cancer cells due to DNA
promoter hypermethylation, which accords with the status of PCDH10
in other types of cancer, and this suggests that the aberrant DNA
methylation of PCDH10 could be a new biomarker for detecting
pancreatic cancer at an early stage. Re-expression of PCDH10
significantly suppresses cell proliferation and colony formation,
markedly inhibits migration and invasion ability, and induces
apoptosis in pancreatic cancer cells. These results suggest that
PCDH10 have tumor suppressor function, which is consistent with
previous studies on PCDH10 in gastric cancer and in colorectal
cancer (23,24). Thus, recovering PCDH10 expression
and restoring its function shows promise as a new therapy for
treating pancreatic cancer. Unfortunately, we did not collect
enough pancreatic tissue samples, thus, we could not analyze the
relationship between aberrant DNA methylation of PCDH10 and
clinical features in pancreatic cancer.
Protocadherins play an important role in signal
transduction, implying that PCDH10 action as tumor suppressor gene
may be via mediating cancer-related signaling pathway. For example,
PCDH10 inhibits the proliferation of multiple myeloma cells via
downregulation of Wnt/β-catenin/BCL-9 signaling pathway (25), and induces apoptosis of multiple
myeloma cells by inhibiting the NF-κB pathway (26). In this study, we explored the
apoptosis mechanisms of PCDH10 in pancreatic cancer cells. The
results showed that PCDH10 may regulate the AKT pathway to induce
the apoptosis of pancreatic cancer cells.
Apoptosis is a regulated cellular suicide mechanism
characterized by nuclear fragmentation, membrane blebbing and
formation of apoptotic bodies (27). Apoptosis pathway has been classified
into mitochondria-mediated pathway and receptor-mediated pathway
(28). The natural death mechanism
can lead to several diseases involving cancer when it is abnormally
regulated. PARP, poly(ADP-ribose) polymerase, appears to be
involved in DNA repair in response to environmental stress. It was
reported to have a key role in caspase-independent cell death
pathway defined as necroptosis (29). Caspases, a family of cysteine
proteases, is divided into initiator and effector caspases.
Initiator caspases such as caspase-9, form apoptosome complex and
activate downstream effector caspases by binding large adaptor
molecules which promote caspase activation. Caspase-3, a member of
effector caspases, is the primary executioner of programmed cell
death, as it is directly or indirectly responsible for cleavage of
many proteins and initiator caspases involved in apoptosis. Bcl-2
family of proteins is another important regulator of apoptosis, it
is divided into anti-apoptosis proteins and pro-apoptosis proteins,
according to the domain and function. Bcl-2 belongs to
anti-apoptosis proteins, locating mainly on the outer mitochondria
membrane. They protect the cells by interacting with mitochondria
protein, preventing damage to the membrane, and they can inhibit
the pro-apoptosis factors released (28,30).
PI3K/AKT signaling pathway has become a major focus
of attention for its critical role in regulating some of the most
fundamental aspects of cell growth, proliferation, survival,
transcription, and protein synthesis, disorder of the PI3K/AKT
pathway is contributing to many diseases including cancer,
diabetes, cardiovascular and neurological diseases (31). In addition, AKT pathway is the
commonly activated signaling pathway in most cancers.
Serine/threonine kinase Akt, the key protein of PI3K/AKT signaling
pathway, is the major mediator of cell apoptosis by directly
inhibiting pro-apoptosis proteins like Bad, caspase-9 and
pro-apoptosis signals generated by transcription factors such as
FoxO1 to regulate cell apoptosis (32).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong SM, Park JY, Hruban RH and Goggins M:
Molecular signatures of pancreatic cancer. Arch Pathol Lab Med.
135:716–727. 2011.PubMed/NCBI
|
|
3
|
Wolffe AP and Matzke MA: Epigenetics:
Regulation through repression. Science. 286:481–486. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singal R and Ginder GD: DNA methylation.
Blood. 93:4059–4070. 1999.PubMed/NCBI
|
|
5
|
Esteller M: Cancer epigenomics: DNA
methylomes and histone-modification maps. Nat Rev Genet. 8:286–298.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merlo A, Herman JG, Mao L, Lee DJ,
Gabrielson E, Burger PC, Baylin SB and Sidransky D: 5′ CpG island
methylation is associated with transcriptional silencing of the
tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med.
1:686–692. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiang T, Li L, Yin X, Yuan C, Tan C, Su X,
Xiong L, Putti TC, Oberst M, Kelly K, et al: The ubiquitin
peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis
through stabilizing p53 and is frequently silenced in breast
cancer. PLoS One. 7:e297832012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Ying J, Tong X, Zhong L, Su X, Xiang
T, Shu X, Rong R, Xiong L, Li H, et al: Epigenetic identification
of receptor tyrosine kinase-like orphan receptor 2 as a functional
tumor suppressor inhibiting β-catenin and AKT signaling but
frequently methylated in common carcinomas. Cell Mol Life Sci.
71:2179–2192. 2014. View Article : Google Scholar
|
|
9
|
Redies C, Vanhalst K and Roy F:
Delta-Protocadherins: Unique structures and functions. Cell Mol
Life Sci. 62:2840–2852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morishita H and Yagi T: Protocadherin
family: Diversity, structure, and function. Curr Opin Cell Biol.
19:584–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu JS, Koujak S, Nagase S, Li CM, Su T,
Wang X, Keniry M, Memeo L, Rojtman A, Mansukhani M, et al: PCDH8,
the human homolog of PAPC, is a candidate tumor suppressor of
breast cancer. Oncogene. 27:4657–4665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu X, Sui X, Li L, Huang X, Rong R, Su X,
Shi Q, Mo L, Shu X, Kuang Y, et al: Protocadherin 17 acts as a
tumour suppressor inducing tumour cell apoptosis and autophagy, and
is frequently methylated in gastric and colorectal cancers. J
Pathol. 229:62–73. 2013. View Article : Google Scholar
|
|
13
|
Lv J, Zhu P, Yang Z, Li M, Zhang X, Cheng
J, Chen X and Lu F: PCDH20 functions as a tumour-suppressor gene
through antagonizing the Wnt/β-catenin signalling pathway in
hepatocellular carcinoma. J Viral Hepat. 22:201–211. 2015.
View Article : Google Scholar
|
|
14
|
Li Z, Chim JC, Yang M, Ye J, Wong BC and
Qiao L: Role of PCDH10 and its hypermethylation in human gastric
cancer. Biochim Biophys Acta. 1823:298–305. 2012. View Article : Google Scholar
|
|
15
|
Zhong X, Zhu Y, Mao J, Zhang J and Zheng
S: Frequent epigenetic silencing of PCDH10 by methylation in human
colorectal cancer. J Cancer Res Clin Oncol. 139:485–490. 2013.
View Article : Google Scholar
|
|
16
|
Tang X, Yin X, Xiang T, Li H, Li F, Chen L
and Ren G: Protocadherin 10 is frequently downregulated by promoter
methylation and functions as a tumor suppressor gene in non-small
cell lung cancer. Cancer Biomark. 12:11–19. 2013.PubMed/NCBI
|
|
17
|
Ying J, Li H, Seng TJ, Langford C,
Srivastava G, Tsao SW, Putti T, Murray P, Chan AT and Tao Q:
Functional epigenetics identifies a protocadherin PCDH10 as a
candidate tumor suppressor for nasopharyngeal, esophageal and
multiple other carcinomas with frequent methylation. Oncogene.
25:1070–1080. 2006. View Article : Google Scholar
|
|
18
|
Redston MS, Caldas C, Seymour AB, Hruban
RH, da Costa L, Yeo CJ and Kern SE: p53 mutations in pancreatic
carcinoma and evidence of common involvement of homocopolymer
tracts in DNA microdeletions. Cancer Res. 54:3025–3033.
1994.PubMed/NCBI
|
|
19
|
Yi JM, Guzzetta AA, Bailey VJ, Downing SR,
Van Neste L, Chiappinelli KB, Keeley BP, Stark A, Herrera A,
Wolfgang C, et al: Novel methylation biomarker panel for the early
detection of pancreatic cancer. Clin Cancer Res. 19:6544–6555.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roperch JP, Incitti R, Forbin S, Bard F,
Mansour H, Mesli F, Baumgaertner I, Brunetti F and Sobhani I:
Aberrant methylation of NPY, PENK, and WIF1 as a promising marker
for blood-based diagnosis of colorectal cancer. BMC Cancer.
13:5662013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balgkouranidou I, Matthaios D,
Karayiannakis A, Bolanaki H, Michailidis P, Xenidis N, Amarantidis
K, Chelis L, Trypsianis G, Chatzaki E, et al: Prognostic role of
APC and RASSF1A promoter methylation status in cell free
circulating DNA of operable gastric cancer patients. Mutat Res.
778:46–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schutte M, Hruban RH, Geradts J, Maynard
R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff
I, Schmiegel W, et al: Abrogation of the Rb/p16 tumor-suppressive
pathway in virtually all pancreatic carcinomas. Cancer Res.
57:3126–3130. 1997.PubMed/NCBI
|
|
23
|
Yu J, Cheng YY, Tao Q, Cheung KF, Lam CN,
Geng H, Tian LW, Wong YP, Tong JH, Ying JM, et al: Methylation of
protocadherin 10, a novel tumor suppressor, is associated with poor
prognosis in patients with gastric cancer. Gastroenterology.
136:640–51.e1. 2009. View Article : Google Scholar
|
|
24
|
Jao TM, Tsai MH, Lio HY, Weng WT, Chen CC,
Tzeng ST, Chang CY, Lai YC, Yen SJ, Yu SL, et al: Protocadherin 10
suppresses tumorigenesis and metastasis in colorectal cancer and
its genetic loss predicts adverse prognosis. Int J Cancer.
135:2593–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Yang Z, Yuan H, Li Z, Li Y, Liu Q
and Chen J: PCDH10 inhibits cell proliferation of multiple myeloma
via the negative regulation of the Wnt/β-catenin/BCL-9 signaling
pathway. Oncol Rep. 34:747–754. 2015.PubMed/NCBI
|
|
26
|
Li Z, Yang Z, Peng X, Li Y, Liu Q and Chen
J: Nuclear factor-κB is involved in the protocadherin-10-mediated
pro-apoptotic effect in multiple myeloma. Mol Med Rep. 10:832–838.
2014.PubMed/NCBI
|
|
27
|
Degterev A and Yuan J: Expansion and
evolution of cell death programmes. Nat Rev Mol Cell Biol.
9:378–390. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drel' VR, Shymans'kyĭ IO, Sybirna NO and
Velykyĭ MM: Role of PARP and protein poly-ADP-ribosylation process
in regulation of cell functions. Ukr Biokhim Zh. 83:5–34. 2011.
|
|
30
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|