Introduction
Breast cancer is a systemic disease. It has become
the most frequently diagnosed cancer and the leading cause of
cancer-related death among females worldwide, with an estimated 1.7
million cases and 521,900 deaths in 2012 (1). There are several obstacles for breast
cancer treatment; for example, chemoresistance, immune escape,
metastasis and recurrence (2,3).
During these obstacles, metastasis is the leading cause of
mortality in breast cancer patients. Nearly 50% of breast cancer
patients treated with chemotherapeutic and/or hormonal agents
develop distant metastatic disease (4,5); these
patients face a 5-year survival rate of only 20% (6). Therefore, there is a great and urgent
need to identify new drugs or treatments for metastatic breast
cancer.
Tumor metastasis is a multi-step process by which
tumor cells disseminate from their primary site and form secondary
tumors at a distant site. In the past decade, a developmental
process, epithelial-mesenchymal transition (EMT), has been
increasingly recognized to play pivotal and intricate roles in
promoting carcinoma invasion and metastasis (7). EMT programs were first observed in the
context of embryonic development, where they function as
transdifferentiation programs that effect critical morphogenetic
steps, such as gastrulation and neural crest formation (8). Specifically, EMTs generate mesenchymal
cell types from epithelial and endothelial precursors. These
epithelial-mesenchymal conversions are crucial for cell movements
that take place during breast cancer metastasis (9,10). By
imparting mesenchymal traits to carcinoma cells, an EMT can
generate cellular traits associated with high-grade malignancy,
including motility, invasiveness and a resistance to apoptosis;
these can lead in turn to metastatic dissemination (9,11).
Moreover, during the process of EMT, epithelial cancer cells
acquire molecular alterations that facilitate the loss of
epithelial features and gain of mesenchymal phenotype. Such
transformation promotes cancer cell migration and invasion
(12–16). For this reason, increasing
therapeutic substances or measures focusing on EMT have been
investigated as potential therapies for preventing breast cancer
metastasis.
Since more and more studies have demonstrated that
herbal plant extracts exert antitumor effects, these herbal plant
extracts have been considered as a practical approach to reduce the
incidence of breast cancer. Moreover, since chemotherapeutics are
limited and chemoresistance occurs frequently, these herbal plant
extracts have also attracted increased attention in the exploration
of effective antitumor drugs for breast cancer metastasis. Ferulic
acid (4-hydroxy-3-methoxycinnamic acid), a widely distributed
constituent of plants, was first isolated from Ferula
foetida in 1866 (17). It has
been described to act as a potent antioxidant by scavenging free
radicals and enhancing the cell stress response through the
upregulation of cytoprotective systems (18). Previous studies have indicated that
ferulic acid could inhibit the expression and activity of cytotoxic
enzymes, including inducible nitric oxide synthase, caspases and
cyclooxygenase-2 (19). However, it
also has been found that ferulic acid exhibits a potential
treatment for many disorders, e.g. Alzheimer's disease (17), colon cancer (20), cardiovascular diseases (21), diabetes mellitus (22) and skin disease (23). Although it was reported that ferulic
acidoctyl and -dodecyl esters, significantly blocked the growth of
breast, lung, colon and central nervous system tumor cells with
IC50 values ranging from 17.05 to 4.29 µg/ml for
the breast and colon, respectively (24), the detailed mechanisms by which
ferulic acid inhibits cell growth and metastasis of breast cancer
have not been fully elucidated.
In the present study, we reported the anticancer
activity of ferulic acid in vitro and in vivo. Our
results indicate that ferulic acid inhibits breast cancer
proliferation and induces apoptosis. We further demonstrated that
ferulic acid suppresses breast cancer metastasis by regulating EMT.
These data collectively suggest that ferulic acid may be a very
promising candidate for breast cancer intervention and
prevention.
Materials and methods
Reagents and cell lines
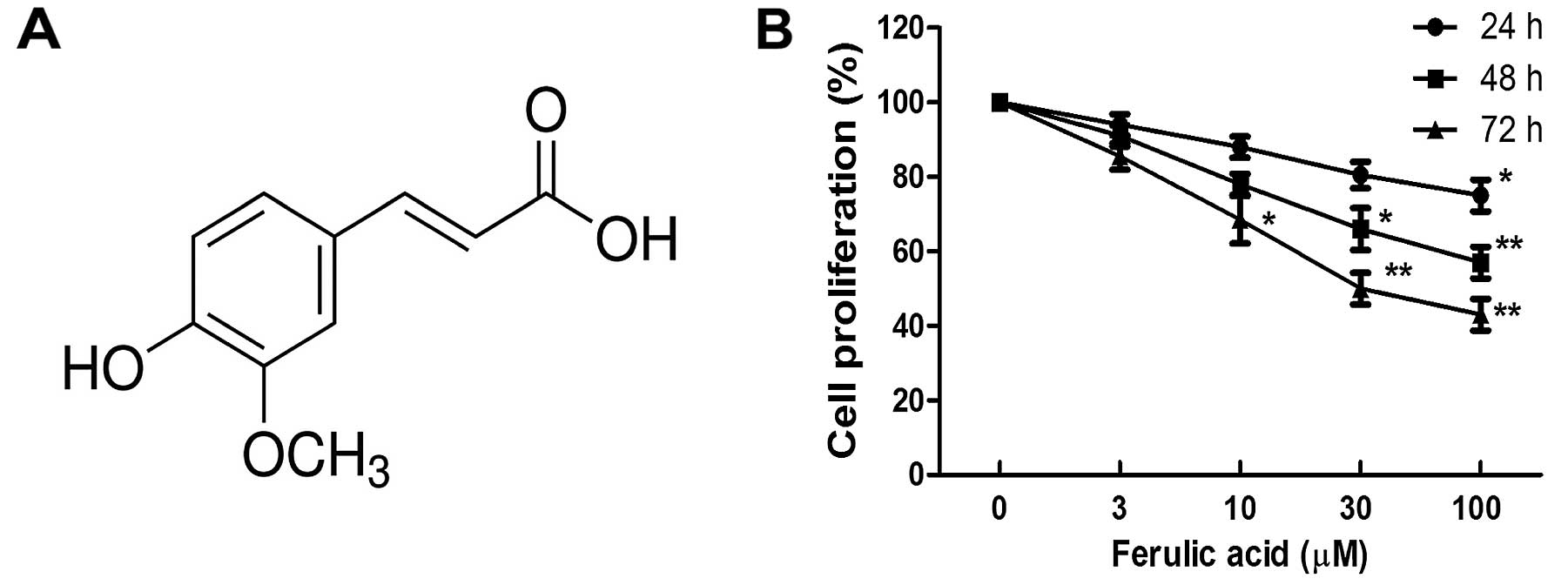
Ferulic acid
(C10H10O4; MW: 194.18; shown in
Fig. 1A) was purchased from Sigma
Chemical Co. (Sigma, St. Louis, MO, USA). Human breast cancer cell
line MDA-MB-231 was obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA), and cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. All the cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2.
Cell viability assay
Cells seeded onto a 96-well plate at a density of
4×103 cells/well were treated with 100 µl medium
plus dimethyl sulfoxide (DMSO; vehicle control, final concentration
<0.1%) or ferulic acid (final concentration: 3, 10, 30 and 100
µM), and incubated for 24, 48 and 72 h, respectively. At the
end of the drug exposure duration, cell viability was assessed
using the Cell Counting Kit-8 (CCK-8) assay according to the
manufacturer's protocols (Dojindo, Gaithersburg, MD, USA). Six
parallel replicates were measured for each sample. Each plate
included a control well that contained medium only, which was used
to obtain a background spectrometric absorbance value that was
subtracted from the test sample readings. The data are expressed as
the mean ratio of live cells (treated vs. control) ± SD for 3
replicates.
Apoptosis analysis by flow cytometry
For apoptosis analysis, human breast cancer cell
line MDA-MB-231 was treated with ferulic acid (final concentration:
10, 30 and 100 µM) and DMSO (vehicle control, final
concentration <0.1%) for 48 h, incubated with fluorescein
isothiocyanate-Annexin V and propidium iodide (BD Biosciences) for
15 min, and then analyzed by flow cytometry.
Caspase activity assay
Caspase-3 activity was measured using colorimetric
assay kits (Beyotime Institute of Biotechnology, Jiangsu, China)
according to the manufacturer's instructions. Briefly, after
isolation by two-step collagenase digestion, the breast cancer
cells were mixed with 50 µl cold lysis buffer. The
supernatant (10 µl) was then mixed with 10 µl caspase
substrate and 80 µl reaction buffer and incubated at 37°C
for 2 h in the dark. The fluorescence intensity of the caspase
substrate at 405 nm was measured using a microplate reader. The
caspase activity was calculated as the OD405/100 µg
protein.
Wound healing and Transwell migration
assays
For the wound healing assay, we drew horizontal
lines across the back of the wells of 6-well plates with a marker
pen. The cells (5×105 cells/well) were plated into the
6-well plates. On the following day, the confluent cell monolayers
were carefully wounded (perpendicular to the horizontal lines) with
sterile pipette tips and washed with phosphate-buffered saline
(PBS) twice to remove cellular debris. Serum-free medium was added
into the wells. Then, the wounded cell monolayers were cultured for
another 48 h treat with or without different concentrations of
ferulic acid. Images were captured under a microscope to observe
the distribution of the cells at the scratch zone at the last time
point. The degree of wound closure was then quantitatively
evaluated using Image-Pro Plus software. Four fields from each well
were documented, and each experiment was performed in
triplicate.
A cell migration assay was performed using Transwell
chambers with a pore size of 0.8 µm. Breast cancer cells
were trypsinized, washed and suspended in medium without FBS. To
the lower wells of the chambers, migration-inducing medium (with
10% FBS) was added. A total of 2×104 cells were seeded
in serum-free medium in the upper chamber, containing various
concentrations of ferulic acid (10, 30 and 100 µM) or
vehicle solvent (DMSO). After incubating for 24 h at 37°C, the
cells in the upper chamber were carefully removed with a cotton
swab, and the cells that had migrated to the reverse face of the
membrane were fixed in methanol, stained with Giemsa solution and
counted.
Immunofluorescence analysis
For the immunofluorescence experiments, cells were
prepared and analyzed under a fluorescence microscope following the
procedures previously described (25). Briefly, cells treated with or
without ferulic acid were incubated with primary antibody against
vimentin (1:100; 5741) or E-cadherin (1:200; 3195) (both from Cell
Signaling Technology), and then incubated with DyLight 594 or 488
(CW Biotech, Beijing, China) secondary antibody against rabbit IgG.
Cells were then counterstained with 4′,6-diamidino-2-phenylindole
(DAPI) (Roche, Basel, Switzerland) and imaged with the fluorescence
microscope.
Western immunoblot analysis and
antibodies
The cell lysate was prepared using a protein
extraction kit (Active Motif Company, Carlsbad, CA, USA) according
to the manufacturer's instructions. Protein concentrations were
determined by BCA protein assay kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), protein was loaded onto 10–12%
Tris-acrylamide gels, electrophoresed and then transferred to
nitrocellulose membranes. After blocking non-specific binding
sites, the membranes were probed with primary antibodies, including
anti-E-cadherin (1:1,000; 3195), anti-claudin-1 (1:1,000; 13255),
anti-N-cadherin (1:1,000; 4061), anti-vimentin (1:1,000; 5741),
anti-Snail (1:1,000; 3897) (all from Cell Signaling Technology),
anti-Slug (1:1,000; ab27568; Abcam) and anti-β-actin (1:1,000;
sc47778; Santa Cruz Biotechnology). Labeled proteins were
visualized by an ECL chemiluminescence kit (Millipore, Billerica,
MA, USA).
RNA extraction and quantitative PCR
(qPCR)
MDA-MB-231 cells were treated with various
concentrations of ferulic acid (3, 10, 30 and 100 µM). At 48
h after treatment, the total RNA was extracted using TRIzol reagent
(Invitrogen), respectively. Reverse transcription reactions were
performed using 2 µg of total RNA and were subsequently
processed using a reverse transcription kit (TransGen Biotech,
Beijing, China). Specific products were amplified by qPCR using the
following primers: E-cadherin, 5′-CGAGAGCTACACGTTCACGG-3′ (forward)
and 5′-GGGTGTCGAGGGAAAAATAGG-3′ (reverse); claudin-1,
5′-CCTCCTGGGAGTGATAGCAAT-3′ (forward) and
5′-GGCAACTAAAATAGCCAGACCT-3′ (reverse); vimentin,
5′-GACGCCATCAACACCGAGTT-3′ (forward) and
5′-CTTTGTCGTTGGTTAGCTGGT-3′ (reverse); N-cadherin,
5′-AGCCAACCTTAACTGAGGAGT-3′ (forward) and
5′-GGCAAGTTGATTGGAGGGATG-3′ (reverse); Snail,
5′-TCGGAAGCCTAACTACAGCGA-3′ (forward) and
5′-AGATGAGCATTGGCAGCGAG-3′ (reverse); Slug,
5′-CGAACTGGACACACATACAGTG-3′ (forward) and
5′-CTGAGGATCTCTGGTTGTGGT-3′ (reverse). Verification of the
expression levels of the genes was performed by qPCR using
TransStart Green qPCR SuperMix kit (TransGen Biotech).
Tissue processing, hematoxylin and eosin
staining, and immunohistochemistry
Mouse tumors, lungs and liver were fixed with
paraformaldehyde, embedded in paraffin, and then sectioned. Some
sections were incubated with hematoxylin and eosin (H&E),
dehydrated and mounted. For immunohistochemical analysis, sections
were boiled in retrieval solutions to expose the antigens, and
incubated at 4°C overnight with anti-Ki67 (1:100; ab16667; Abcam)
and anti-cleaved caspase-3 (1:300; 9661; Cell Signaling
Technology). The section-affixed slides were counterstained with
hematoxylin, dehydrated and mounted. The immunostaining results
were independently evaluated by two pathologists.
Animal experiments
Five-week-old female BALB/c nude mice were purchased
from the Institute of Laboratory Animal Science (Chinese Academy of
Medical Science, Beijing, China). All procedures for the animal
experiments were conducted according to the Animal Ethics Committee
of Chongqing Medical University. All the animals were randomly
divided into two groups of 9 mice each and housed according to the
national and institutional guidelines for humane animal care. At 6
weeks of age, the mice were perorally (p.o.) gavaged with either
100 µl ferulic acid (100 mg/kg weight) or normal saline
(control) and the animals were gavaged daily during the experiment.
At 7 weeks of age, the MDA-MB-231 cells were inoculated
subcutaneously into the right flanks of the mice
(1.5×106 cells/mouse). Body weights were monitored
weekly as an indicator of overall health. Tumor diameter was
measured every week, and tumor volumes were calculated with the
formula: tumor volume (mm3) = 0.5 × length (mm) ×
width2 (mm2). The mice were sacrificed via
CO2 asphyxiation 6 weeks after the transplant. Tumors
were then removed, weighed and sent for immunohistochemistry (IHC)
analysis.
For the tumor metastatic assay, six-week-old female
BALB/c nude mice were p.o. gavaged with 100 µl ferulic acid
(100 mg/kg weight) or normal saline (control) for one week. Then,
they were administered MDA-MB-231 cells (2.5×106 cells)
via the tail vein (6 mice in each group), and the treatment
continued until the end of the experiment. At the end of the
experiment, on day 28, the mice were sacrificed via CO2
asphyxiation. The lungs and livers were removed, and sent for
H&E staining.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
software. Data from all the experiments are presented as means ± SD
and represent 3 independent experiments. One-way analysis of
variance (ANOVA) or Student's t-test was used to compare means
between treatment groups. A P-value of <0.05 was considered to
indicate a statistically significant result.
Results
Effect of ferulic acid on breast cancer
proliferation and apoptosis
Breast cancer cell line MDA-MB-231 was treated with
various concentrations of ferulic acid (3, 10, 30 and 100
µM). As shown in Fig. 1B, we
observed that ferulic acid inhibited the cell proliferation of
breast cancer cell line MDA-MB-231 in a dose-dependent manner. To
further explore the effects of ferulic acid on cell apoptosis,
MDA-MB-231 cells were used for the analysis of apoptosis by FACS.
Following treatment with ferulic acid (10, 300 and 100 µM)
for 48 h, significantly increased apoptosis was observed when
breast cancer cells were treated with ferulic acid, which may
partly contribute to the decreased cell viability (Fig. 2A and B). Moreover, the effect of
ferulic acid on apoptosis was further confirmed by analyzing the
activation of caspase-3 in the apoptosis pathway. In addition, the
results showed that ferulic acid considerably enhanced the activity
of caspase-3 in breast cancer cells (Fig. 2C). Briefly, these data suggest that
ferulic acid suppresses breast cancer cell proliferation and
induces apoptosis.
Ferulic acid inhibits breast cancer cell
migration
To investigate the role of ferulic acid in breast
cancer metastasis, we investigated the migratory capacity of high
metastatic cell line MDA-MB-231. We firstly carried out wound
healing assays to evaluate the effects of ferulic acid on breast
cancer cell migration at the concentrations of 10, 30 and 100
µM. As shown in Fig. 3A and
B, the migration across the wound edges was markedly slower
after treatment with different concentrations of ferulic acid. From
the Transwell assay, we also found that ferulic acid
dose-dependently inhibited breast cancer cell migration (Fig. 3C and D). These results indicate that
ferulic acid inhibits breast cancer migration even at a low
concentration (10 µM) and that ferulic acid indeed possesses
the ability to inhibit tumor metastasis in vitro.
Ferulic acid induces reversal of EMT
EMT is thought to be a driver of invasion and
metastasis in different types of epithelial cancers (26). The most comprehensive theory
describing how initially quiescent tumor cells acquire metastatic
capability is EMT (27). A group of
pleiotropic transcription factors (TFs) have been found capable of
orchestrating EMT programs. Most EMT-TFs are transcriptional
repressors and many, such as Snail, Slug, ZEB1 and Twist, directly
repress mediators of epithelial adhesion, the most important of
which is E-cadherin, an integral component of adherens junctions
and claudins, which are necessary for the assembly of tight
junctions between adjacent cells (28–33).
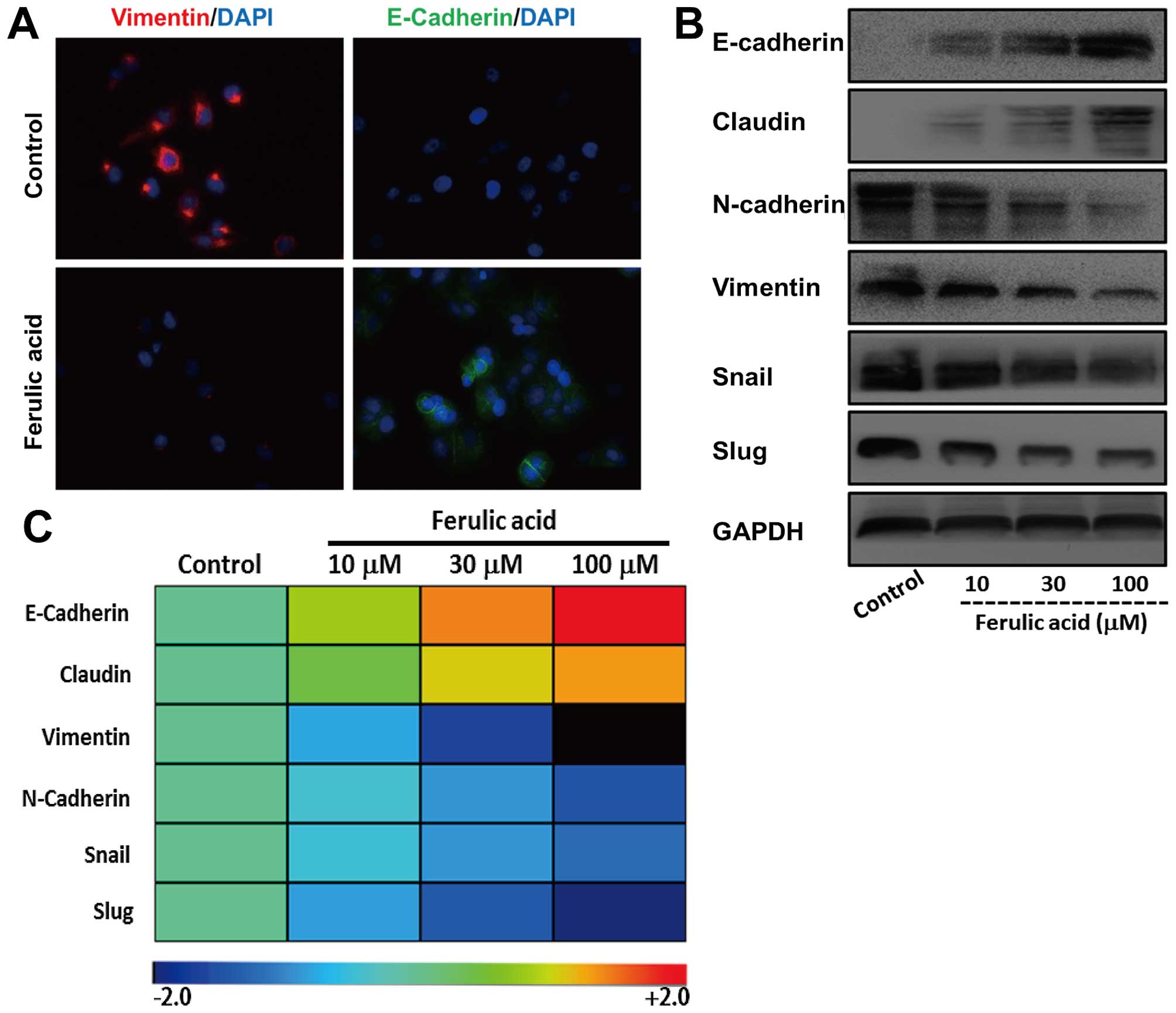
To evaluate the effect of ferulic acid on EMT, we treated
mesenchymal-like breast cancer cell line MDA-MB-231 with ferulic
acid at different concentrations (10, 30 and 100 µM). We
first detected the expression of E-cadherin and vimentin, a
mesenchymal intermediary filament by immunofluorescence. As shown
in Fig. 4A, the expression of
vimentin was markedly decreased after treatment with ferulic acid.
However, we observed a significant increase of E-cadherin.
Moreover, we investigated the effects of ferulic acid on the
expression of these EMT markers at protein and mRNA levels
(Fig. 4B and C). Consistent with
immunofluorescence, increased expression of epithelial markers and
decreased mesenchymal markers were demonstrated in ferulic
acid-treated cancer cells. Collectively, these observations support
that ferulic acid induces an effective switch from a mesenchymal to
an epithelial phenotype in breast cancer cells.
Ferulic acid exerts antitumor activities
in vivo
Based on the in vitro findings described
above, MDA-MB-231 xenograft model was employed to evaluate the
antitumor potential of ferulic acid in vivo. Ferulic acid
was orally initiated one week prior to tumor cell injection and
then continued until the end of the experiment (Fig. 5A). Oral administration of ferulic
acid had no detectable toxicity, as there were no differences in
body weight between the control and treatment groups, and no signs
of adverse health reactions, pain or distress were observed. As
shown in Fig. 5B and C, the tumors
observed from the xenografts treated with ferulic acid exhibited
smaller tumor volumes and lower tumor weights as compared with the
control mice. Consistent with the in vitro experiments, we
detected a significant decrease in proliferation (Ki67 staining)
and increase in apoptosis (active caspase-3 staining) in tumors
from the ferulic acid-treated mice (Fig. 5D). These in vivo data were
consistent with the in vitro results and confirmed that
ferulic acid exerts a marked antitumor activity against breast
cancer.
Ferulic acid suppresses the metastatic
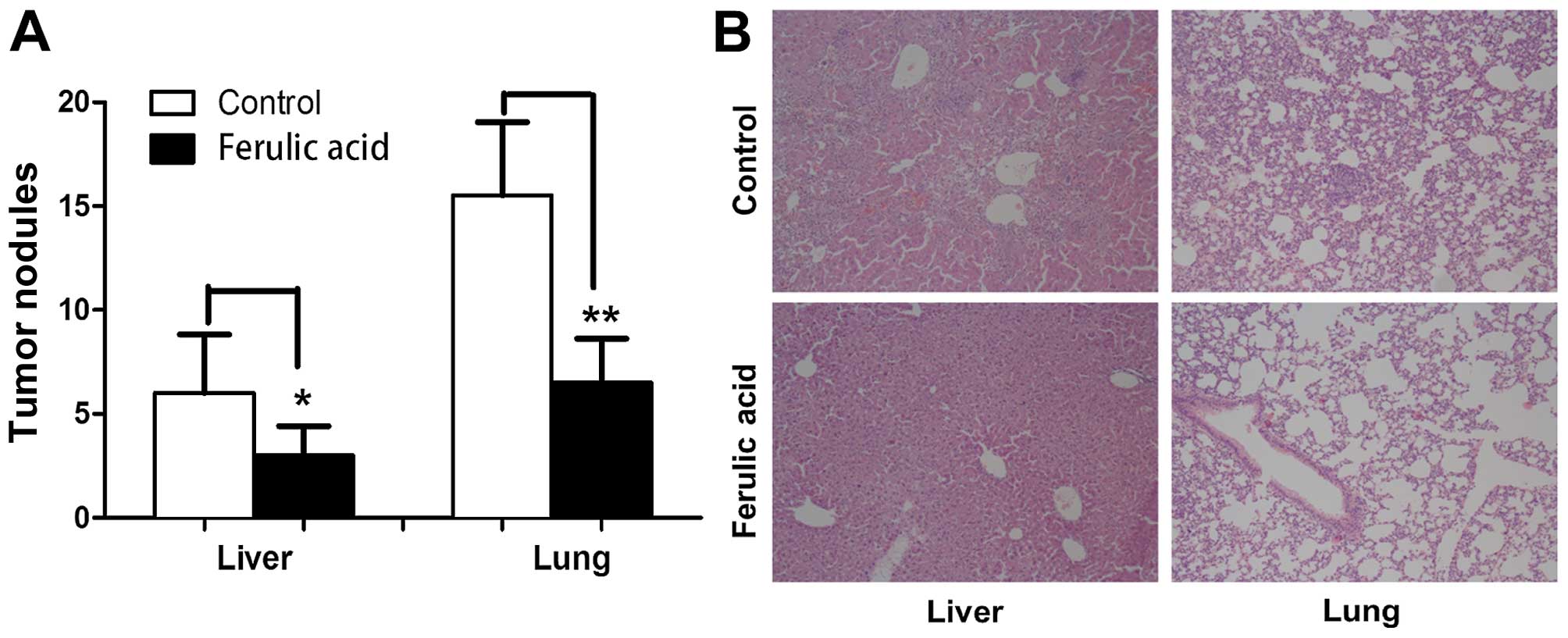
potential of breast cancer in vivo
To investigate whether ferulic acid can affect
breast cancer metastasis in vivo, we established a
metastatic model by injecting human breast cancer MDA-MB-231 cells
into the tail vein of BALB/c nude mice. The metastasis of
MDA-MB-231 cells into the lungs and liver was measured by H&E
staining 4 weeks after injection. As shown in Fig. 6A and B, the tumor nodules on the
surface of the lungs and liver were significantly decreased in the
ferulic acid-treated mice (100 mg/kg, daily). In summary, our data
demonstrated that ferulic acid significantly inhibits breast cancer
metastasis in vivo.
Discussion
The standard treatment for breast cancer includes
surgery, chemotherapy, hormonal therapy and radiotherapy. However,
there are several ̔bottle necks̓ for the treatment of breast
cancer, and metastasis is one of the main causes leading to
treatment failure. Since metastastic breast cancer patients have a
poor prognosis and a high mortality rate, preventing and inhibiting
the metastasis itself is crucial for controlling the disease,
prolonging survival, and enhancing the patient quality of life.
Chemotherapy is the main treatment strategy for metastatic breast
cancer. Since effective therapeutic drugs are limited and drug
resistance occurs frequently, to explore natural and alternative
cancer treatments which can improve the treatment of metastatic
breast cancer itself and mitigate the undesirable side-effects of
its treatment becomes more and more important. In the present
study, we found that ferulic acid, exhibited an antimigration
effect in vitro and in vivo.
Numerous studies have shown that ferulic acid is an
efficient scavenger of both reactive oxygen and nitrogen species
(ROS and RNS, respectively), and several lines of evidence have
also demonstrated that the cytoprotective effects of ferulic acid
could be attributable to the downregulation of pathways involved in
cell death and the upregulation of gene/proteins which are able to
enhance the cell stress response (34). It is widely accepted that
carcinogenesis is a multi-stage and complex process, such as
enhanced cell proliferation, chronic inflammation, free radical
formation and the following damage to DNA, abnormal activation of
proinflammatory pathways including cyclooxygenases and NOS. In
addition, each of these events not only plays a key role in
carcinogenesis per se, but also contributes to strengthening
the toxic potential of the others, thus amplifying the cell
proliferative cascade. In light of this, the ability of ferulic
acid to scavenge free radicals, stimulate cytoprotective enzymes
and inhibit cytotoxic systems account for the potential adjuvant
role of ferulic acid in cancer therapy. Additionally, previous
studies have reported the antineoplastic activity of ferulic acid
in various types of cancers. For example, it was reported that
ferulic acid exhibited antiproliferative effects on Caco-2 colon
cancer cells by upregulating RABGAP1 and CEP2, which were involved
in centrosome assembly as well as the gene for the S phase
checkpoint protein SMC1L1 (20).
Additionally, another study demonstrated that ferulic acid had
significantly reduced plasma levels of lactic dehydrogenase and
alkaline phosphatase in nicotine-treated rats by counteracting the
nicotine-induced lipoperoxidation and DNA damage (35,36).
Although studies concerning the antitumor activity of ferulic acid
are limited, a growing body of evidence supports the potentially
important role in free radical-induced cancers. In the present
study, we showed that ferulic acid effectively inhibited breast
cancer cell proliferation and induced apoptosis, which support
ferulic acid as an antitumor agent in breast cancer treatment.
EMT occurs during the early stages of the
transformation of a tumor into a malignant neoplasm and is required
for breast cancer metastasis (37).
The concept that EMT involves the formation of metastatic cancer
cells is based on the observation that acquisition of mesenchymal
markers such as vimentin, Snail and Twist by epithelial carcinoma
cells is associated with increased metastatic potential (38), as is nuclear overexpression of
β-catenin (39) and loss of
epithelial cell adhesion molecules such as E-cadherin (38–40).
Moreover, accumulating evidence has demonstrated that EMT could
result in cancer stem cell transformation, drug resistance and
poorer prognosis for many types of human cancers. Therefore,
pharmacologic inhibition of EMT or shutting down the function of
EMT-TFs such as Twist, Snail and ZEB1 may be instrumental in cancer
prevention and treatment (41,42).
In the present study, we verified the reversal effect of ferulic
acid on EMT in metastatic breast cancer cell lines. Our results
indicated that inhibition of EMT or inhibiting the function of
EMT-TFs may be another mechanism involved in the antitumor activity
of these herbal plant extracts.
Taken together, we first demonstrated the antitumor
activity of ferulic acid in vitro and in vivo, and
then we confirmed that ferulic acid could suppress breast cancer
migration and metastasis by inhibiting the EMT process. These
results strongly indicate the potential role of ferulic acid to
inhibit both the growth and the metastasis of human breast cancer
cells by mediating EMT. Furthermore, our findings support the
conclusion that ferulic acid may be a potential candidate for
metastastic breast cancer therapy.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81472475).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vanneman M and Dranoff G: Combining
immunotherapy and targeted therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nicolini A, Giardino R, Carpi A, Ferrari
P, Anselmi L, Colosimo S, Conte M, Fini M, Giavaresi G, Berti P, et
al: Metastatic breast cancer: An updating. Biomed Pharmacother.
60:548–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rubens RD: 7. Management of advanced
breast cancer. Int J Clin Pract. 55:676–679. 2001.
|
|
6
|
Yardley DA: Visceral disease in patients
with metastatic breast cancer: Efficacy and safety of treatment
with ixabepilone and other chemotherapeutic agents. Clin Breast
Cancer. 10:64–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieto MA: The ins and outs of the
epithelial to mesenchymal transition in health and disease. Annu
Rev Cell Dev Biol. 27:347–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: Concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shook D and Keller R: Mechanisms,
mechanics and function of epithelial-mesenchymal transitions in
early development. Mech Dev. 120:1351–1383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP and Lim CT: Tumor dissemination:
An EMT affair. Cancer Cell. 23:272–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bill R and Christofori G: The relevance of
EMT in breast cancer metastasis: Correlation or causality? FEBS
Lett. 589:1577–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
15
|
Armstrong AJ, Marengo MS, Oltean S, Kemeny
G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ and
Garcia-Blanco MA: Circulating tumor cells from patients with
advanced prostate and breast cancer display both epithelial and
mesenchymal markers. Mol Cancer Res. 9:997–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Sarkissyan M and Vadgama JV:
Epithelial-mesenchymal transition and breast cancer. J Clin Med.
5:pii: E13. 2016. View Article : Google Scholar
|
|
17
|
Sgarbossa A, Giacomazza D and di Carlo M:
Ferulic Acid: A hope for Alzheimer's disease therapy from plants.
Nutrients. 7:5764–5782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanski J, Aksenova M, Stoyanova A and
Butterfield DA: Ferulic acid antioxidant protection against
hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal
cell culture systems in vitro: Structure-activity studies. J Nutr
Biochem. 13:273–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mancuso C and Santangelo R: Ferulic acid:
Pharmacological and toxicological aspects. Food Chem Toxicol.
65:185–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janicke B, Hegardt C, Krogh M, Onning G,
Akesson B, Cirenajwis HM and Oredsson SM: The antiproliferative
effect of dietary fiber phenolic compounds ferulic acid and
p-coumaric acid on the cell cycle of Caco-2 cells. Nutr Cancer.
63:611–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alam MA, Sernia C and Brown L: Ferulic
acid improves cardiovascular and kidney structure and function in
hypertensive rats. J Cardiovasc Pharmacol. 61:240–249. 2013.
View Article : Google Scholar
|
|
22
|
Roy S, Metya SK, Sannigrahi S, Rahaman N
and Ahmed F: Treatment with ferulic acid to rats with
streptozotocin-induced diabetes: Effects on oxidative stress,
pro-inflammatory cytokines, and apoptosis in the pancreatic β cell.
Endocrine. 44:369–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin FH, Lin JY, Gupta RD, Tournas JA,
Burch JA, Selim MA, Monteiro-Riviere NA, Grichnik JM, Zielinski J
and Pinnell SR: Ferulic acid stabilizes a solution of vitamins C
and E and doubles its photoprotection of skin. J Invest Dermatol.
125:826–832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jayaprakasam B, Vanisree M, Zhang Y,
Dewitt DL and Nair MG: Impact of alkyl esters of caffeic and
ferulic acids on tumor cell proliferation, cyclooxygenase enzyme,
and lipid peroxidation. J Agric Food Chem. 54:5375–5381. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cole L, Anderson M, Antin PB and Limesand
SW: One process for pancreatic beta-cell coalescence into islets
involves an epithelial-mesenchymal transition. J Endocrinol.
203:19–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomes LR, Terra LF, Sogayar MC and
Labriola L: Epithelial-mesenchymal transition: Implications in
cancer progression and metastasis. Curr Pharm Biotechnol.
12:1881–1890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eger A, Aigner K, Sonderegger S, Dampier
B, Oehler S, Schreiber M, Berx G, Cano A, Beug H and Foisner R:
DeltaEF1 is a transcriptional repressor of E-cadherin and regulates
epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: Direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martínez-Estrada OM, Cullerés A, Soriano
FX, Peinado H, Bolós V, Martínez FO, Reina M, Cano A, Fabre M and
Vilaró S: The transcription factors Slug and Snail act as
repressors of Claudin-1 expression in epithelial cells. Biochem J.
394:449–457. 2006. View Article : Google Scholar :
|
|
34
|
Barone E, Calabrese V and Mancuso C:
Ferulic acid and its therapeutic potential as a hormetin for
age-related diseases. Biogerontology. 10:97–108. 2009. View Article : Google Scholar
|
|
35
|
Adluri RS, Nagarajan D, Periyaswamy V and
Venugopal PM: Dose-response effect of ferulic acid against
nicotine-induced tissue damage and altered lipid levels in
experimental rats: A pathohistological evaluation. Fundam Clin
Pharmacol. 22:557–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sudheer AR, Muthukumaran S, Kalpana C,
Srinivasan M and Menon VP: Protective effect of ferulic acid on
nicotine-induced DNA damage and cellular changes in cultured rat
peripheral blood lymphocytes: A comparison with N-acetylcysteine.
Toxicol In Vitro. 21:576–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thompson EW, Torri J, Sabol M, Sommers CL,
Byers S, Valverius EM, Martin GR, Lippman ME, Stampfer MR and
Dickson RB: Oncogene-induced basement membrane invasiveness in
human mammary epithelial cells. Clin Exp Metastasis. 12:181–194.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brabletz T, Jung A, Hermann K, Günther K,
Hohenberger W and Kirchner T: Nuclear overexpression of the
oncoprotein beta-catenin in colorectal cancer is localized
predominantly at the invasion front. Pathol Res Pract. 194:701–704.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xue C, Plieth D, Venkov C, Xu C and
Neilson EG: The gatekeeper effect of epithelial-mesenchymal
transition regulates the frequency of breast cancer metastasis.
Cancer Res. 63:3386–3394. 2003.PubMed/NCBI
|
|
41
|
Dave B, Mittal V, Tan N; Toxicol In Vitro
M; Chang JC: Epithelial-mesenchymal transition, cancer stem cells
and treatment resistance. Breast Cancer Res. 14:2022012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar
|