Introduction
There is a subpopulation of cancer cells in tumors
that possess the ability to initiate neoplasms and have the
property of self-renewal. These are called cancer stem cells (CSCs)
or tumor-initiating cells (TICs) (1). Meantime, somatic cells can be
reprogrammed into induced pluripotent stem cells (iPSCs) by defined
transduction factors Oct4, Klf4, Sox2 and c-Myc (OKSM) (2). Incomplete reprogramming is the process
by which cells are dedifferentiated by defined factors but do not
reach the pluripotent stage (3).
However, these partially reprogrammed cells have the ability of
tumorigenesis in vivo (4).
In addition, the formation of teratomas in immunodeficient mice is
one of the properties of identical iPSCs (5). Therefore, there is a parallel pathway
between the reprogramming of iPSCs and tumorigenesis. TICs in
cancers could be recognized as the products of endogenous
reprogramming (6).
Among the defined factors (OKSM), c-Myc is a
pro-oncogene (7) whereas Klf4 could
be an oncogene or tumor suppressor (8). In addition, c-Myc can cause genetic
instability in iPSC reprogramming but re-expression of Klf4 could
counteract the genetic instability in these cells (9). This neutralization guarantees the
reprogramming of cells toward the direction to iPSCs but not toward
the way to neoplasms. Oct4 and Sox2 are demonstrated to be good
indicators of stem-like capacity (10). Neither Oct4- nor Sox2-knockout mice
survive during development of the embryo. Oct4 alone can reprogram
neural mouse stem cells into iPSCs in the presence of endogenous
Sox2 expression (11) suggesting
that Oct4 and Sox2 are indispensable on the road to reprogramming.
However, it is not clear, apart from stem cell function, whether
Oct4 or Sox2 plays a crucial role in the development and
progression of human cancer.
In our previous studies, Oct4 and Sox2
double-positive cells (Oct4+Sox2+) were found
in the precancerous lesions of the oral mucosa (12), implying that these cells may be
undergoing reprogramming into TICs. In addition, in another study,
we established an immortalized oral epithelial cell line
(hTERT+-OME) by human telomerase reverse transcriptase
(hTERT) transduction and discovered that this cell line is an ideal
model for the study of parallels of reprogramming and tumorigenesis
(13). In the present study, we
proposed that Oct4+Sox2+ cells may be
reprogrammed TICs inducing oral carcinogenesis, and this hypothesis
was studied using a cell model. This hypothesis was examined by
detecting the increasing tumorigenesis of Oct4/Sox2 transduction
into the hTERT+-OME cell line. In addition, two oral
squamous cell carcinoma (OSCC) cell lines were used to examine the
decreased tumorigenesis by Oct4/Sox2 knockdown.
Materials and methods
Cell lines
Twelve cell groups from three cell lines were used
in the present study. hTERT+-OME is an immortalized cell
line created by hTERT gene transduction into primary cultured oral
mucosal epithelial (OME) cells (13). Human tongue squamous cell carcinoma
cell line (Cal27) was obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). Gca1551 is a cell line
established by primary cultured cells from a 64-year-old man with
gingival squamous cell carcinoma with lymph node metastasis
(T2N2M0). hTERT+-O+-OME,
hTERT+-S+-OME,
hTERT+-OS+-OME, Cal27-Olow,
Cal27-Slow, Cal27-OlowSlow,
Gca1551-Olow, Gca1551-Slow and
Gca1551-OlowSlow cells were derived by our
group (see below). Ethical approval was obtained from the Ethics
Committee of Zhengzhou University (reference no.,
20130523-10-2).
Establishment of Gca1551 cells
Human gingival carcinoma primary tumor samples were
obtained within 1 h after surgery. The tissues were minced with
blades into small pieces. These pieces were enzymatically digested
using 0.25% dispase II (Sigma, St. Louis, MO, USA) at 4°C
overnight. After digestion with 0.25% trypsin (Sigma) for 10 min at
37°C, the tissue was triturated with a pipette and passed through a
200-mm cell strainer. Then, the cells were centrifuged at 300 × g
for 5 min, re-suspended in Dulbecco's modified Eagle's
medium:nutrient mixture (DMEM/F12) with 10% fetal bovine serum
(FBS), and plated in 6-well plates. Once the cell clones emerged,
they were removed by 0.25% trypsin digestion and cultured in
plates. The cells that were not attached after 20 min were
collected to purify floating cancer cells from the more rapidly
adhering fibroblasts. The collected cells were centrifuged and
plated in the new flasks at a density of 1,000
cells/cm2. The process was repeated several times. The
purified cancer cells were acquired and this cell line was named as
Gca1551.
Cell culture
All the cell lines were cultured in a basic medium
that was comprised of DMEM/F12 supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin (all from Sigma). This
basic medium was named as 'A' in the present study and the
culturing details of all cell lines are described in Table I.
 | Table ICulture medium of the cell lines. |
Table I
Culture medium of the cell lines.
| Cell line | Culture medium |
|---|
|
hTERT+-OME | A + 200
µg/ml G418 |
|
hTERT+-O+-OME | A + 200
µg/ml G418 + 2.5 µg/ml puromycin |
|
hTERT+-S+-OME | A + 200
µg/ml G418 + 200 µg/ml hygromycin |
|
hTERT+-OS+-OME | A + 200
µg/ml G418 + 2.5 µg/ml puromycin + 200 µg/ml
hygromycin |
| Cal27, Gca1551 | A |
|
Cal27-Olow,
Gca1551-Olow | A + 2.5
µg/ml puromycin |
|
Cal27-Slow,
Gca1551-Slow | A + 2.5
µg/ml puromycin |
|
Cal27-OlowSlow,
Gca1551-OlowSlow | A + 2.5
µg/ml puromycin |
In the process of cell reprogramming,
hTERT+-OME, hTERT+-O+-OME,
hTERT+-S+-OME and
hTERT+-OS+-OME were cultured in TeSR™-E7 and
TeSR™-E8 media (StemCell Technologies, Vancouver, BC, Canada)
according to the manufacturer's protocol.
The sphere medium and culture method were the same
as previously described (13).
Briefly, the medium contained no FBS and was replaced by B27
(serum-free supplement from Invitrogen, Carlsbad, CA, USA). In
addition, 20 ng/ml of epidermal growth factor (Invitrogen) and
basic fibroblast growth factor (BD Biosciences, San Jose, CA, USA)
were added.
Cell transduction and reprogramming
hTERT+-OME cells were transduced with
lentiviral vectors encoding nuclear reprogramming factors. Briefly,
the lentiviral vector plasmids pMXs-hOCT4-GFP (GenePharma,
Shanghai, China) and mCMV-hSOX2-mCherry (BioWit Technologies,
Shenzhen, China) were introduced into 293T cells using RNAi-Mate
transfection reagent. After 48 h, the virus-containing supernatants
were passed through a 0.45-mm filter and supplemented with 5 mg/ml
Polybrene. hTERT+-OME cells were seeded at
6×105 cells/100-mm dish 24 h before incubation in the
virus/Polybrene-containing supernatants for 24 h. The cells were
then washed and returned to fresh DMEM/F12 medium. Subsequently,
the GFP and mCherry-positive clones were acquired by adding 2.5
µg/ml puromycin and 200 µg/ml hygromycin,
respectively. After 7 days, the cells were cultured in ultra-low
attachment dishes and the medium was replaced with TeSR™-E7 medium.
After 1 week, the cells were attached on Matrigel (BD
Biosciences)-coated plates with TeSR™-E7 medium for 7 days. Then,
the culture medium was replaced with TeSR™-E8 medium for an
additional 7 days. After transduction and reprogramming, cell
colonies were plated in an attachment dish with DMEM/F12 medium
supplemented with 10% FBS. The cells were maintained at 37°C in a
5% CO2 incubator for these days. Thereafter, three cell
lines (hTERT+-O+-OME,
hTERT+-S+-OME and
hTERT+-OS+-OME) were obtained.
Cell transfection and gene knockdown
Two different shRNA sequences were inserted into
plasmid vector pVSV-G. 48–72 h after transient transfection. The
transfection efficiency was examined using fluorescence microscopy
(Axio Observer A1; Carl Zeiss, Jena, Germany) to choose the cells
with the most effective transfection. The sequence was then cloned
into the pGLV3/H1 and pGLV10/U6 vectors for lentiviral-mediated
knockdown of Oct4 and Sox2, respectively. The chosen short hairpin
sequence specific for Oct4 was as follows:
(5′-CCTTCGCA1551AGCCCTCATTT-3′) and Sox2
(5′-GCA1551GACTTCACATGTCCCAGC-3′). These complete vectors were
named as LV3 (H1/GFP&Puro)-Oct4 and LV10
(U6/RFP&Puro)-Sox2. The Cal27 and Gca1551 cells were stably
transfected with LV3 (H1/GFP&Puro)-Oct4 and LV10
(U6/RFP&Puro)-Sox2, respectively, according to the
manufacturer's protocol (GenePharma). Subsequently, the single GFP-
or RFP-positive clones were acquired by adding 2.5 µg/ml
puromycin. The double GFP- and RFP-positive clones were selected by
sorting using a flow cytometer (Aria II; BD Biosciences). After
that, 6 fresh cell lines (Cal27-Olow,
Cal27-Slow, Cal27-OlowSlow,
Gca1551-Olow, Gca1551-Slow and
Gca1551-OlowSlow) were formed. All cells were
tested for Oct4 and Sox2 protein expression by western
blotting.
Western blotting
Eight cell lines (Cal27, Cal27-Olow,
Cal27-Slow, Cal27-OlowSlow,
Gca1551, Gca1551-Olow, Gca1551-Slow and
Gca1551-OlowSlow) were analyzed by western
blotting. The total protein was extracted from the cultured cells
using lysis buffer (Bio-Rad, Hercules, CA, USA). Proteins (40
µg) were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) with 10% polyacrylamide gels and
transferred to polyvinylidene fluoride membranes (Bio-Rad). The
membranes were then incubated with primary antibodies overnight at
4°C, washed twice and incubated with horseradish
peroxidase-conjugated secondary antibody (Bio-Rad) for 2 h at room
temperature. Subsequently, the protein bands were detected by
enhanced chemiluminescence, visualized using a VersaDoc-MP Imaging
system (Bio-Rad). The antibodies used were goat anti-rabbit-Oct4,
goat anti-rabbit-Sox2 (both from Stemgent, San Diego, CA, USA) and
goat anti-mouse-GAPDH (Boster, Wuhan, China). The concentrations
used were 1:500 (Oct4), 1:500 (Sox2) and 1:1,000 (GAPDH),
respectively.
In vivo tumor formation assay
One hundred and twenty 5-week-old non-obese diabetic
severe combined immunodeficiency (NOD/SCID) mice (16–18 g, all
males) (Vital River Laboratories, Beijing, China) were divided into
12 groups, with 10 mice in each group. Twelve groups of cells were
washed twice with antibiotic- and serum-free cell culture medium
and finally resuspended in 0.1 ml of serum-free culture medium. The
cell suspension was then mixed with an equal volume of Matrigel (BD
Biosciences) and injected subcutaneously into the mice. The tumors
that formed were surgically removed after 21 days. All the
xenografts of oral squamous cell carcinoma were weighed.
Representative tumor tissues were fixed in 3% formalin, embedded in
paraffin wax and sectioned at a thickness of 10 µm. The
sections were stained with hematoxylin and eosin (H&E) for
pathological examination, or processed for immunohistochemical
analysis. The experiments were carried out in accordance with the
guidelines issued by the ethics Committee of Zhengzhou
University.
Immunohistochemistry of tumor tissue
sections
For immunohistochemistry, paraffin-embedded tissue
sections were removed of paraffin in xylene, and dehydrated in
alcohol. Afterwards, the sections were subjected to antigen
retrieval with a steam pressure cooker (120°C, 5 min) in citrate
buffer (pH 6.0), washed with phosphate-buffered saline (PBS) and
then blocked with 5% goat serum. Primary antibodies were diluted in
0.1% bovine serum albumin (BSA) and incubated with the sections
overnight at 4°C. After incubation with the secondary antibody at
room temperature for 60 min, the sections were stained with SP link
detection kits (ZSGB-BIO, Beijing, China) according to the
manufacturer's instructions. The primary antibodies used in the
present study were as follows: goat anti-rabbit-Oct4 (1:200), goat
anti-rabbit-Sox2 (1:200) (both from Stemgent), goat anti-rabbit-CK5
(1:100), goat anti-rabbit-CK19 (1:100), goat anti-rabbit-vimentin
(1:100), goat anti-rabbit-Ki-67 (1:100), goat anti rabbit-CD31
(1:100) and goat anti rabbit-calponin (1:100) (all from Bioss,
Beijing, China).
Statistical analysis
All values for tumors weight are presented as mean ±
standard deviation. Comparisons between four groups of two cell
lines were performed by one-way ANOVA. The significance level was
assigned at P<0.05. The statistical tests were performed with
the program, Statistical Package for Social Sciences (IBM SPSS
22.0; IBM SPSS, Armonk, NY, USA).
Results
Reprogramming of human immortalized oral
mucosal epithelial cells by defined reprogramming factors
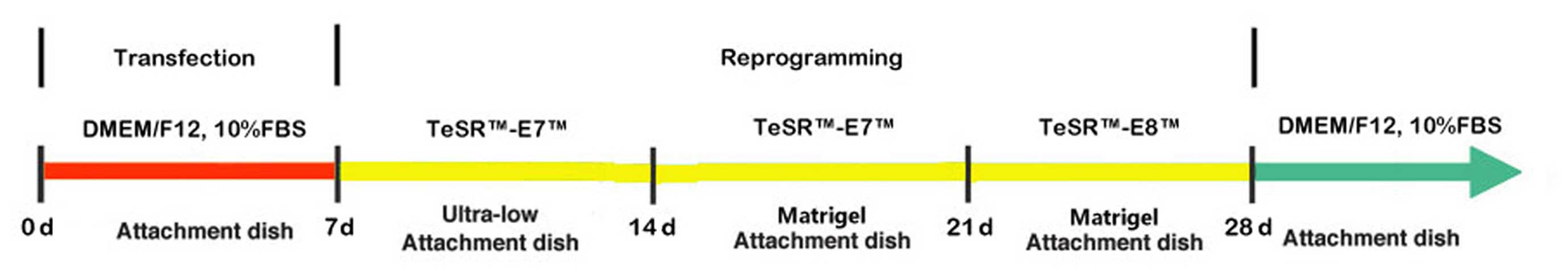
The lentiviral-mediated introduction of Oct4 and
Sox2 (both or individual) into hTERT+-OME cells,
followed by a series of reprogramming steps (Fig. 1), gave rise to the expression of
Oct4 and Sox2 proteins in the nuclei of the cells after 28 days.
The entire process was recorded by fluorescence microscopy
(Fig. 2).
Tumorigenesis of
hTERT+-OS+-OME cells in vivo
The ability of hTERT+-OS+-OME
cells to form tumors in the xenograft model was assessed by
injection of hTERT+-OME,
hTERT+-O+-OME,
hTERT+-S+-OME and
hTERT+-OS+-OME cells subcutaneously into
NOD/SCID mice, respectively and then the tumors were monitored for
21 days. As a result, only the hTERT+-OS+-OME
group of cells was able to initiate tumor formation in the mice
(Fig. 3A). Histological
examinations of the tumors revealed that
hTERT+-OS+-OME cells produced tumors with
local invasion (Fig. 3B).
Immunohistochemical studies demonstrated that the tumors had
differentiation from epithelial cells (CK5- and CK19-positive) with
mesenchymal properties (vimentin-positive). In addition, we
detected that Oct4 and Sox2 transduced into the
hTERT+-OS+-OME cells were positively
expressed in the xenografts (Fig.
3B).
Tumorigenesis of shRNA-mediated OSCC
cells in vivo
Western blot analysis showed that the expression
levels of Oct4 and Sox2 were effectively altered by shRNA
transfection in the Cal27 and Gca1551 cells. Downregulation of Sox2
by shRNA led to the slight downregulation or no change of Oct4
expression, while shRNA-mediated repression of Oct4 induced the
upregulation of Sox2 expression (Fig.
4). In the xenograft model, Sox2low and
Oct4lowSox2low cells decreased the size of
tumors in the immunodeficient mice equally. In contrast, the single
knockdown Oct4low cancer cells had increased tumor size
when compared to the untreated cells (Fig. 5). The weight of each group of tumors
was measured and the results are detailed in graphical
representation (Fig. 6A). Moreover,
the histological examinations demonstrated that Sox2low
or Oct4lowSox2low cells caused low
histological grade carcinoma in the xenografts, while the
Oct4low cancer cells formed more aggressive xenografts
comprised of high grade carcinoma (Fig.
6B).
Discussion
The ambiguous origin of tumor-initiating cells
(TICs) makes these cells difficult to be eliminated by conventional
adjuvant therapy and induces tumor relapse and metastasis. One of
the possible origins of TICs is from adult stem cells since adult
stem cells can survive for several years with accumulation of
epigenetic and genetic alterations (14). In contrast, the origin of induced
pluripotent stem cells that are reprogrammed from somatic cells is
under debate. It is argued that these parental reprogrammed cells
actually are adult stem cells which reside in tissues when the
samples are collected for primary culture (15). Adult stem cells own limited
differentiation behavior in contrast to embryonic stem cells. They
may be the residues of the embryo in the body since various adult
stem cells express the same molecules that are found in embryonic
stem cells (16). It is possible
that there is a common origin of adult stem cells, TICs and iPSCs.
Cell reprogramming between these cells may be a potential way to
tumorigenesis.
Nishi et al acquired cells with malignant
stem cell properties from MCF-10A mammary epithelial cells by OSKM
transduction (17). Both MCF-10A
(18) and hTERT+-OME
cells were non-tumorigenic immortalized epithelial cells. In
contrast to the findings of Nishi et al, the present study
showed that hTERT+-OS+-OME cells initiated
tumors in a mouse model but not invasive cancer. A possible
explanation is that factors Klf4 and c-Myc control the property of
the neoplasm but factors Oct4 and Sox2 play a pivotal role in
neoplasm derivation. This viewpoint is supported by another
independent study that reported that dysplastic epithelial cells
(pre-cancer) were observed in several organs in one mouse when Oct4
was forciby expressed in gene-editing mice (19).
Oct4 expresses its Oct4B variant in the cytoplasm of
embryonic stem cells and numerous somatic cells without stem cell
function (20). Sox2 plays the role
of the cytoskeleton when it is expressed in the cytoplasm (21). Oct4 and Sox2 expressed in the
cytoplasm cannot play a role of reprogramming since chromatin
remodeling occurs in cell nuclei. The cellular nuclear
translocation of Oct4 was noted when hTERT+-OME cells
were cultured in a 3-dimentional environment as we previously
demonstrated (13). However,
neither hTERT+-O+-OME nor
hTERT+-S+-OME cells triggered tumor formation
in immunodeficient mice although the
hTERT+-S+-OME cells particularly underwent
3-dimentional culture to acquire spontaneous Oct4 nuclear
expression in the present study. These findings showed that
hTERT+-OME cells cannot move into reprogramming
spontaneously without exogenous Oct4 gene transduction. In
pathological examination, we found that human oral precancerous
lesions cannot induce xenograft tumors in immunodeficient mice
despite the fact that Oct4+Sox2+ profile
cells were observed in oral lichen planus and oral leukoplakia
(12,22). This raised the question of why
hTERT+OS+-OME cells have the ability of
tumorigenesis in the present study. Arnold et al reported
that Sox2+ adult stem cells in several epithelia
originate from fetal Sox2+ tissue progenitors by
developmental fate mapping (23).
This implies that Sox2+ adult stem cells, not
Oct4+ cells, in normal epithelial cells of oral mucosa
may be residues of the embryo. The
hTERT+-S+-OME cells cultured in suspension to
acquire Oct4 nuclear expression resembles a situation in which
specific Sox2+ adult stem cells in tissues accumulate
active Oct4 variant with nuclear expression. This may occur when
there are changes in the surrounding microenvironment, such as an
inflammatory reaction, to form Oct4+Sox2+
profile cells in vivo. Only by transducing exogenous Oct4
into cells in vitro, can the genome be reprogrammed into the
direction of neoplasm.
Oct4 is known to bind in partnership with Sox2 to
form Oct-Sox enhancers. Downregulation of Sox2 causes
downregulation of Oct4 activators and upregulation of Oct4
repressors, resulting in the gradual downregulation of Sox2
(24). In melanoma cells, RNA
interference-mediated knockdown of Oct4 led to diminished TIC
phenotypes (25). Sox2 knockdown
delayed tumor formation in xenograft tumor initiation models of
breast cancer (26). An independent
study demonstrated that when Sox2 was elevated ~2-fold in embryonic
stem cells, the levels of Oct4 did not change (27). Notably, another independent study
indicated that once the level of Oct4 in embryonic stem cells was
increased, the Sox2 level began to decrease at the RNA level
(28). In the present study, by
knocking down the expression of Oct4 and Sox2 in Cal27 and Gca1551
OSCC cells, Sox2low and
Oct4lowSox2low cells exhibited equal tumor
inhibition in vivo and low grade carcinoma in the
xenografts. In contrast, the single knockdown Oct4low
cancer cells increased tumor size in mouse models and the cancer
cells formed higher grade carcinoma in xenografts than the
untreated cells. Our results indicated that, at least at the
protein level, RNA interference of Sox2 may suppress Sox2 and
further Oct4 expression via a positive loop, while downregulation
of Oct4 could upregulate Sox2 expression via negative feedback in
Oct4low cancer cells. An explanation is that elevating
or decreasing either Sox2 or Oct4 in specific cells changes the
critical ratio between these two master regulators. This imbalance
then induces the cell differentiation in embryonic stem cells and
dedifferentiation in tumor cells. However, this hypothesis has not
been well studied in the scientific world. Since Sox2+
cells were noted in primary sites of oral cancer with lymph node
metastasis in our previous study (22), combined with these findings
discovered in the present study, it is suggested that Sox2
inhibition, not Oct4, should be a therapeutic target for oral
cancer. In addition, Oct4 may have a dual-character (oncogene or
tumor suppressor) in OSCC development. Exploring this hypothesis is
the subject of further study.
To conclude, somatic cells can be reprogrammed into
TICs as represented by the hTERT+-OS+-OME
cells in the present study. In addition, oral carcinogenesis may be
derived from Oct4+Sox2+ reprogrammed TICs in
which Oct4 plays the role of derivation while Sox2 plays the role
of stem cell property. In the absence of Oct4 expression, neoplasms
could not be initiated from normal tissues. Without Sox2
expression, the neoplastic cells could not be self-renewed to
maintain tumor growth. By studying these processes of induced
reprogramming, novel insights into the origins and control of
epigenetic alterations in human neoplasms may be achieved.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81200796), the
Zhengzhou Department for Science and Technology (grant no.
131PPTGG409-20), and funding from the Youth Foundation of The First
Affiliated Hospital of Zhengzhou University.
References
|
1
|
González-Moles MA, Scully C, Ruiz-Ávila I
and Plaza-Campillo JJ: The cancer stem cell hypothesis applied to
oral carcinoma. Oral Oncol. 49:738–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mattout A, Biran A and Meshorer E: Global
epigenetic changes during somatic cell reprogramming to iPS cells.
J Mol Cell Biol. 3:341–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohnishi K, Semi K, Yamamoto T, Shimizu M,
Tanaka A, Mitsunaga K, Okita K, Osafune K, Arioka Y, Maeda T, et
al: Premature termination of reprogramming in vivo leads to cancer
development through altered epigenetic regulation. Cell.
156:663–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan X, Qin H, Qu C, Tuan RS, Shi S and
Huang GT: iPS cells reprogrammed from human mesenchymal-like
stem/progenitor cells of dental tissue origin. Stem Cells Dev.
19:469–480. 2010. View Article : Google Scholar :
|
|
6
|
Hobbs RM and Polo JM: Reprogramming can be
a transforming experience. Cell Stem Cell. 14:269–271. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Biol. 6:635–645. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Karim EA, Hagos EG, Ghaleb AM, Yu B and
Yang VW: Krüppel-like factor 4 regulates genetic stability in mouse
embryonic fibroblasts. Mol Cancer. 12:892013. View Article : Google Scholar
|
|
9
|
Yamanaka S: Pluripotency and nuclear
reprogramming. Philos Trans R Soc Lond B Biol Sci. 363:2079–2087.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin SL: Concise review: Deciphering the
mechanism behind induced pluripotent stem cell generation. Stem
Cells. 29:1645–1649. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JB, Sebastiano V, Wu G, Araúzo-Bravo
MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et
al: Oct4-induced pluripotency in adult neural stem cells. Cell.
136:411–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Islam F, Qiao B, Smith RA, Gopalan V and
Lam AK: Cancer stem cell: Fundamental experimental pathological
concepts and updates. Exp Mol Pathol. 98:184–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiao B, Gopalan V, Chen Z, Smith RA, Tao Q
and Lam AK: Epithelial-mesenchymal transition and
mesenchymal-epithelial transition are essential for the acquisition
of stem cell properties in hTERT-immortalised oral epithelial
cells. Biol Cell. 104:476–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smalley M and Ashworth A: Stem cells and
breast cancer: A field in transit. Nat Rev Cancer. 3:832–844. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugii S, Kida Y, Berggren WT and Evans RM:
Feeder-dependent and feeder-independent iPS cell derivation from
human and mouse adipose stem cells. Nat Protoc. 6:346–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellis P, Fagan BM, Magness ST, Hutton S,
Taranova O, Hayashi S, McMahon A, Rao M and Pevny L: SOX2, a
persistent marker for multipotential neural stem cells derived from
embryonic stem cells, the embryo or the adult. Dev Neurosci.
26:148–165. 2004. View Article : Google Scholar
|
|
17
|
Nishi M, Sakai Y, Akutsu H, Nagashima Y,
Quinn G, Masui S, Kimura H, Perrem K, Umezawa A, Yamamoto N, et al:
Induction of cells with cancer stem cell properties from
nontumorigenic human mammary epithelial cells by defined
reprogramming factors. Oncogene. 33:643–652. 2014. View Article : Google Scholar
|
|
18
|
Soule HD, Maloney TM, Wolman SR, Peterson
WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF and Brooks
SC: Isolation and characterization of a spontaneously immortalized
human breast epithelial cell line, MCF-10. Cancer Res.
50:6075–6086. 1990.PubMed/NCBI
|
|
19
|
Hochedlinger K, Yamada Y, Beard C and
Jaenisch R: Ectopic expression of Oct-4 blocks progenitor-cell
differentiation and causes dysplasia in epithelial tissues. Cell.
121:465–477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo CL, Liu L, Jia YD, Zhao XY, Zhou Q and
Wang L: A novel variant of Oct3/4 gene in mouse embryonic stem
cells. Stem Cell Res. 9:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Wei X, Ling J, Wu L and Xiao Y:
Expression pattern of Oct-4, Sox2, and c-Myc in the primary culture
of human dental pulp derived cells. J Endod. 37:466–472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiao B, He B, Cai J and Yang W: The
expression profile of Oct4 and Sox2 in the carcinogenesis of oral
mucosa. Int J Clin Exp Pathol. 7:28–37. 2013.
|
|
23
|
Arnold K, Sarkar A, Yram MA, Polo JM,
Bronson R, Sengupta S, Seandel M, Geijsen N and Hochedlinger K:
Sox2+ adult stem and progenitor cells are important for
tissue regeneration and survival of mice. Cell Stem Cell.
9:317–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masui S, Nakatake Y, Toyooka Y, Shimosato
D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, et
al: Pluripotency governed by Sox2 via regulation of Oct3/4
expression in mouse embryonic stem cells. Nat Cell Biol. 9:625–635.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ,
Gimotty PA, Guerra M, Guo W and Xu X: Acquired cancer stem cell
phenotypes through Oct4-mediated dedifferentiation. Oncogene.
31:4898–4911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leis O, Eguiara A, Lopez-Arribillaga E,
Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R
and Martin AG: Sox2 expression in breast tumours and activation in
breast cancer stem cells. Oncogene. 31:1354–1365. 2012. View Article : Google Scholar
|
|
27
|
Kopp JL, Ormsbee BD, Desler M and Rizzino
A: Small increases in the level of Sox2 trigger the differentiation
of mouse embryonic stem cells. Stem Cells. 26:903–911. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|