Introduction
The inhibitors of
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCoAR) and
fatty acid synthase (FAS) enzymes are known to have selective
cytotoxic activity against cancer cells both in vitro and in
various in vivo models (1–3).
Several studies have demonstrated that statins, inhibitors of
HMGCoAR, and orlistat, an inhibitor of FAS, inhibit tumor cell
growth by restricting cholesterol and fatty acid synthesis,
respectively (4–8).
Previously, we demonstrated in an HepG2 cell line
(9) a synergistic effect in the
inhibition of cancer cell proliferation obtained by combination of
eicosapentaenoic acid (EPA, an omega (ω)-3-polyunsaturated fatty
acid) and lovastatin, demonstrating an inhibition at the lower
doses with respect to the substances used separately.
In an in vivo model of colon carcinogenesis,
ApcMin/+ mice, we previously demonstrated that natural
compounds such as olive oil and ω-3-polyunsaturated fatty acids
(ω-3-PUFAs), when administered to mice that spontaneously develop
intestinal polyps, were able to reduce the polyp number and volume
by decreasing proliferation and increasing pro-apoptotic activity
(10). These biological effects
were associated with inhibition of HMGCoAR and FAS gene expression
and activity and with an increase in the ratio of estrogen receptor
β/estrogen receptor α (ERβ/ERα ratio).
Estrogens and relative receptors are involved in the
aetiology and/or progression of many types of cancers, including
colon cancer (11). ERβ is
abundantly expressed in the normal colon but shows progressively
decreased expression in human adenomatous sporadic polyps (11) and in ApcMin/+ mice
(10). Downregulation of ERβ
expression has also been detected in individuals with familial
adenomatous polyposis and colorectal cancer, and was found to be
correlated with disease progression and aggressiveness (12–15).
In contrast, ERα is a well-known mediator of cell
proliferation activity (16,17);
it acts by enhancing the transcription of factors associated with
cell proliferation and shows an increased expression in colon
cancer as compared to normal surrounding tissue (17). In particular, ERα protein expression
has been demonstrated to play a role in the regulation of the
hedgehog (Hh) signaling pathway which, in turn, regulates
proliferation, angiogenesis, matrix remodeling and stem-cell
renewal (18). Alterations of the
Hh pathway have been found in patients with various types of
cancers including colorectal cancer (19). Moreover, in gastric cancer a
biologically significant linkage has been shown between the ERα and
Hh pathways; estrogens activate the ERα pathway, which induces
sonic hedgehog (Shh) production, responsible for the activation of
Hh that increases cell proliferation (20). Finally, activation of the Hh pathway
induces an overexpression of Gli1, the glioma-associated
oncogene homolog family of transcription factors, that is known to
play a role in tumorigenesis (21).
Recently, dysregulated expression of PES1, an estrogen-inducible
protein also known as Pescadillo, was found to be associated with
cancer development (22–24); PES1 seems to exert differential
actions on the transcriptional responses of the ER subtypes in
breast cancer, increasing the transcriptional activity of ERα and
decreasing that of ERβ (25).
On the basis of this experimental evidence, in the
present study, we evaluated whether lovastatin and orlistat exert
effects on polyp formation in ApcMin/+ mice similar to
the effects obtained with the use of natural compounds, such as
olive oil and ω-3-PUFAs. In addition, since preliminary results
using these drugs indicated a decrease in ERβ associated with an
increase in ERα, we focused our attention on ERα and its related
molecular targets, PES1, Shh and Gli1.
Materials and methods
Animals and experimental study
design
Five-week-old C57BL/6J male mice with a heterozygote
mutation for the Apc gene (ApcMin/+) were
obtained from Charles River Laboratories Italia (Calco, LC, Italy).
Mice were maintained under temperature-, air- and light-controlled
conditions and received food and water ad libitum; they did
not receive any surgical or hormonal manipulation. All animals
received care in compliance with the Guide for the Care and Use of
Laboratory Animals by the Italian Ministry of Health. The
procedures related to animal use were communicated to and approved
by the Italian Ministry of Health.
The ApcMin/+ mice were randomly divided
into 3 groups of 10 animal each and fed for 10 weeks as follows:
control (ST) group, that received a standard diet (12.5% protein,
12% soybean oil, 3% fiber); lovastatin (LOVA) group, that received
a standard diet supplemented with lovastatin (20 mg/kg); orlistat
(OR) group, that received a standard diet supplemented with
orlistat (200 mg/kg). All diets were isocaloric and supplied as
pellets (Mucedola Srl, Settimo Milanese, Italy). Mouse body weight
and food intake were measured every 3 days.
After 10 weeks of dietary treatment, all animals
were sacrificed by cervical dislocation and the entire intestinal
tract was immediately removed and washed with cold
phosphate-buffered saline (PBS).
In the ApcMin/+ mice, the volume of
polyps was calculated considering polyps as hemispheres (1/2×3/4
πr3). The small intestine and colon were cut along the
mesenteric insertion, placed on a paper strip at 0°C to 4°C and
analyzed through a stereomicroscope at ×3 magnification by two
independent observers. For our evaluations, the small intestine was
further divided into proximal, medial and distal segments. One
portion of the intestinal segments of all animals was immediately
put into RNAlater® and stored at -20°C, and another
portion was placed in liquid nitrogen in order to run real-time PCR
and western blot analyses, respectively. The remaining portion of
the intestinal segments was fixed in 10% neutral buffered formalin
for 24 h and embedded in paraffin in a 'Swiss roll' fashion.
Paraffin-embedded tissues were processed for light and confocal
microscopy studies.
Histological studies
To evaluate the grade of dysplasia, hematoxylin and
eosin-stained sections were examined in a blinded fashion by two
pathologists. Dysplasia was defined as the occurrence of
disorganized glandular architecture, depletion of mucin-producing
cells and goblet cells, nuclear atypia and increased mitotic
activity, and was graded mild, moderate or severe as previously
described (12). The further
presence of tissue hypercellularity, enhanced cell polymorphism and
degenerative/necrotic phenomena allowed us to recognize cancerous
lesions.
Proliferating cell nuclear antigen (PCNA)
assay
Cell proliferation was evaluated by PCNA assay.
Distal tissue sections underwent antigen retrieval [Tris EDTA, pH
9, in a microwave (850 W) for 10 min], followed by processing with
the primary polyclonal anti-PCNA antibody (Ab 2496; Abcam,
Cambridge, UK) first and then with the secondary antibody (Alexa
555 anti-rabbit; Invitrogen, OR, USA) as previously described
(10). All sections were observed
at x400 magnification by confocal microscopy (Leica TCS SP2
confocal laser scanning microscope). The percentage of
PCNA-positive cells over the total number of counted cells, i.e.,
the PCNA labeling index (PCNA-LI), was used to quantify epithelial
cell proliferation.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick end labeling
(TUNEL) assay
Apoptotic cells were detected by TUNEL method,
according to the manufacturer's instructions (In Situ Cell Death
Detection kit; Roche) in 10 randomly selected fields, as previously
described (12).
Western blotting
ERα, ERβ, PES1, Shh, Gli1 and β-actin protein
expression levels were evaluated by western blot analysis in distal
intestinal specimens. Briefly, 50 μg of aliquots of total
protein were separated on 4–12% pre-cast polyacrylamide gels
(Invitrogen, Life Technologies) and transferred onto a PVDF
membrane with Trans-Blot Turbo (both from Bio-Rad Laboratories,
Milan, Italy). The primary antibodies (anti-ERα, -ERβ, -PES1, -Shh,
-Gli1 and -β-actin; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
were diluted 1:500 in blocking buffer. After overnight incubation,
the membranes were further incubated with a horseradish
peroxidase-conjugated secondary antibody (Bio-Rad Laboratories).
The proteins were detected by chemiluminescence (ECL; Thermo
Scientific, Rockford, IL, USA) and densitometric analysis of each
protein-related signal was obtained using the Molecular Imager
Chemidoc™ (Bio-Rad Laboratories) and normalized against β-actin
expression.
Lipogenic gene expression analysis and
apoptotic death assay
To study the effects of the diets on gene expression
of lipogenic enzymes and on apoptosis in the intestinal distal
tract from treated mice, the mRNA levels of FAS and
HMGCoAR genes, as well as the levels of Bax and
Bcl-2, were assessed by real-time PCR (RT-PCR) as previously
described (10). The reactions were
obtained using Master Mix with SYBR Green (iQ SYBR Green Supermix;
Bio-Rad Laboratories) and sense and antisense primers for the
target genes and the β-actin gene (Table I). Real-time PCR was carried out in
a CFX96 Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.)
using the following protocol: 45 cycles at 95°C for 3 min, 95°C for
10 sec, 55°C for 30 sec followed by a melting curve step at 65–95°C
at a heating rate of 0.5°C per cycle for 80 cycles. The PCR
products were quantified by external calibration curves, one for
each tested gene, obtained with serial dilutions of known copy
numbers of molecules (102–107 molecules). All
expression data were normalized by dividing the target amount by
the amount of β-actin used as internal control for each sample. The
specificity of the PCR products was confirmed by gel
electrophoresis.
 | Table ISequences of the amplification
primers. |
Table I
Sequences of the amplification
primers.
| Gene | Primers |
|---|
| FAS | Sense:
5′-GATCCTGGAACGAGAACACGA-3′ |
| Antisense:
5′-GAGACGTGTCACTCCTGGACTTG-3′ |
| HMGCoAR | Sense:
5′-GCTTGAGCATCCTGACATAC-3′ |
| Antisense:
5′-GAACCATAGTTCCCACGTCT-3′ |
| Bax | Sense:
5′-CAGGATGCGTCCACCAAGAA-3′ |
| Antisense:
5′-GCTCCCGGAGGAAGTCCAAT-3′ |
| Bcl-2 | Sense:
5′-GTGGAGGAGCTCTTCAGGGA-3′ |
| Antisense:
5′-AGGCACCCAGGGTGATGCAA-3′ |
| β-actin | Sense:
5′-GCCTCTGGTCGTACCACTGGC-3′ |
| Antisense:
5′-AGGGAGGAAGAGGATGCGGCA-3′ |
Microsomal HMGCoAR activity and FAS
activity assay
Microsomal HMGCoAR and FAS activities were
determined on frozen distal intestinal samples, as previously
described (10) and expressed as
picomoles (pmol) of 14C mevalonate/min/mg of microsomal
proteins and picomoles (pmol) of incorporated
2-14C-malonyl-CoA/min/mg of total proteins,
respectively.
Statistical analysis
The significance of the differences among
experimental groups was evaluated by one-way analysis of variance
(ANOVA) and Tukey's multiple comparison test. t-test for paired
data was used to compare polyp and 'normal' mucosa parameters in
the same group of animals. Differences were considered significant
at a 5% probability level.
Results
Dietary treatment and gross anatomy
evaluations
After 10 weeks of dietary treatment, no
statistically significant difference in food consumption and body
weight was found among the three groups of mice (data not shown).
Gross anatomy evaluations demonstrated no significant variation in
the numbers of polyps among the three groups of mice (Fig. 1A), whereas polyp volume was
significantly reduced in the LOVA group (Fig. 1B, p=0.002, Tukey's multiple
comparison test), but not in the OR group as compared to the ST
group.
Histological studies
Notably, 40% of the mice treated with orlistat
showed ulcerated polyps and widespread red petechiae. Upon
histological observation, while polyps in the sST and LOVA groups
showed only moderate-severe grade dysplasia (Fig. 2A), notable, in the OR group we found
the presence of cancerous foci in the ulcerated areas (Fig. 2B).
Intestinal epithelial cell
proliferation
Cell proliferation, assessed by PCNA
immunohistochemical assay, was similar in the 'healthy' mucosa of
the three groups but was significantly increased in the polyp
tissues as compared to that noted in the 'healthy' mucosa (Fig. 3, p=0.002, p=0.002 and p=0.001,
paired t-test in ST, LOVA and OR groups, respectively). In
particular, a statistically significant increase in cell
proliferation was observed in the polyp tissues of the
orlistat-treated mice compared with that noted in the mice fed a
standard diet (Fig. 3, p=0.01,
Tukey's multiple comparison test).
Intestinal epithelial cell apoptosis
Fig. 4 shows the
apoptotic activity in the small intestine, expressed as TUNEL-LI
(Fig. 4A) and as Bax/Bcl-2 mRNA
levels (Fig. 4B). TUNEL-LI in the
polyps was significantly higher as compared to the corresponding
'healthy' adjacent mucosa in the LOVA and OR groups (Fig. 4A, p=0.01, p=0.02, paired t-test,
respectively) but not in the ST group. The increase in TUNEL-LI in
the LOVA and OR mouse polyps was also significantly higher as
compared to the ST mouse polyps (p=0.03, p=0.02, Tukey's multiple
comparison test, respectively). To confirm the results obtained by
TUNEL, Bax and Bcl2 gene expression were evaluated demonstrating an
increased Bax/Bcl-2 ratio in both the LOVA and OR mice, that
reached statistical significance only in those animals receiving
the diet supplemented with lovastatin (Fig. 4B, p=0.03, Tukey's multiple
comparison test).
Lipogenic enzyme activity/gene
expression
As expected, in the OR group, FAS activity and gene
expression were significantly decreased as compared to the ST group
(Fig. 5A, p=0.001 and 5B, p=0.001,
Tukey's multiple comparison test, respectively). A similar result
was found when FAS activity and gene expression in the OR group
were compared to these parameters in the LOVA group (Fig. 5A, p=0.02 and 5B, p=0.01, Tukey's
multiple comparison test, respectively). Similarly, lovastatin
significantly inhibited its target HMGCoAR, demonstrating a
significant reduction in both enzyme activity and mRNA as compared
to ST (Fig. 5A, p=0.001 and 5B,
p=0.002, Tukey's multiple comparison test, respectively).
ERα, PES1, Shh and Gli1 protein
expression
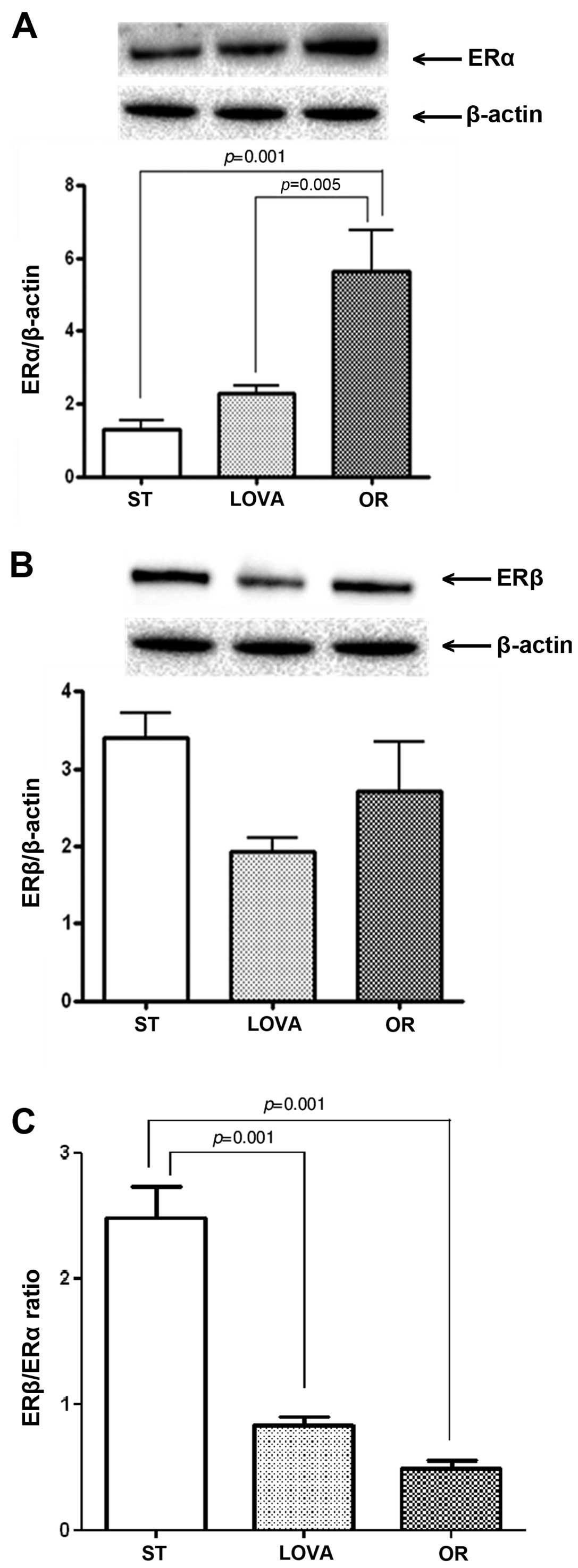
Fig. 6A shows a
striking increase in ERα protein expression in the OR group as
compared to that noted in the ST and LOVA groups (p=0.001 and
p=0.005, Tukey's multiple comparison test, respectively). Moreover,
in both treatment groups, this increase was associated with a
reduction in ERβ expression, that did not reached statistical
significance as compared to the ST group (Fig. 6B). Consequently, in the LOVA and OR
groups, the ERβ/ERα ratio was significantly reduced as compared to
that noted in the ST group (Fig.
6C, p=0.001 and p=0.001, Tukey's multiple comparison test,
respectively).
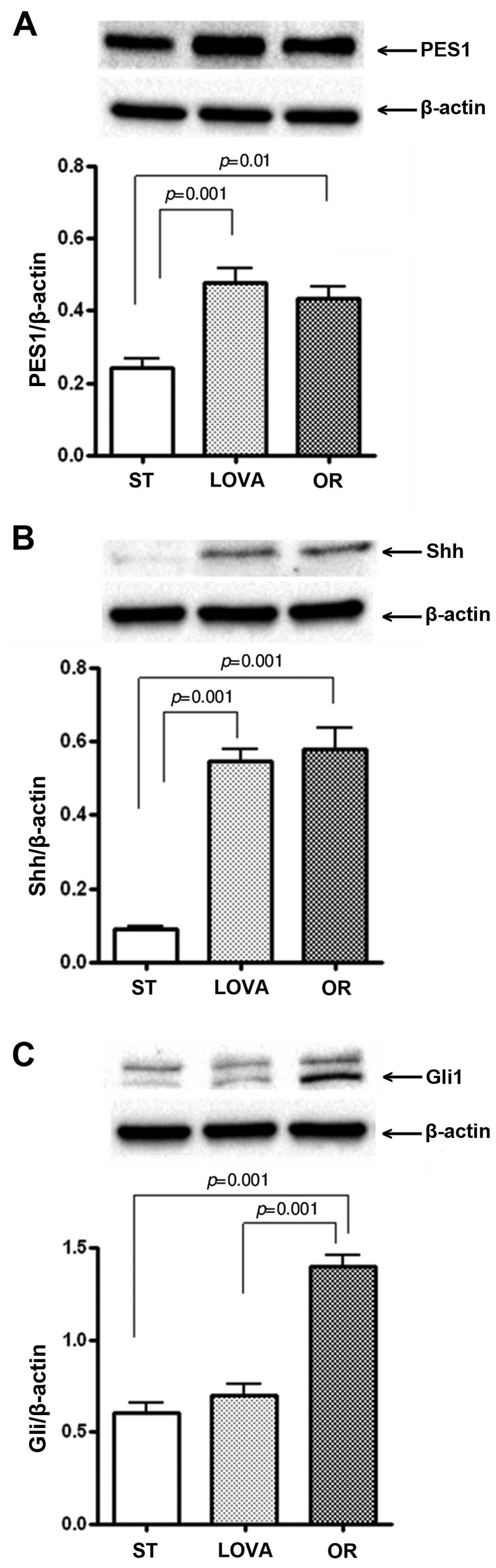
Fig. 7A shows the
protein levels of PES1 in the mouse treatment groups. PES1
expression was significantly increased both in the LOVA and OR
groups as compared to that in the ST group (p=0.001 and p=0.01,
Tukey's multiple comparison test, respectively). Similarly, a
statistically significant increase in Shh protein levels was
observed in both treatment groups as compared to the mice fed the
ST diet (Fig. 7B, p=0.001 and
p=0.001, Tukey's multiple comparison test, respectively). In
contrast, the level of Gli1 protein was significantly increased
only in the OR group as compared to the level in the ST and LOVA
groups (Fig. 7C, p=0.001 and
p=0.001, Tukey's multiple comparison test, respectively).
Discussion
The aim of the present study was to evaluate the
effects of orlistat or lovastatin on polyp formation in
ApcMin/+ mice, in order to confirm the same findings
previously obtained with natural compounds, such as olive oil and
ω-3-PUFAs (10).
The use of orlistat and lovastatin significantly
reduced FAS and HMGCoAR enzymatic activities and gene expression in
the mouse colonic mucosa, but did not affect the number of
intestinal polyps, while there was a statistically significant
reduction in polyp volume only in the LOVA group. This result could
be partially related to the fact that in mice receiving orlistat,
the significant increase in cell proliferation in the polyp tissue
was not balanced by an increase in apoptosis as observed in the
LOVA group. Moreover, in the OR group, we observed the development
of cancerous foci.
Moreover, lipogenic enzyme inhibition was not
associated with an increase in the ERβ/ERα ratio as observed in our
previous study using olive oil and ω-3-PUFAs (10). In contrast, the treatment with
orlistat and lovastatin induced a modest decrease in ERβ and a
significant overexpression of ERα, leading to a significant
reduction in the ERβ/ERα ratio, more evident in the OR group.
In order to clarify the involvement of ERα and its
possible mechanism(s) of action in polyp development, we found
that, in the OR group, the increased ERα expression was associated
with a statistically significant increase in PES1, Shh and Gli1
protein levels.
PES1 has been demonstrated to increase the
transcriptional activity of ERα and to decrease that of ERβ in
breast cancer, resulting in an increased expression of
estrogen-responsive genes, known to promote cell proliferation and
survival (25). Since there are
similarities in the carcinogenesis pathways for breast and
colorectal carcinoma (26,27), this study suggests an involvement of
PES1 in the mechanisms regulating estrogen receptor expression in
intestinal polyps of ApcMin/+ mice.
An oncogenic role of the Hh pathway in promoting the
proliferation of colon cancer tissue has also been widely described
(18–20,28).
Moreover, it has been demonstrated that overexpression of the Hh
pathway in intestinal adenoma increases both the incidence and the
size of the adenomas in ApcMin/+ mice (28). Our recent study demonstrated that
Shh protein expression also plays a role in molecular mechanisms of
polyp reversion in ApcMin/+ mice (29).
In the present study, in the mice treated with
lovastatin, the Gli1 protein remained unmodified and only the PES1
and Shh proteins were overexpressed. The fact that in the LOVA
group we did not find a correlation between the increased
expression of Shh and Gli1 was not surprising since it is already
known that Gli1 expression may not be necessarily associated with
an upregulation of Shh expression (18).
Our present data clearly demonstrated that
lovastatin, but not orlistat, reduced intestinal polyp volume in
the ApcMin/+ mouse model, probably due to an increase in
ERα expression, known to be a positive modulator of cell
proliferation.
Our findings are in agreement with research
demonstrating that orlistat treatment is associated with a
significant increase in the number of colonic aberrant crypt foci
as well as the induction of colonic cell proliferation and severe
crypt alterations (30). In this
scenario, the apparent discordance with other data in the
literature demonstrating the anti-proliferative effect of orlistat
could be related to the different experimental models used
(6–8).
Finally, from our present data, we suggest that, in
addition to the inhibition of FAS enzyme activity and gene
expression, other intestinal tissue molecular changes occur during
orlistat treatment in ApcMin/+ mice. Therefore, further
research is warranted to elucidate the negative or beneficial side
effects of this drug.
References
|
1
|
Shibata MA, Kavanaugh C, Shibata E, Abe H,
Nguyen P, Otsuki Y, Trepel JB and Green JE: Comparative effects of
lovastatin on mammary and prostate oncogenesis in transgenic mouse
models. Carcinogenesis. 24:453–459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuhajda FP, Pizer ES, Li JN, Mani NS,
Frehywot GL and Townsend CA: Synthesis and antitumor activity of an
inhibitor of fatty acid synthase. Proc Natl Acad Sci USA.
97:3450–3454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orita H, Coulter J, Tully E, Kuhajda FP
and Gabrielson E: Inhibiting fatty acid synthase for
chemoprevention of chemically induced lung tumors. Clin Cancer Res.
14:2458–2464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pizer ES, Chrest FJ, DiGiuseppe JA and Han
WF: Pharmacological inhibitors of mammalian fatty acid synthase
suppress DNA replication and induce apoptosis in tumor cell lines.
Cancer Res. 58:4611–4615. 1998.PubMed/NCBI
|
|
5
|
Wong WW, Dimitroulakos J, Minden MD and
Penn LZ: HMG-CoA reductase inhibitors and the malignant cell: The
statin family of drugs as triggers of tumor-specific apoptosis.
Leukemia. 16:508–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kridel SJ, Axelrod F, Rozenkrantz N and
Smith JW: Orlistat is a novel inhibitor of fatty acid synthase with
antitumor activity. Cancer Res. 64:2070–2075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chuang HY, Chang YF and Hwang JJ:
Antitumor effect of orlistat, a fatty acid synthase inhibitor, is
via activation of caspase-3 on human colorectal carcinoma-bearing
animal. Biomed Pharmacother. 65:286–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carvalho MA, Zecchin KG, Seguin F, Bastos
DC, Agostini M, Rangel AL, Veiga SS, Raposo HF, Oliveira HC, Loda
M, et al: Fatty acid synthase inhibition with Orlistat promotes
apoptosis and reduces cell growth and lymph node metastasis in a
mouse melanoma model. Int J Cancer. 123:2557–2565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Notarnicola M, Messa C, Refolo MG, Tutino
V, Miccolis A and Caruso MG: Synergic effect of eicosapentaenoic
acid and lovastatin on gene expression of HMGCoA reductase and LDL
receptor in cultured HepG2 cells. Lipids Health Dis. 9:1352010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barone M, Notarnicola M, Caruso MG, Scavo
MP, Viggiani MT, Tutino V, Polimeno L, Pesetti B, Di Leo A and
Francavilla A: Olive oil and omega-3 polyunsaturated fatty acids
suppress intestinal polyp growth by modulating the apoptotic
process in ApcMin/+ mice. Carcinogenesis. 35:1613–1619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barone M, Tanzi S, Lofano K, Scavo MP,
Guido R, Demarinis +L, Principi MB, Bucci A and Di Leo A:
Estrogens, phytoestrogens and colorectal neoproliferative lesions.
Genes Nutr. 3:7–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barone M, Scavo MP, Papagni S, Piscitelli
D, Guido R, Di Lena M, Comelli MC and Di Leo A: ERβ expression in
normal, adenomatous and carcinomatous tissues of patients with
familial adenomatous polyposis. Scand J Gastroenterol.
45:1320–1328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konstantinopoulos PA, Kominea A, Vandoros
G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G
and Papavassiliou AG: Oestrogen receptor beta (ERbeta) is
abundantly expressed in normal colonic mucosa, but declines in
colon adenocarcinoma paralleling the tumour's dedifferentiation.
Eur J Cancer. 39:1251–1258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudolph A, Toth C, Hoffmeister M, Roth W,
Herpel E, Jansen L, Marx A, Brenner H and Chang-Claude J:
Expression of oestrogen receptor β and prognosis of colorectal
cancer. Br J Cancer. 107:831–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Leo A, Barone M, Maiorano E, Tanzi S,
Piscitelli D, Marangi S, Lofano K, Ierardi E, Principi M and
Francavilla A: ER-beta expression in large bowel adenomas:
Implications in colon carcinogenesis. Dig Liver Dis. 40:260–266.
2008. View Article : Google Scholar
|
|
16
|
Weihua Z, Andersson S, Cheng G, Simpson
ER, Warner M and Gustafsson JA: Update on estrogen signaling. FEBS
Lett. 546:17–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas C and Gustafsson JA: The different
roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer.
11:597–608. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gulino A, Ferretti E and De Smaele E:
Hedgehog signalling in colon cancer and stem cells. EMBO Mol Med.
1:300–302. 2009. View Article : Google Scholar
|
|
19
|
Wang H, Li YY, Wu YY and Nie YQ:
Expression and clinical significance of hedgehog signaling pathway
related components in colorectal cancer. Asian Pac J Cancer Prev.
13:2319–2324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kameda C, Nakamura M, Tanaka H, Yamasaki
A, Kubo M, Tanaka M, Onishi H and Katano M: Oestrogen
receptor-alpha contributes to the regulation of the hedgehog
signalling pathway in ERalpha-positive gastric cancer. Br J Cancer.
102:738–747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bian YH, Huang SH, Yang L, Ma XL, Xie JW
and Zhang HW: Sonic hedgehog-Gli1 pathway in colorectal
adenocarcinomas. World J Gastroenterol. 13:1659–1665. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinoshita Y, Jarell AD, Flaman JM, Foltz
G, Schuster J, Sopher BL, Irvin DK, Kanning K, Kornblum HI, Nelson
PS, et al: Pescadillo, a novel cell cycle regulatory protein
abnormally expressed in malignant cells. J Biol Chem.
276:6656–6665. 2001. View Article : Google Scholar
|
|
23
|
Maiorana A, Tu X, Cheng G and Baserga R:
Role of pescadillo in the transformation and immortalization of
mammalian cells. Oncogene. 23:7116–7124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie W, Feng Q, Su Y, Dong B, Wu J, Meng L,
Qu L and Shou C: Transcriptional regulation of PES1 expression by
c-Jun in colon cancer. PLoS One. 7:e422532012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng L, Li J, Han Y, Lin J, Niu C, Zhou
Z, Yuan B, Huang K, Li J, Jiang K, et al: PES1 promotes breast
cancer by differentially regulating ERα and ERβ. J Clin Invest.
122:2857–2870. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dey P, Barros RP, Warner M, Ström A and
Gustafsson JA: Insight into the mechanisms of action of estrogen
receptor β in the breast, prostate, colon, and CNS. J Mol
Endocrinol. 51:T61–T74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papapolychroniadis C, Kaimakis D,
Giannoulis K, Papadopoulos V, Zatagias A, Halvatzoulis O,
Kostopoulos J, Kokkonis G, Giala M and Harlaftis N: Metachronous
breast carcinoma (second malignancy), following 'cure' from
colorectal carcinoma. Tech Coloproctol. 8(Suppl 1): s129–s131.
2004. View Article : Google Scholar
|
|
28
|
Büller NV, Rosekrans SL, Metcalfe C,
Heijmans J, van Dop WA, Fessler E, Jansen M, Ahn C, Vermeulen JL,
Westendorp BF, et al: Stromal Indian hedgehog signaling is required
for intestinal adenoma formation in mice. Gastroenterology.
148:170–180.e6. 2015. View Article : Google Scholar
|
|
29
|
Notarnicola M, Tutino V, Caruso MG and
Francavilla A: n-3 polyunsaturated fatty acids reverse the
development of polyps in ApcMin/+ transgenic mice. Oncol
Rep. 35:504–510. 2016.
|
|
30
|
Garcia SB, Barros LT, Turatti A,
Martinello F, Modiano P, Ribeiro-Silva A, Vespúcio MV and Uyemura
SA: The anti-obesity agent Orlistat is associated to increase in
colonic preneoplastic markers in rats treated with a chemical
carcinogen. Cancer Lett. 240:221–224. 2006. View Article : Google Scholar
|