Introduction
Lung cancer is the first ranked malignant tumor with
high incidence and mortality worldwide, and more than one million
patients are diagnosed with lung cancer each year. Among the
various types of lung cancer, NSCLC accounts for 75–85%, and
elderly patients aged more than 65 years account for more than 50%
(1). Generally when patients are
diagnosed at a late stage (stage III or IV) the 5-year survival
rate is only 12–15% (2,3), as the patients lost the opportunity
for surgical treatment (4,5).
For elderly advanced NSCLC patients, chemotherapy is
still the first choice of treatment (6,7), it is
relatively effective treatment for elderly NSCLC patients who
cannot tolerate surgical operation. It can effectively reduce the
progress of lung cancer and the recurrence, enhance the effect of
clinical treatment, prolong patient survival rate and improve their
quality of life (8).
There are various chemotherapy regimens and drugs
for elderly NSCLC patients, such as cisplatin, VP-16, gemcitabine,
paclitaxel and docetaxel (9).
However, there is still lack of a mature and effective way for
NSCLC due to the side-effects of the drugs. Thus, how to choose a
drug with benefits and little side effects is a hotspot in tumor
research (10,11). Docetaxel is a type of
anti-microtubule taxane drug, and it is the only one approved for
first- and second-line chemotherapy for NSCLC treatment by the US
Food and Drug Administration (FDA), and the EU. According to the
existing clinical data of current studies, DTX has been proven very
effective for NSCLC (12,13).
Biodegradable albumin nanoparticles are thought more
and more important during the past few decades (14). Previous studies have been proven
that drug-loaded polymeric nanoparticles accumulated in certain
tumors more efficiently than other carriers by enhancing
permeability and retention (EPR) effect (15,16).
Furthermore, another advantage is long circulating half-life and
lower systemic toxicity which is superior to conventional drug
formulations (17–20).
Polyethylene glycol (PEG) is one of medicinal
synthetic polymer injections which can be used for the body as
approved by FDA. There are numerous advantages of PEG drugs such
as: i) extending biologic half-life of drugs, enhancing long-acting
and sustained-release effect; ii) improving the solubility and
stability of the drug; iii) reducing immunogenicity and
antigenicity; iv) reducing enzyme degradation; v) enhancing the
targeting function of drugs; and vi) reducing the toxicity of
various drugs. Based on the above advantages, we chose PEG to
formulate polymeric nanoparticles due to its excellent
biocompatibility and biodegradability.
Aiming to develop a good nanoparticle carrier for
DTX, we synthesized PEG-DANPs via the emulsion-evaporation
cross-link method, and we carried out a series of experiments both
in vitro and in vivo, the results demonstrated that
PEG-DANPs were quite a promising modality for NSCLC.
Materials and methods
Materials
All reagents and solvents were used as received,
without further purification. Monomethoxy PEG with a molecular
weight of 20,000 kDa (mPEG 20,000), D,L-lactide and stannous
octoate were purchased from Sigma-Aldrich Chemical Corp. (Shanghai,
China); DTX was purchased from Beijing Norzer Pharmaceutical Co.,
Ltd. (Beijing, China); free-DTX (Aisu®) is manufactured
by Hengrui Pharmaceutical Co., Ltd. (Jiangsu, China); and
3-(4,5)-dimethylthiazol(z-y1)-3,5-di-phenyltetrazolium
bromide (MTT) was obtained from Amresco (Solon, OH, USA); Annexin
V-FITC apoptosis detection kit was purchased from 4A Biotech Co.,
Ltd. (Beijing, China); VivoGlo® luciferin was purchased
from Promega Corporation (Madison, WI, USA). Trypsin, fetal bovine
serum (FBS) and RPMI-1640 medium were purchased from HyClone
(Logan, UT, USA), and culture flasks and dishes were from Corning
(Corning, NY, USA).
Cell line and animals
Human non-small lung cancer A549 cell line was
provided by the Department of Pathology in Institute of Medicinal
Biotechnology in Peking Union Medical College. A549 cells were
cultured in RPMI-1640 medium supplemented with 10% heat-inactivated
FBS and incubated in a humidified atmosphere of 5% CO2
and 95% air at 37°C. Female BALB/c mice (6–8-weeks old) were used
for antitumor efficacy studies and were purchased from Beijing
Vital River Laboratories (Beijing, China).
Animals were acclimatized in the holding facility
prior to beginning of the present study. All animal procedures were
approved by the Institutional Animal Care and Use Committee of the
Chinese Academy of Medical Sciences. All surgeries were performed
under sodium pentobarbital anesthesia (5 mg/ml solution), and all
efforts were made to minimize suffering. Lung tumor and other
sections were routinely stained with hematoxylin and eosin
(H&E) and evaluated under a light microscope.
Preparation of DANPs and PEG-DANPs
DTX was dissolved in chloroform and ethanol to form
solution A, albumin was dissolved in sterile water to form solution
B. The solution A and B was mixed and stirred by homogenate machine
5 min to form raw milk, the raw milk was moved into high pressure
homogenizer, under the 20,000 psi for 12 cycles. The chloroform of
mixture was eliminated using rotary evaporator for 25 min and
followed by filtration through a 0.22 µm filter.
DANPs and mPEG (20,000 kDa) were added to the
solution of boric acid buffer (0.1 mol/l, pH 9.0) according to the
ratio of 3:1 with stirring and the reaction was terminated via
adding glycine (1 mg/ml) 3 h later. Unbound HAS and PEG were
removed by ultrafiltration (MWCO, 70 kDa) (21,22).
Determination via PAGE gel electrophoresis: DANPs
and PEG-DANPs were determined via PAGE gel electrophoresis with
iodine staining and Mas blue staining.
Cell viability
The in vitro cytotoxic activity of DANPs and
PEG-DANPs was evaluated by the MTT assay. Briefly, the A549 cells
(8×104 cells/ml) were seeded into 96-well plates and
incubated for 24 h to allow cell attachment. The cells were then
treated with a series of phosphate-buffered saline (PBS) (control),
Aisu®, DANPs and PEG-DANPs at 37°C (0.001, 0.01, 0.1, 1,
10 and 100 µg/ml). At the incubation-time points of 48 h, 20
µl of MTT (5 mg/ml) was added and incubated for 4 h, MTT was
aspirated off and 180 µl/well of dimethyl sulfoxide (DMSO)
was added to dissolve the formazan crystals, and the plate was
gently shaken for 10 min. The optical density (OD) was measured at
490 nm by Synergy H1m monochromator-based multi-mode microplate
reader (BioTek, Winooski, VT, USA). The cell inhibition was
calculated according to the below formula: Cell inhibition (%) = [1
− (ODsample − ODblank)/(ODsample −
ODblank)] × 100%. The results were expressed as means ±
SD of 3 measurements. No precipitation of DTX was found during
incubation procedure (23).
Cell apoptosis assay
Apoptotic cells were determined by dual staining
with an Annexin V and propidium iodide (PI) kit (4A Biotech Co.,
Ltd.) according to the manufacturer's instructions (24). After 48 h of incubation in the
exponential stage, A549 cells seeded in 12-well plates were treated
for a further 48 h with 10 nmol/ml Aisu®, DANPs and
PEG-DANPs, respectively. After treatment, cells were washed twice
with warm PBS, detached by trypsin without EDTA, then through the
following steps: collection, centrifugation, washing with warm PBS,
further staining with PI and Annexin V-FITC for 15 min at room
temperature in the dark. Apoptosis was then analyzed using a
FACScan cytometer. Quadrant analysis was performed and cells that
stained positive for both Annexin V-FITC and PI were designated as
apoptotic, while unstained cells were designated as viable.
In vivo antitumor and metastasis
inhibition
All experimental procedures were performed in
conformity with institutional guidelines and protocols for the care
and use of laboratory animals. We chose 40 BALB/c mice to divide
into two parts, one part was to establish the transplantation tumor
model (25,26) to observe the inhibition of drugs for
transplantation tumor and weight changes of nude mice; the other
part was to establish the in situ carcinoma model to observe
the inhibition of drugs for in situ carcinoma and
metastasis. Each group contained 20 mice and they were equally
divided into four groups, respectively, negative control (glucose
injection group), positive control (Aisu® group), DANPs
and PEG-DANPs groups with 5 animals each. The lung cancer A549
cells were suspended in BD Matrigel, and the mice in each group
were implanted with 3×106 cells in alar or in the lung.
When the tumor volume in transplantation tumor group reached ~120
mm3, the mice were treated 4 times at 7-day intervals
with 5% glucose injection (negative control), with
Aisu®, DANPs or PEG-DANPs at the same time in both
parts, respectively. All formulations were injected intravenously
via the tail vein at a DTX dose of 20 mg/kg. The body weight and
tumor volume were measured simultaneously. Tumor volume was
calculated using the equation of V = w2 × l/2. Here, w
and l are the width and length of the tumor. Forty-eight hours
after the last treatment, the mice were sacrificed. The tumors with
lung and other major organs (including heart, liver, spleen and
kidney) were removed, fixed in 10% formalin solution, and subjected
to paraffin embedding for H&E staining.
Survival rate of the nude mice after
treatment
Additional 32 nude mice were used to establish the
transplantation tumor model and divided into negative control group
(group glucose injection), positive control group
(Aisu®), DANPs and PEG-DANPs group, the mice were
treated until natural death, observe and the survival rate was
drawn.
Statistical analysis
Results are presented as means ± SD. Statistical
comparisons were carried out by t-test or ANOVA analysis. The level
of significance was set at p<0.05.
Results
Determination of DANPs and PEG-DANPs via
PAGE gel electrophoresis
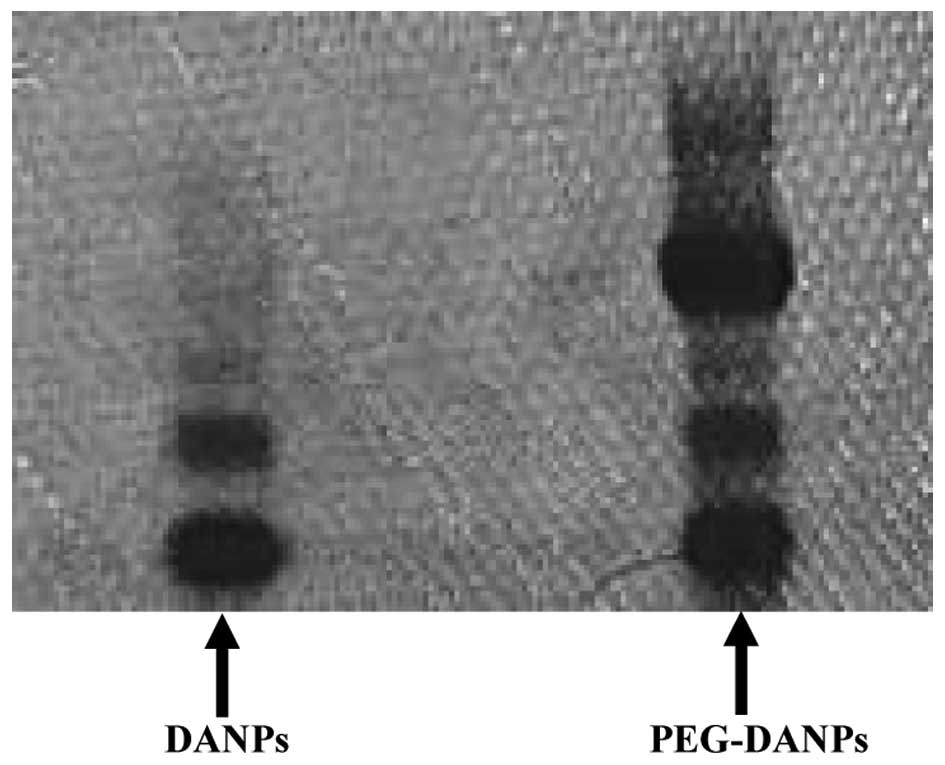
The PAGE gel electrophoresis displayed PEG-DANPs
existing in two bands, PEG and protein, respectively; whereas there
was only one band in DANPs, which represents the protein (Fig. 1).
Cell viability
Fig. 2 shows the
result of the cytotoxicity of Aisu®, DANPs and PEG-DANPs
against A549 lung cancer cells. A549 cells were exposed to a series
of equivalent concentrations of Aisu®, DANPs and
PEG-DANPs for 48 h, and the inhibition rates were determined via
the MTT method. The cell survival rate had a dose-dependent inverse
relationship with the drug concentrations. PEG-DANPs accelerated
cellular uptake of the drug and induced higher cytotoxicity in
cancer cells than Aisu® and DANPs, particularly at lower
DTX concentrations (0.001–0.1 µg/ml). However, A549 cells
were more sensitive to Aisu® than DANPs and PEG-DANPs at
higher concentrations (1–100 µg/ml). Nanoparticles are
internalized into cancer cells via endocytic mechanisms (27), while the free-drug diffuses into
cells according to the concentration gradient between the
intracellular and extracellular environments. It is why
Aisu® is more cytotoxic at higher concentrations.
PEG-DANPs increase DTX-induced apoptosis
in A549 cells
DTX was described as an antimitotic agent which
could bind to β-tubulin, resulting in blocking the cell cycle at
the G2/M phase and apoptosis of cells (28,29).
According to a previous study, encapsulation of DTX in
nanoparticles could increase apoptosis of prostate cancer cells
(30). Given that PEG-DTX-HANPs
demonstrated stronger in vitro cytotoxicity than DANPs and
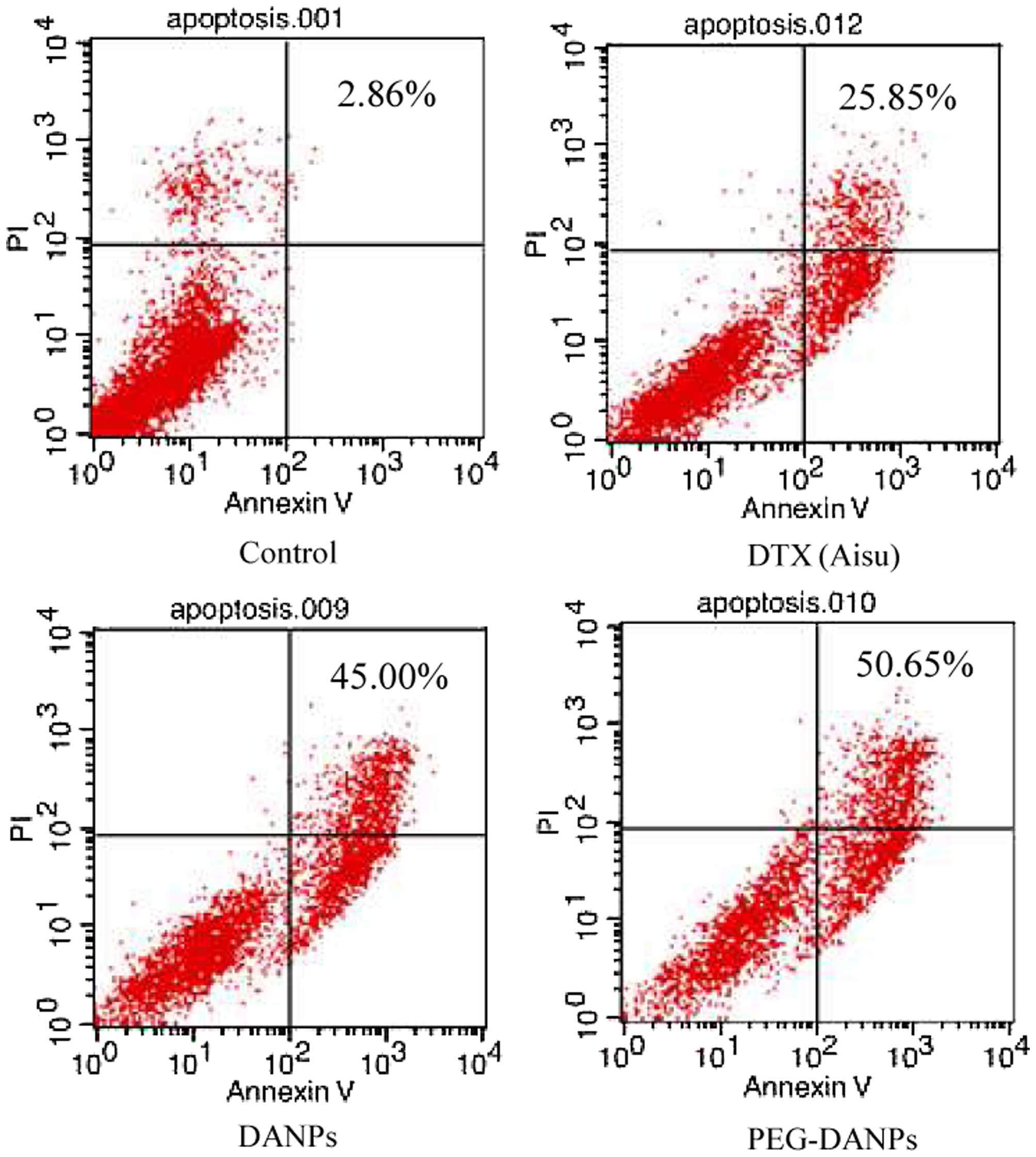
Aisu®, we performed apoptosis assays using Annexin
V-FITC and PI staining to compare apoptosis induction. As
predicted, PEG-DANPs (55.65%) increased late apoptosis in A549
cells compared with DANPs and Aisu® (25.85 and 43.00%)
(Fig. 3).
Anticancer and metastasis inhibition of
PEG-DANPs in vivo
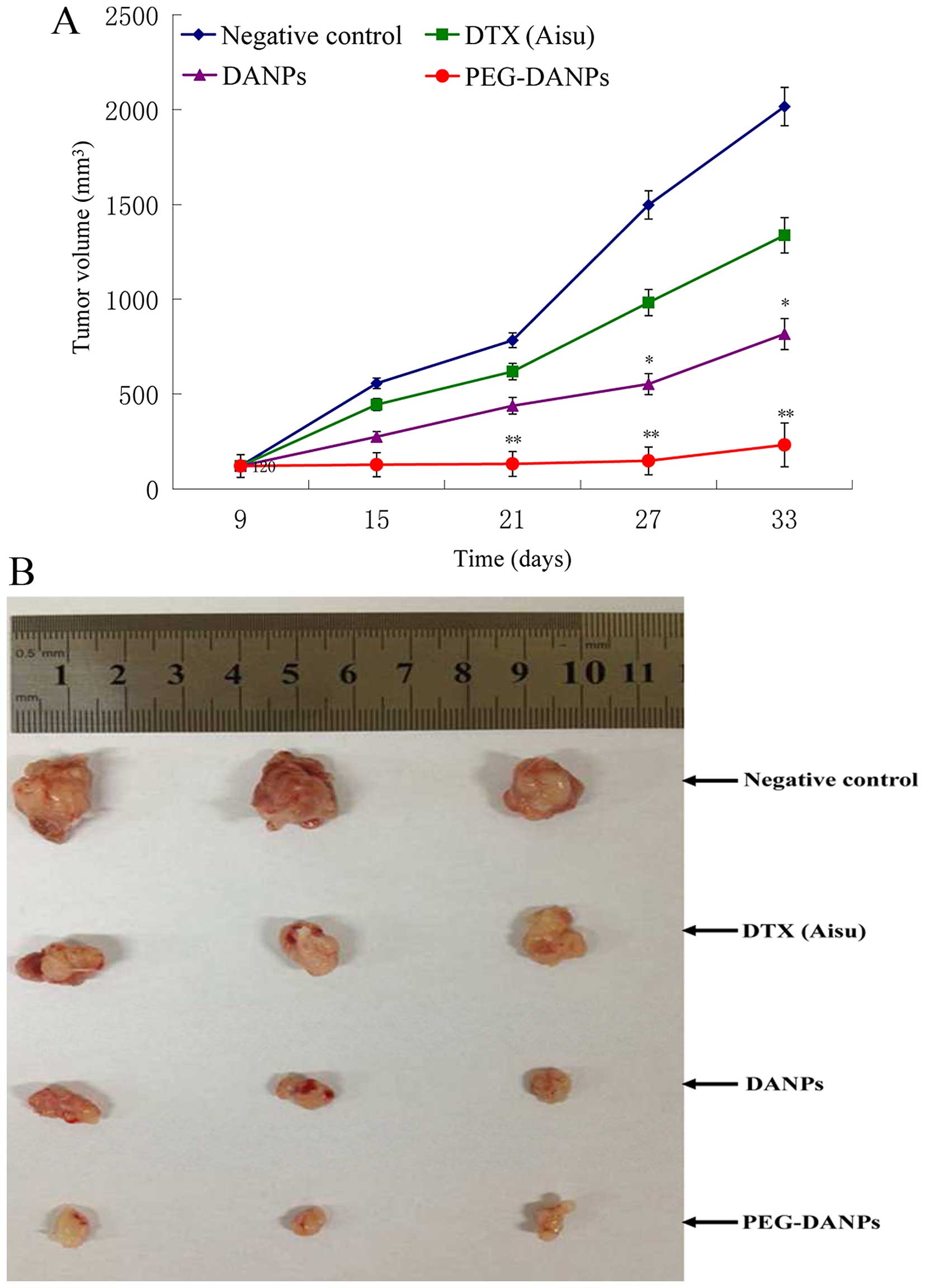
Tumor-bearing nude mice were injected with
Aisu®, DANPs and PEG-DANPs, and their therapeutic
effects were examined by measuring the suppression of body weight
and tumor growth. The body weight of the mice in the negative
control groups was basically unchanged or slightly increased, while
the mice in the drug groups all lost weight in the process of the
treatment. However, the reduction range in the Aisu®
group was more significant than the other two groups (p<0.01)
(Fig. 4), suggesting severe
systemic toxicity in addition to tumor toxicity. It was found that
all the tumor volumes treated with Aisu®, DANPs and
PEG-DANPs were much smaller than those of negative control groups
treated with the same dose (p<0.05), and the tumors of PEG-DANPs
groups were obviously smaller than those of Aisu® groups
(Fig. 5A and B), indicating that
PEG-DANPs is the most effective to inhibit tumor growth among the
three.
A typical tumor cellular morphology was seen under
the microscope in the negative control groups both in transplant
model and in situ. Regardless of the tissue, tumor or lung,
we could see that the tumor cells were of different sizes with
abundant deep blue stained nuclei, and the nuclear division was
significant. There was also loss of polarity, tightly packed cells
with ill-defined nuclei and visible focal degeneration or necrosis
of cancer cells. This showed that the model had been established
successfully (Fig. 6A and B). In
the PEG-DANPs groups, the number of cancer cells was significantly
reduced, the color of nuclei was stained red rather than light blue
in DANPs and Aisu® group, and large necrotic areas could
be seen without any nuclear division.
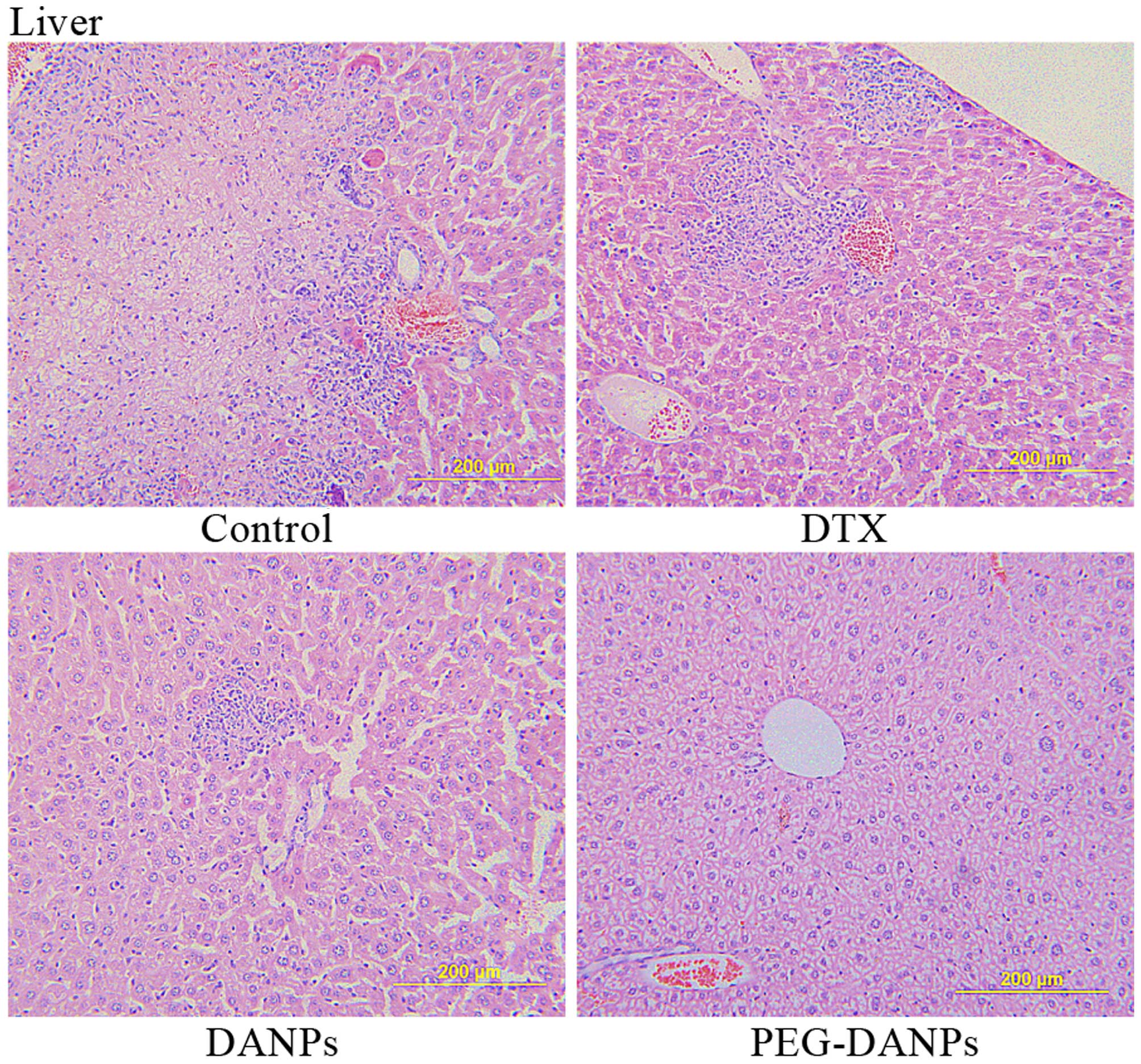
Extensive metastases were found in the livers of
control group mice, and there were large tumor nests, we could also
see liver metastases both in Aisu® and DANPs-treated
mice which were significant smaller than the control groups. In
contrast, liver tissue from PEG-DANP-treated mice showed barely
measurable levels of tumor cells but only few neutrophils
infiltrated by tumor cells (Fig.
7), these results suggest that PEG-DANPs could suppress
metastases in other organs such as liver more effectively than
DANPs and Aisu®.
Survival rate of the nude mice after
treatment
Nude mice in control group were all dead on the 36th
day, and the mice in PEG-DANPs group were dead on the 56th day.
Fig. 8 shows that the mice which
were treated with PEG-DANPs survived longer than the other groups,
and the differences are significant.
Discussion
According to the results of our previous experiments
(31), we demonstrated that
PEG-DANPs presented a more sustained manner of release in
vitro, this is since DTX is encapsulated in the core portion,
it has to go through the process of diffusion before release which
leads to the delayed effect. Furthermore, PEG-DANPs are superior to
DANPs in vitro drug release due to the PEG on the surface of
the nanoparticles. Moreover, PEG-DANPs could be also used as a
platform for the incorporation of active targeting moiety (28). PEG-DANPs could not only minimize the
exposure of normal tissues but also increase the accumulation of
the therapeutic drug in the tumor site compared to Aisu®
(32), thus showing its potential
applicability as a drug delivery system.
The PEG chain of PEG-DANPs is hydrophilic, it is
currently thought to act as a protector to achieve long circulation
time of drugs in the blood. It accumulated in the liver, spleen and
lung, and finally was released from these organs to blood
circulation according to the drug concentration gradient (29), which resulted in a sustained blood
level compared to Aisu® and DANPs. From the above
results, we can make a conclusion that PEG-DANPs can not only
increase the concentration and uptake of antitumor drugs in the
tumors, but also prolong the time that drugs are sustained in the
blood (33,34), which are the main approaches to
increase antitumor activity and inhibit tumor growth in
chemotherapy.
PEG-DANPs with an appropriate particle size can
significantly accumulate in the tumor via the EPR effect, this is
called size-dependent passive targeting. The size of PEG-DANPs is
~169 nm (31), it can
preferentially accumulate and stay in tumor tissues compared to
normal tissues. Moreover, the higher DTX concentration in blood of
the PEG-DANPs group could lead to delay tumor development
significantly better than the other two drugs both in transplant
model and in situ. In Fig.
4, the body weight changes in the tested mouse groups are
shown. The body weight loss of the mice in Aisu® groups
was more significant than the DANPs and PEG-DANPs groups,
particularly the PEG-DANPs group. These results show that PEG-DANPs
has less toxicity to normal organs and less systemic toxicity so
that it can provide longer survival time.
Collectively, our experiments were reported on the
PEG-DANPs against NSCLC in vitro and in vivo. The
in vitro cytotoxicity study proved the dose- and
time-dependent manner against A549 lung cancer cells; the in
vivo PEG-DANPs had superior antitumor and metastasis effects
compared to DANPs and Aisu®, and also relatively lower
side-effects providing longer survival time. PEG-DANPs exerted
promising therapeutic effects on NSCLC, and it is a good
drug-delivery platform for the treatment of NSCLC.
References
|
1
|
Gridelli C, Maione P, Comunale D and Rossi
A: Adjuvant chemotherapy in elderly patients with non-small-cell
lung cancer. Cancer Control. 14:57–62. 2007.PubMed/NCBI
|
|
2
|
Wu SH: Effect of docetaxel with cisplatin
in treating advanced non-small cell lung cancer. J Hainan Med Coll.
16:1615–1617. 2010.In Chinese.
|
|
3
|
Li XT: Docetaxel and cisplatin treatment
of non-small cell lung cancer analysis. Chin J Mod Drug Appl.
4:34–35. 2010.
|
|
4
|
Lu XM and Mao GX: A study of docetaxel
plus cisplatin versus gemcitabine plus cisplatin in treating
advanced non-small cell lung cancer. J Basic Clin Oncol.
22:308–310. 2009.
|
|
5
|
Xu Y: Docetaxel combined with cisplatin in
the treatment of advanced non-small cell lung cancer. Chin J Mod
Drug Applic. 4:26–28. 2010.
|
|
6
|
Shao JH: Efficacy of docetaxel combined
with nedaplatin or cisplatin in patients with advanced non-small
cell lung cancer. Chin J N Drugs. 19:599–601. 2010.
|
|
7
|
Zhang CH, Ren ZH, Li M, et al: Cisplatin
plus docetaxel combination in the first-line treatment of advanced
non-small cell lung cancer. Chin J Clin Oncol Rehab. 17:54–56.
2010.
|
|
8
|
Stinchcombe TE and Socinski MA:
Considerations for second-line therapy of non-small cell lung
cancer. Oncologist. 13(Suppl 1): S28–S36. 2008. View Article : Google Scholar
|
|
9
|
Xu YZ and Xu LW: The exploration of 70
years of age or older chemotherapy against non-small cell lung
cancer cells. Zhejiang Clin Med. 9:1226–1227. 2007.In Chinese.
|
|
10
|
Li JJ: A study of cisplatin plus docetaxel
or gemcitabine in treating advanced non small cell lung cancer. Mod
Med. 18:54–55. 582011.
|
|
11
|
Xiong Y, Zhou TC, Liu Y, Wang ZW, Lin XL,
Song XL, Shi XY and Liao ZW: Clinical analysis of efficacy in
docetaxel plus cisplatin chemotherapy with 3-DCRT treating the
patients with locally advanced NSCLC. China J Cancer Prev Treat.
17:699–702. 2010.
|
|
12
|
Greco FA, Spigel DR, Burris HA III,
Shipley DL, Farley C, Gandhi J, Houston GA and Hainsworth JD:
Weekly docetaxel versus docetaxel/gemcitabine in elderly/poor
performance status (PS) patients (pts) with stage III B/IV
non-small cell lung cancer (NSCLC): Randomized phase III trial of
the Minnie Pearl Cancer Research Network. J Clin Oncol. 25(Suppl):
S75342007.
|
|
13
|
Kudoh S, Takeda K, Nakagawa K, Takada M,
Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, et al:
Randomized phase study of docetaxel versus vinorelbine for elderly
patients with advanced non-small cell lung cancer (NSCLC): Results
of a west Japan thoracic oncology group trial (WJTOG 9904). J Clin
Oncol. 24:3657–3663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Li R, Zhu Z, Qian X, Guan W, Yu L,
Yang M, Jiang X and Liu B: Enhanced antitumor efficacy,
biodistribution and penetration of docetaxel-loaded biodegradable
nanoparticles. Int J Pharm. 430:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh SC, Neslihan Alpay S and
Klostergaard J: CD44: A validated target for improved delivery of
cancer therapeutics. Expert Opin Ther Targets. 16:635–650. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao W, Xiang B, Meng TT, Liu F and Qi XR:
Chemotherapeutic drug delivery to cancer cells using a combination
of folate targeting and tumor microenvironment-sensitive
polypeptides. Biomaterials. 34:4137–4149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Zhang D, Zhang Q, Chen Y, Zheng D,
Hao L, Duan C, Jia L, Liu G and Liu Y: Synergistic effect of
folate-mediated targeting and verapamil-mediated P-gp inhibition
with paclitaxel-polymer micelles to overcome multi-drug resistance.
Biomaterials. 32:9444–9456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ganesh S, Iyer AK, Gattacceca F, Morrissey
DV and Amiji MM: In vivo biodistribution of siRNA and cisplatin
administered using CD44-targeted hyaluronic acid nanoparticles. J
Control Release. 172:699–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iyer AK, Greish K, Seki T, Okazaki S, Fang
J, Takeshita K and Maeda H: Polymeric micelles of zinc
protoporphyrin for tumor targeted delivery based on EPR effect and
singlet oxygen generation. J Drug Target. 15:496–506. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang J, Nakamura H and Maeda H: The EPR
effect: Unique features of tumor blood vessels for drug delivery,
factors involved, and limitations and augmentation of the effect.
Adv Drug Deliv Rev. 63:136–151. 2011. View Article : Google Scholar
|
|
21
|
Tang N, Du G, Wang N, Liu C, Hang H and
Liang W: Improving penetration in tumors with nanoassemblies of
phospholipids and doxorubicin. J Natl Cancer Inst. 99:1004–1015.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li RY: Docetaxel-loaded PEG-albumin
nanoparticles for anti-metastasis in murine 4T1 breast cancer.
Yanbian University PhD Thesis. 2012
|
|
23
|
Li JQ, Yang ZZ, Meng TT and Qi XR: The use
of cationic liposomes to co-deliver docetaxel and siRNA for
targeted therapy of hepatocelluar carcinoma. J Chin Pharm Sci.
23:667–673. 2014. View Article : Google Scholar
|
|
24
|
Li Y, Jin M, Shao S, Huang W, Yang F, Chen
W, Zhang S, Xia G and Gao Z: Small-sized polymeric micelles
incorporating docetaxel suppress distant metastases in the
clinically-relevant 4T1 mouse breast cancer model. BMC Cancer.
14:329–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim D, Gao ZG, Lee ES and Bae YH: In vivo
evaluation of doxorubicin-loaded polymeric micelles targeting
folate receptors and early endosomal pH in drug-resistant ovarian
cancer. Mol Pharm. 6:1353–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao ZG, Fain HD and Rapoport N: Controlled
and targeted tumor chemotherapy by micellar-encapsulated drug and
ultrasound. J Control Release. 102:203–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao L, Xiong X, Sun X, Zhu Y, Yang H,
Chen H, Gan L, Xu H and Yang X: Role of cellular uptake in the
reversal of multidrug resistance by PEG-b-PLA polymeric micelles.
Biomaterials. 32:5148–5157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Zhan C, Gu B, Meng Q, Wang H, Lu W
and Hou H: Targeted brain delivery of itraconazole via RVG29
anchored nanoparticles. J Drug Target. 19:228–234. 2011. View Article : Google Scholar
|
|
29
|
Mu L, Teo MM, Ning HZ, Tan CS and Feng SS:
Novel powder formulations for controlled delivery of poorly soluble
anticancer drug: Application and investigation of TPGS and PEG in
spray-dried particulate system. J Control Release. 103:565–575.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Li R, Qian X, Ding Y, Tu Y, Guo R,
Hu Y, Jiang X, Guo W and Liu B: Superior antitumor efficiency of
cisplatin-loaded nanoparticles by intratumoral delivery with
decreased tumor metabolism rate. Eur J Pharm Biopharm. 70:726–734.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin G, Jin M, Yin X, Jin Z, Chen L and Gao
Z: A comparative study on the effect of docetaxel-albumin
nanoparticles and docetaxel-loaded PEG-albumin nanoparticles
against non-small cell lung cancer. Int J Oncol. 47:1945–1953.
2015.PubMed/NCBI
|
|
32
|
Gong C, Xie Y, Wu Q, Wang Y, Deng S, Xiong
D, Liu L, Xiang M, Qian Z and Wei Y: Improving anti-tumor activity
with polymeric micelles entrapping paclitaxel in pulmonary
carcinoma. Nanoscale. 4:6004–6017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Zhang D, Zhang Q, Guo S, Zheng D,
Hao L, Guo H and Li C: Tissue distribution and pharmacokinetics
evaluation of DOMC-FA micelles for intravenous delivery of PTX. J
Drug Target. 21:137–145. 2013. View Article : Google Scholar
|
|
34
|
Wang J, Huo MR and Zhang XY: Progress in
hyaluronic acidbased targeted nano-drug delivery systems. Chin J
Pharm. 44:828–835. 2013.
|