Introduction
Gastric cancer is the second leading cause of
cancer-related deaths worldwide (1). Although remarkable progress has been
made in the treatment of gastric cancer, the prognosis of gastric
cancer patients remains poor (2).
The main reasons for the unsatisfactory prognosis of gastric cancer
patients include lack of symptoms for patients in the early stages
and malignant growth and systemic metastasis for patients in the
advanced stages (3). Therefore, it
is of great importance to elucidate the molecular mechanisms
involved in the development and progression of gastric cancer,
which may contribute to the identification of novel biomarkers and
therapeutic targets, thus promoting the early diagnosis and more
effective treatment of gastric cancer.
MicroRNAs (miRNAs) are a group of endogenous short
non-coding nucleotide RNAs which modulate the expression of
downstream targets by binding to complementary sites within the
3′-untranslated region (3′-UTR) of target mRNAs (4). It has been well-recognized that miRNAs
play crucial roles in various biological processes, including cell
proliferation, apoptosis, and metastatic activity (5). Recently, various studies (6,7) have
confirmed that miRNAs play critical roles in the development and
progression of gastric cancer. The expression status, clinical
significance and the biological function of miRNAs in gastric
cancer remains largely unknown.
MicroRNA-340 (miR-340) has been found to play a
critical role in human cancers, including breast (8,9),
prostate (10,11), and esophageal cancer (12), hepatocellular carcinoma (13) and glioma (14). Functionally, miR-340 was found to
inhibit the migration and invasion of breast cancer cells by
inhibiting the Wnt pathway (8). In
prostate cancer, miR-340 was found to inhibit cell proliferation
and promote cell apoptosis by inhibiting high-mobility group
nucleosome-binding domain 5 (11).
Studies of glioma have shown that miR-340 inhibited the stem-like
cell function by targeting tissue plasminogen activator (14). Notably, the expression of miR-340
has been found highly expressed in gastric cancer tissues (15,16).
miR-340 can promote gastric cancer cell growth and reduce cell
apoptosis effectively (17).
However, the clinical significance and underlying mechanisms of
miR-340 in human gastric cancer remain unknown.
In this study, miR-340 expression was found to be
aberrantly increased in human gastric cancer tissues and cells.
Elevated levels of miR-340 were found to be correlated with adverse
clinicopathological features and poor prognosis of gastric cancer
patients. Functionally, miR-340 promoted cell viability,
proliferation, colony formation and cell cycle progression by
targeting cyclin G2 (CCGN2).
Materials and methods
Clinical specimens and cell culture
Tumor tissues and the adjacent non-cancer tissues
were obtained from 80 gastric cancer patients who received surgical
resection at the Department of General Surgery, of the First
Affiliated Hospital of Xi'an Jiaotong University. All clinical
specimens were frozen and stored at −80°C. The patients did not
receive any chemotherapy or radiotherapy before surgery. The
demographic and clinicopathological features of the 80 patients are
presented in Table I. All protocols
involving clinical samples were approved by the Ethics Committee of
Xi'an Jiaotong University according to the Declaration of Helsinki
(as revised in Tokyo 2004).
 | Table IThe clinical significance of miR-340
expression in gastric cancer. |
Table I
The clinical significance of miR-340
expression in gastric cancer.
| Clinicopathologic
features | Total no. of patients
(n=80) | miR-340
| P-value |
|---|
| Low n | High n |
|---|
| Age (years) |
| <65 | 44 | 24 | 20 | 0.369 |
| ≥65 | 36 | 16 | 20 | |
| Gender |
| Male | 55 | 27 | 28 | 0.809 |
| Female | 25 | 13 | 12 | |
| Histology |
| Well, moderate | 36 | 13 | 23 | 0.025a |
| Poor, signet | 44 | 27 | 17 | |
| Tumor Size (cm) |
| <5 | 33 | 10 | 23 | 0.003a |
| ≥5 | 47 | 30 | 17 | |
| Depth of
invasion |
| T1 | 15 | 7 | 8 | 0.775 |
|
T2–T4 | 65 | 33 | 32 | |
| Lymph node
metastasis |
| Absent | 29 | 12 | 17 | 0.245 |
| Present | 51 | 28 | 23 | |
| Lymphatic
invasion |
| Absent | 26 | 10 | 16 | 0.152 |
| Present | 54 | 30 | 24 | |
| Venous
infiltration |
| Absent | 56 | 25 | 31 | 0.143 |
| Present | 24 | 15 | 9 | |
| TNM stage |
| I, II | 43 | 14 | 29 | 0.001a |
| III, IV | 37 | 26 | 11 | |
Human gastric cancer cell lines (SGC-7901, MGC-803,
MKN-45, and AGS) and a normal gastric epithelium cell line (GES-1)
were obtained from the Cell Bank of the Type Culture Collection of
the Chinese Academy of Sciences. All cells were maintained in
Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY, USA)
supplemented with 10% FBS (Invitrogen Life Technologies, Carlsbad,
CA, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin
(Invitrogen Life Technologies) at 37°C with 5% CO2.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from the gastric cancer
tissues and cells using TRIzol (Invitrogen Life Technologies)
reagent. The TaqMan Human MiRNA Assay kit (Applied Biosystems,
Foster City, CA, USA) and a SYBR® Premix Ex
Taq™ II kit (Takara Bio, Shiga, Japan) were used for the
PCR amplification. Primers for miR-340 (HmiRQP0434), CCGN2
(HQP021882), U6 (HmiRQP9001) and GAPDH (HQP006347) were purchased
from Genecopoeia, Inc. (Guangzhou, China). U6 was used as an
internal control for the relative expression level of miR-340,
while GAPDH was used as the internal control for the relative
expression of CCNG2.
Cell transfection
The plasmids and siRNA used in this study include
miR-340 mimics (HmiR0090-MR04) and control vector (CmiR0001-MR03),
miR-340 inhibitors (HmiR-AN0434-AM03) and negative control vector
(CmiR-AN0001-AM03; all from Genecopoeia, Inc.) and CCNG2 siRNA
(SR300634) and scrambled negative control siRNA (SR30004; both from
OriGene, Technologies, Inc., Beijing, China). These vectors
aforementioned were transfected into the gastric cancer cells with
Lipofectamine 2000 (Invitrogen Life Technologies) based on the
manufacturer's protocol.
Western blot analysis
RIPA buffer was used to extract the total protein
from the gastric cancer cells, and 20–30 µg of isolated
protein was separated by 10% SDS-PAGE and transferred onto
0.22-µm NC membranes (Sigma-Aldrich, St. Louis, MO, USA).
After membrane transfer, the membranes were incubated with the
GAPDH and CCNG2 antibodies (both from Cell Signaling Technology,
Inc., Danvers, MA USA), overnight at 4°C. Then, the membranes were
incubated with the secondary goat anti-mouse or anti-rabbit IgG
antibody (ZSGB-BIO Co., Ltd., Beijing, China). GAPDH was used as
control.
MTT, deoxyuridine (BrdU) incorporation
and colony formation assays
For assessment of cell viability, gastric cancer
cells (4×103) were seeded into 96-well plates and
stained with sterile MTT for 4 h at 37°C, following which the
culture medium was discarded and an extra 150 µl DMSO (both
from Sigma-Aldrich) were then added into each well. The absorbance
at 490 nm was examined 24, 48 and 72 h after transfection.
For assessment of proliferation, BrdU incorporation
and colony formation assays were performed. Regarding the BrdU
incorporation assay, cells after transfection were incubated with
BrdU and then were stained with anti-BrdU antibody (Sigma-Aldrich)
according to the manufacturer's instructions. Regarding the colony
formation assay, gastric cancer cells were seeded on 6-well plates.
Two weeks after cell seeding, the colonies were stained with 1%
crystal violet and the number of colonies was counted.
Cell cycle assays
For the cell cycle analysis, 48 h after
transfection, the gastric cancer cells were collected, washed with
PBS, and fixed with 80% ethanol overnight at 4°C. Then, the cells
were incubated with RNaseA for 30 min at 37°C, followed by
incubation with propidium iodide (both from Sigma-Aldrich) for 20
min at room temperature. Next, the cells were subjected to flow
cytometric analysis using a FACSCalibur (BD Biosciences, Bedford,
MA, USA).
Tumor formation assay in a nude mouse
model
Female BALB/c nude mice, 4–6 weeks old were used to
establish the nude mouse xenograft model. MGC-803 cells transfected
with miR-340 inhibitor or negative control vectors were suspended
in 100 µl of PBS and were injected subcutaneously into the
flank of each nude mouse. Tumor volumes were determined by
measuring two of its dimensions with calipers every 3 days, and the
equation for calculating the tumor volume was V = 0.5 × D ×
d2 (V, volume; D, longitudinal diameter; d, latitudinal
diameter). All nude mice were sacrificed at 3 weeks after the
injection of MGC-803 cells. All in vivo protocols were
approved by the Institutional Animal Care and Use Committee of
Xi'an Jiaotong University.
Luciferase reporter assay
The wild-type 3′-UTR sequence of CCNG2 or the
mutated sequence within the predicted target sites was synthesized
and inserted into the pGL3 control vector (Promega Corp., Madison,
WI, USA), to construct the wt CCNG2-3′-UTR or mt CCNG2-3′-UTR,
respectively. Then, SGC-7901 cells were seeded into 24-well plates,
and were cultured in OptimMEM reduced serum media (Invitogen Life
Technologies), and were cotransfected with each luciferase reporter
construct (the wild-type or mutant 3′-UTR of CCNG2) and miR-340
overexpression vector using FuGENE (Promega Corp). Forty-eight
hours after transfection, SGC-7901 cells were harvested and
luciferase activity was measured using the dual-luciferase reporter
assay system (Promega Corp.). Results were obtained from three
independent experiments performed in triplicate.
Statistical analysis
Data are presented as the mean ± SEM. GraphPad Prism
5 software (GraphPad Software, Inc., San Diego, CA, USA) was used
for statistical analysis. The differences between two or more
groups were compared with a two-tailed Student's t-test or ANOVA.
Pearson's correlation analysis was used to analyze the clinical
correlation. To compare the difference of patient survival between
two groups, the Kaplan-Meier method and the log-rank test were
performed. Differences were considered statistically significant at
P<0.05.
Results
miR-340 is aberrantly upregulated in
gastric cancer tissues and cell lines
First, we measured the expression level of miR-340
in gastric cancer tissues and matched tumor-adjacent tissues with
qRT-PCR. The results showed that miR-340 expression in gastric
cancer tissues was significantly higher than that in the non-tumor
tissues (P<0.05, Fig. 1A). As
compared with GES-1 cells, miR-340 was upregulated in a panel of
gastric cancer cell lines (SGC-7901, AGS, MKN-45, and MGC-803)
(P<0.05, Fig. 1B). The obvious
elevation of miR-340 in gastric cancer tissues and cells suggest
that miR-340 may play an oncogenic role in the development and
progression of gastric cancer.
Elevated expression of miR-340 is
correlated with adverse clinicopathological features and poor
prognosis of the gastric cancer patients
The gastric cancer patients were divided into two
groups (miR-340 high or low expression group) based on the median
expression level of miR-340. Then we compared the
clinicopathological features and prognosis of gastric cancer
patients between the two groups. As shown in Table I, high expression of miR-340 was
associated with poor histological features (P=0.025), large tumor
size (P=0.003), and advanced tumor-node-metastasis (TNM) stage
(P=0.001). Furthermore, Kaplan-Meier analysis showed that increased
miR-340 expression was significantly associated with shorter
overall survival (P=0.003, Fig. 1C)
and disease-free survival (P=0.008, Fig. 1D). Multi-variant Cox regression
analysis demonstrated that miR-340 expression was an independent
prognostic marker for predicting both overall survival and
disease-free survival in gastric cancer patients (P=0.001 and
P=0.045, respectively, Table II).
These data indicate that miR-340 can serve as a novel biomarker for
the survival of gastric cancer patients.
 | Table IIMultivariate Cox regression analysis
of the 5-year overall and disease-free survival of 80 gastric
cancer patients. |
Table II
Multivariate Cox regression analysis
of the 5-year overall and disease-free survival of 80 gastric
cancer patients.
| Variables | Overall survival
| Disease-free
survival
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Histology | 0.557 | 0.319–0.971 | 0.039a | 0.686 | 0.390–1.205 | 0.190 |
| Tumor Size
(cm) | 2.344 | 1.482–3.706 | <0.001a | 1.355 | 0.825–2.225 | 0.229 |
| TNM stage | 3.188 | 1.964–5.175 | <0.001a | 2.660 | 1.588–4.457 | <0.001a |
| miR-340
expression | 2.269 | 1.472–3.498 | 0.001a | 1.604 | 1.011–2.543 | 0.045a |
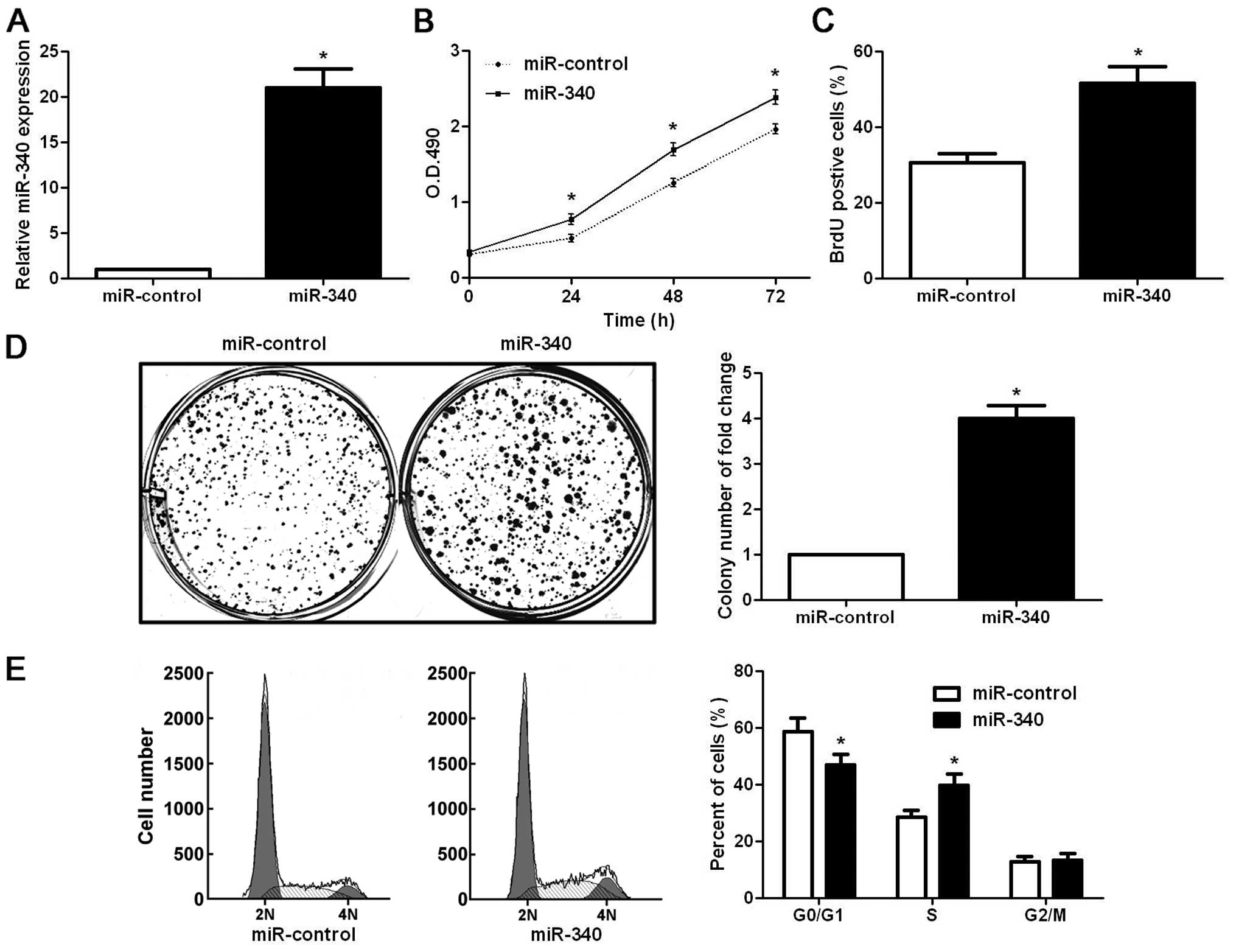
miR-340 enhances the growth of gastric
cancer cells by promoting cell viability, proliferation and cell
cycle progression
To investigate the biological functions of miR-340
in gastric cancer cells, we transfected gastric cancer cell line
SGC-7901 with miR-340 mimics. Transfection of miR-340 mimics
significantly increased the miR-340 level in the SGC-7901 cells
(P<0.05, Fig. 2A). MTT assays
showed that ectopic expression of miR-340 significantly increased
cell viability of the SGC-7901 cells (P<0.05, Fig. 2B). Furthermore, BrdU incorporation
assay showed that the overexpression of miR-340 increased the
proliferative ability of the SGC-7901 cells (P<0.05, Fig. 2C). Furthermore, miR-340
overexpression markedly increased the colony number of the SGC-7901
cells (P<0.05, Fig. 2D). Cell
cycle analysis revealed that upregulation of miR-340 increased the
percentage of cells in the S phase while it decreased the
percentage of cells in the G0/G1 phase (P<0.05, Fig. 2E). In contrast, we downregulated the
miR-340 level with corresponding inhibitors in the MGC-803 cells
and examined the cell viability, proliferation, colony formation
and cell cycle. miR-340 inhibitor transfection significantly
inhibited the expression level of miR-340 in the MGC-803 cells
(P<0.05, Fig. 3A). Functionally,
inhibition of miR-340 expression resulted in significantly
decreased cell viability (P<0.05, Fig. 3B), proliferation (P<0.05,
Fig. 3C), colony formation
(P<0.05, Fig. 3D) and cell cycle
progression (P<0.05, Fig. 3E).
To further confirm these functional effects of miR-340 on gastric
cancer cells, we performed tumor formation assay in a nude mouse
model. Inhibition of the miR-340 expression level with its
inhibitor significantly decreased the tumor growth of MGC-803 cells
in the nude mice (P<0.05, Fig.
4). Collectively, these data indicate that miR-340 can promote
the tumor growth of gastric cancer cells both in vitro and
in vivo.
CCNG2 is a direct target of miR-340 in
gastric cancer cells
To elucidate the underlying molecular mechanisms
involved in the biological function of miR-340 in gastric cancer
cells, we used TargetScanHuman 7.0 to search for the downstream
targets of miR-340. The data in the TargetScanHuman 7.0 showed that
3′-UTR of CCNG2 contained the highly conserved putative binding
sites for miR-340 (Fig. 5A),
indicating that CCNG2 is a potential downstream target of miR-340.
Then, we performed luciferase assays to elucidate whether miR-340
could interact with the 3′-UTR region of CCNG2. The results of the
luciferase assays showed that miR-340 overexpression significantly
decreased the luciferase acitivity of the wt 3′-UTR of CCNG2
(P<0.05, Fig. 5B), while it had
no effects on that of the mt 3′-UTR of CCNG2 in SGC-7901 cells.
Next, we used qRT-PCR and western blot analysis to determine
whether miR-340 could modulate the expression of CCNG2 in gastric
cancer cells. Overexpression of miR-340 significantly reduced the
expression level of CCNG2 mRNA in the SGC-7901 cells (P<0.05,
Fig. 5C). The results from western
blot analysis demonstrated that the protein level of CCNG2 in the
SGC-7901 cells was also significantly decreased after miR-340
overexpression (P<0.05, Fig.
5D). Downregulation of miR-340 with its inhibitor significantly
increased the mRNA level (P<0.05, Fig. 5E) and protein level (P<0.05,
Fig. 5F) of CCNG2 in the MGC-803
cells. These data indicate that CCNG2 is a direct downstream target
of miR-340 in gastric cancer cells.
CCNG2 is critical for the functional
effects of miR-340 in gastric cancer cells
As CCNG2 is a direct downstream target of miR-340,
we further evaluated whether CCNG2 could mediate the biological
function of miR-340 in gastric cancer cells. CCNG2 siRNA
significantly reduced the protein level of CCNG2 in the MGC-803
cells transfected with miR-340 inhibitors (P<0.05, Fig. 6A). Knockdown of CCNG2 abrogated the
effects of miR-340 knockdown on the MGC-803 cells with increased
cell viability (P<0.05, Fig.
6B), proliferation (P<0.05, Fig.
6C), colony formation (P<0.05, Fig. 6D) and cell cycle progression
(P<0.05, Fig. 6E). These data
indicate that CCNG2 is not only a downstream target of miR-340, but
also a functional mediator of miR-340 in gastric cancer cells.
Discussion
Accumulating studies demonstrate that aberrant
expression and function of miRNAs play a critical role in the
development of various types of human cancers (6,18),
including gastric cancer (18).
Examination of the expression status, biological function and
underlying mechanisms of specific miRNAs in gastric cancer can
contribute to the identification of novel biomarkers and
therapeutic targets in human gastric cancer. Previous studies have
shown that miR-340 plays an important role in human cancers
(8–14). It was found to inhibit the
metastatic behavior of breast cancer cells (14) while it decreased the proliferation
and promoted the cell apoptosis of prostate cancer cells (13,18).
Moreover it was also found to modulate the stem-like cell function
of glioma cells (10). However, the
clinical significance and underlying mechanisms of miR-340 in
gastric cancer remain unclear.
In the present study, we found that miR-340
expression was significantly increased in gastric cancer tissues
compared with that in the paired non-tumor-adjacent tissues.
Significant elevation of miR-340 was also observed in gastric
cancer cells. Association analysis showed that elevated expression
of miR-340 was significantly correlated with adverse
clinicopathological features, including poor histological
differentiation, large tumor size, and advanced TNM stage.
Moreover, survival analysis showed that an elevated level of
miR-340 was associated with poorer overall and disease-free
survival of gastric cancer patients. These data indicate that
miR-340 can serve as a novel prognostic indicator of the prognosis
of gastric cancer patients.
Increased proliferative ability and cell cycle
progression are important in the functional foundation for the
development and progression of human cancers (19). The functional assays in this study
showed that ectopic expression of miR-340 facilitated cell
viability, proliferation, colony formation, and cell cycle
progression in gastric cancer cells, while inhibition of miR-340
decreased these cellular processes in the gastric cancer cells.
Therefore, these results demonstrated that miR-340 contributes to
the development and progression of gastric cancer by promoting cell
viability, proliferation and cell cycle progression.
CCNG2, which is an important regulator of cell
proliferation and cell cycle progression (20), has been found to be decreased in
gastric cancer tissues (21,22).
Moreover, CCGN2 was found to influence the cell viability and cell
cycle progression of gastric cancer cells (22). In this study, we presented concrete
data showing that CCGN2 is a novel downstream target of miR-340 in
human gastric cancer cells. First, the 3′-UTR of CCNG2 contained
the complementary sequence of miR-340. Second, overexpression of
miR-340 significantly inhibited the luciferase activity of the wt
3′-UTR of CCNG2 while had no influence on that of the mt 3′-UTR of
CCNG2. Third, overexpression of miR-340 significantly reduced the
expression level of CCNG2, while inhibition of miR-340 increased
the expression level of CCNG2 in the gastric cancer cells.
Furthermore, this study showed that CCNG2 knockdown could reverse
the effects of miR-340 inhibitors on cell viability, proliferation,
colony formation and cell cycle progression. Therefore, CCNG2 is a
direct functional target of miR-340 in gastric cancer cells.
In conclusion, we demonstrated that miR-340 is
frequently overexpressed in gastric cancer tissues and cell lines.
miR-340 plays an important role in the progression of gastric
cancer by modulating the expression of CCNG2 in gastric cancer
cells. Therefore, this study indicates that miR-340 can potentially
serve as a promising prognostic factor and therapeutic target in
gastric cancer.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendell JT: MicroRNAs: Critical regulators
of development, cellular physiology and malignancy. Cell Cycle.
4:1179–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohammadi-Yeganeh S, Paryan M, Arefian E,
Vasei M, Ghanbarian H, Mahdian R, Karimipoor M and Soleimani M:
MicroRNA-340 inhibits the migration, invasion, and metastasis of
breast cancer cells by targeting Wnt pathway. Tumour Biol. Jan
12–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CP, Sun ZL, Lu X, Wu WX, Guo WL, Lu
JJ, Han C, Huang JQ and Fang Y: miR-340 suppresses cell migration
and invasion by targeting MYO10 in breast cancer. Oncol Rep.
35:709–716. 2016.
|
|
10
|
Huang K, Tang Y, He L and Dai Y:
MicroRNA-340 inhibits prostate cancer cell proliferation and
metastasis by targeting the MDM2-p53 pathway. Oncol Rep.
35:887–895. 2016.PubMed/NCBI
|
|
11
|
Wei P, Qiao B, Li Q, Han X, Zhang H, Huo Q
and Sun J: microRNA-340 suppresses tumorigenic potential of
prostate cancer cells by targeting high-mobility group
nucleosome-binding domain 5. DNA Cell Biol. 35:33–43. 2016.
View Article : Google Scholar
|
|
12
|
Yan S, Jiang H, Fang S, Yin F, Wang Z, Jia
Y, Sun X, Wu S, Jiang T and Mao A: MicroRNA-340 inhibits esophageal
cancer cell growth and invasion by targeting phosphoserine
aminotransferase 1. Cell Physiol Biochem. 37:375–386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi L, Chen Z-G, Wu LL, Zheng JJ, Yang JR,
Chen XF, Chen ZQ, Liu CL, Chi SY, Zheng JY, et al: miR-340 reverses
cisplatin resistance of hepatocellular carcinoma cell lines by
targeting Nrf2-dependent antioxidant pathway. Asian Pac J Cancer
Prev. 15:10439–10444. 2014. View Article : Google Scholar
|
|
14
|
Yamashita D, Kondo T, Ohue S, Takahashi H,
Ishikawa M, Matoba R, Suehiro S, Kohno S, Harada H, Tanaka J, et
al: miR340 suppresses the stem-like cell function of
glioma-initiating cells by targeting tissue plasminogen activator.
Cancer Res. 75:1123–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroenterol
Hepatol. 24:652–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni
L, Zhang WG, Nan KJ, Song TS and Huang C: MicroRNA profiling of
human gastric cancer. Mol Med Rep. 2:963–970. 2009.PubMed/NCBI
|
|
17
|
Hou X and Qiao H: Effect of miR-340 on
gastric cancer cell proliferation and apoptosis. Int J Clin Exp
Pathol. 8:13108–13113. 2015.
|
|
18
|
Petrocca F, Pilozzi E, Rapazzotti M,
Aurello P, Mercantini P, Volinia S, Ruco LP, Croce CM and Vecchione
A: MicroRNAs deregulation in gastric cancer. Cancer Res. 66:1338.
2006.
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bates S, Rowan S and Vousden KH:
Characterisation of human cyclin G1 and G2: DNA damage inducible
genes. Oncogene. 13:1103–1109. 1996.PubMed/NCBI
|
|
21
|
Shi W, Yu K, Wu G and Zhang H: Expression
of CCNG2 in gastric carcinoma and its relationship with prognosis.
Chin J Cell Biol. 33:994–997. 2011.
|
|
22
|
Sun GG, Hu WN, Cui DW and Zhang J:
Decreased expression of CCNG2 is significantly linked to the
malignant transformation of gastric carcinoma. Tumour Biol.
35:2631–2639. 2014. View Article : Google Scholar
|