Introduction
Bladder cancer (BC) is one of the most common
cancers with high morbidity and mortality worldwide. BC seriously
threatens human health and life (1). Endothelial cells (ECs) play critical
roles in the tumor microenvironment and in angiogenesis, which are
essential for the growth, proliferation and migration of cancer
cells (2,3). ECs are regarded as a significant
target for antitumor angiogenesis and various anti-angiogenic drugs
have been applied in the clinic (4). For this reason, identification of new
molecules targeting ECs may become effective treatment strategies
for BC.
The Warburg effect states that cancer cells can
secrete lactic acid (5). ECs are
surrounded by a high lactic acid environment, which can activate
numerous signaling pathways and promote EC proliferation (6,7).
Recently, a study demonstrated that ECs rely on glycolysis for ATP
production, and deletion of the
phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3) gene
in ECs could suppress angiogenesis (8). PFKFB enzymes synthesize
fructose-2,6-bisphosphate (F2,6P2), as an allosteric activator of
PFK-1, an essential molecule in glycolysis and vascularization
(9). As a single carboxylic acid
transporter, monocarboxylate transporter 1 and 4 (MCT1 and MCT4)
can transfer the lactic acid produced by tumor cells to the
surrounding microenvironment, and then into ECs to cause a series
of molecular events such as increased invasive activity (10). Targeting lactate metabolism has been
reported to be studied in regards to cancer therapeutics, and
PFKFB3 as well as MCTs have been studied as targets for cancer
treatment (9,11). However, the effects of PFKFB3 and
MCT in the tumor microenvironment of BC remain unclear and warrant
further research.
Three-dimensional (3D) co-culture models are
constructed to simulate the cell in vivo environment when
cells are cultured in vitro. The cells are grown within
extracellular matrix (ECM) gels in a fluidic chip, and the chip is
highly porous for seeding cells inside and providing large areas
for cell-to-cell interactions (12). 3D co-culture allows cells to
maintain normal shape, structure and function, in order to well
reflect the microstructure, dynamic mechanical properties and
biochemical functionalities to simulate a natural microenvironment
(13). The microfluidic chip is an
efficient experimental platform which unites numerous experiments
into one chip and realizes 3D co-culture (14).
In the present study, in order to simulate the human
tumor microenvironment, we conducted a co-culture with human
umbilical vein endothelial cells (HUVECs) and human BC T24 cells,
on a microfluidic chip. HUVEC activity was examined under a
co-culture situation. The roles of PFKFB3 and MCT1 in cell
proliferation, apoptosis and lactic acid synthesis were also
investigated.
Materials and methods
Cell culture
HUVECs and T24, a BC cell line, were obtained from
the Central Laboratory of the Affiliated Hospital of Qingdao
University. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) (Gibco, Carlsbad, CA, USA) containing 10% fetal
bovine serum (FBS), supplemented with 100 U/ml penicillin and 100
μg/ml streptomycin (Sigma, St. Louis, MO, USA) in a 37°C
incubator containing 5% CO2.
Preparation of matrix gel and
microfluidic chip platform (co-culture and transfer chip)
Firstly, the matrix gel was put into a refrigerator
at 4°C overnight to enable it to thaw out completely. Then, the
pre-cooled matrix gel was sufficiently mixed with DMEM (FBS-free)
in a ratio of 1:1, and 10 μl of mixed liquor was seeded into
the central microchannel of the microfluidic chip lightly using a
pipettor. The microfluidic chip was placed into a 37°C thermostatic
incubator overnight to enable the matrix gel to solidify.
The microfluidic platform is exhibited in Fig. 1 and it has 3 major functions:
co-culture of two types of cells, matrix gels between chambers to
avoid mixed cells and high flux. The microfluidic chip has chambers
and each chamber has two microchannels. The cells were seeded into
chambers through one microchannel and the second channel ensures a
stable pressure. Four chambers were separated by two major square
crossing microchannels filled with Matrigel to simulate the
membrane in the human body and made it easier to observe the
behavior of a single type of cells. The chip was surrounded by a
perfusion microchannel that could supply medium for cells in the
chambers.
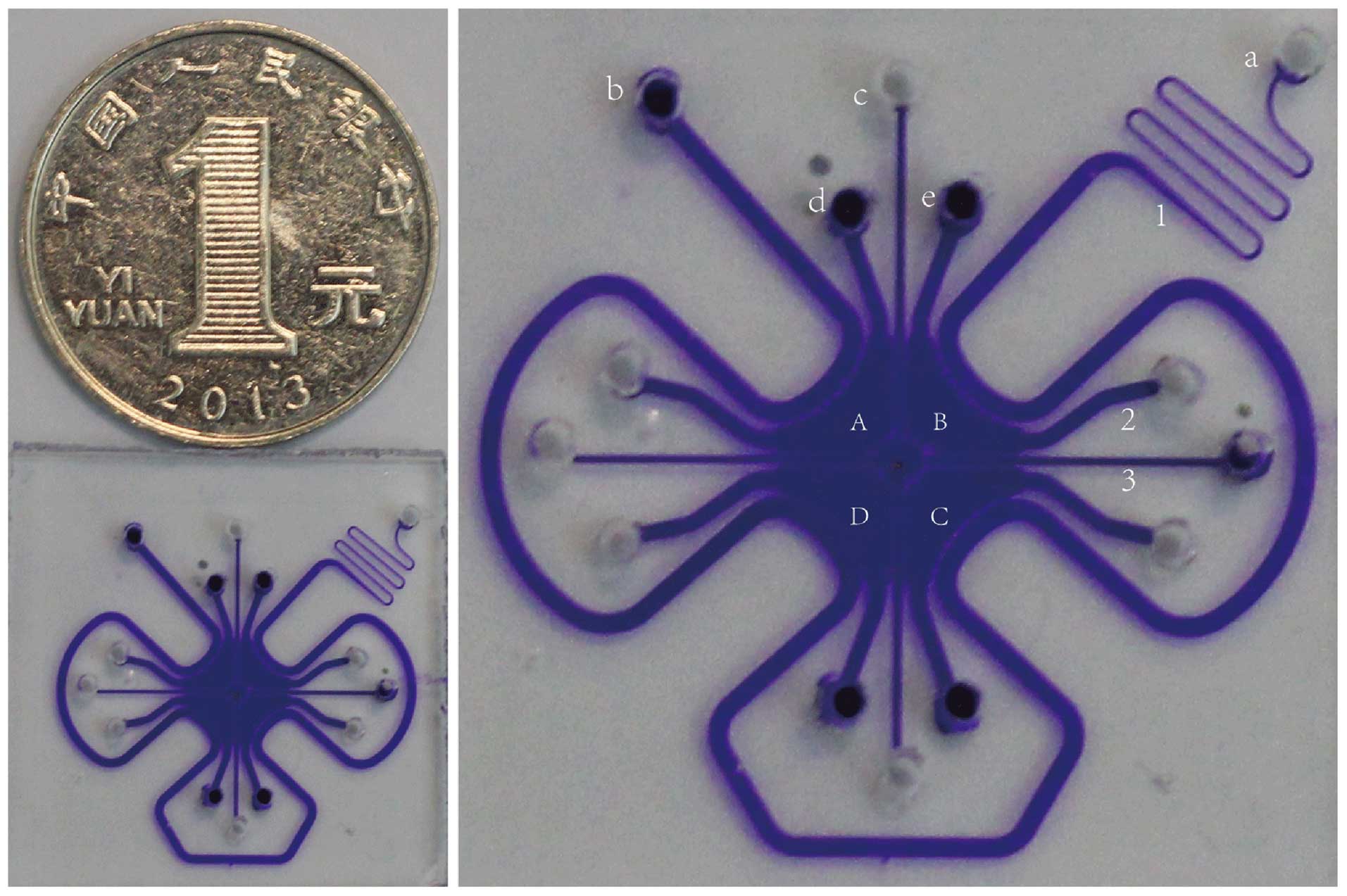 | Figure 1Image of the microfluidic chip for
cell culture. The diameter is shorter than 1 yuan coin RMB ~2 cm.
(A–D) Four chambers: 1, perfusion channel for nutrition support; 2,
channels for planting cells; 3, central cross channel serving as
membrane: a, entrance for inputting medium; b, exit for outputting
medium; c, entrance for inputting Matrigel; d and e, two holes for
planting cells. In the present study, A and C were planted with
HUVECs, while B and D were planted with T24 cells. Small molecules
and factors can pass through the channels between the different
chambers, and medium can supply energy to the cells from the
surrounding perfusion channels. |
3D co-culture of HUVECs and T24
Three groups were designated in this experiment,
namely control (HUVECs in complete medium), 2D (HUVECs co-cultured
with T24 in complete solution) and 3D (HUVECs co-cultured with T24
in Matrigel) groups. In the 3D group, the suspension of HUVECs and
T24 was prepared with a high density of 107 cells/ml and
mixed with matrix gel in a proportion of 1:1. Then, the cell-matrix
gel mixed suspension was separately seeded into two opposite
chambers of the chip and the chip was cultured in a 37°C
thermostatic incubator for 30 min to form a co-culture net.
Meanwhile, complete culture solution was injected into the
irrigation channel to support nutrition for cells in the 3D-matrix
gel. The cells in the control and 2D groups were treated using the
same method.
Immunofluorescence
After washing with phosphate-buffered saline (PBS),
the cells were fixed with 4% paraformaldehyde in PBS and then
permeabilized with 0.05% Triton X-100 in PBS. They were blocked
with 3% BSA for 2 h, and stained with primary antibodies
(anti-CD31, anti-CD105, anti-MCT1 or anti-PFKFB3; abcam) at 4°C
overnight. After washing with PBS, the chip was incubated with
TRITC-conjugated secondary antibodies (BD, San Diego, CA, USA) for
40 min, followed by staining with 1 μg/ml of DAPI (Sigma)
for 5 min at room temperature. The chip was then rinsed with PBS 3
times and observed using fluorescence microscopy. Confocal imaging
was performed using Zeiss510 Meta system. The red and blue
fluorescence was observed at 543 and 408 nm, respectively.
Fluorescence cell viability assay
Cells were divided into 5 groups, namely the control
group, quercetin (cells were treated with 5 μl 0.02
nmol/μl quercetin), MCT1KD group (MCT1 was
knocked down with 5 μl 0.02 nmol/μl siMCT1),
PFKFB3KD group (PFKFB3 was knocked down with 5 μl
0.02 nmol/μl siPFKFB3) and MKD + PKD
group (MCT1 and PFKFB3 were both knocked down with 2.5 μl
0.02 nmol/μl siMCT1 and 2.5 μl 0.02 nmol/μl
siPFKFB3). The control group was treated with the same amount of
DMEM. The siRNA treatment was performed according to the
manufacturer's instructions when cells reached 60% confluency, and
the second siRNAs were transfected 48 h later. Then, the cells were
transferred into the microfluidic chip after 48 h. After planting
HUVECs into the microfluidic chip for 3 days, the cells were
stained with 2 μmol/l calcein and 10 μmol/l propidium
iodide to observe the cell apoptosis rate and death rate under
fluorescence microscopy immediately. Conventional chemical
synthesis of siRNAs as 21–25 nt of the double chain small molecule
RNA was carried out by Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). Our previous study verified that they can effectively block
MCT1 (15). In addition, the siRNAs
for PFKFB3 were purchased from RiboBio; 3 pairs of different siRNAs
were designed for different sites of the same target gene. The 3
pairs of siRNAs in accordance with a specific proportion were mixed
into highly efficient silencing target gene products, that is, a
cocktail.
Immunoblotting
The proteins were extracted from the lysates of the
HUVECs with lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium
deoxycholate, 2.5 mM sodium pyrophosphate and protease inhibitor.
After centrifugation, the protein concentration was determined
using the Bradford assay (Bio-Rad, Hercules, CA, USA).
Approximately 20 μg of the proteins was loaded and separated
using 8% SDS-PAGE and transferred to a polyvinylidene difluoride
(PVDF) membrane. Then, the members were blocked with 5% BSA,
followed by incubation with primary antibodies at 4°C overnight.
Immunodetection was accomplished using horseradish
peroxidase-conjugated secondary antibody, followed by processing
using an enhanced chemiluminescence (ECL) detection system.
Cell proliferation (CCK-8) assay
Cell proliferation of the HUVECs was evaluated using
Cell Counting Kit-8 (CCK-8) assay (Dojindo) according to the
manufacturer's instructions. Briefly, HUVECs were seeded into the
microfluidic chip and co-cultured for 3 days. Then, the cells were
digested and removed into a 96-well cell culture plate containing
100 μl DMEM complete and grown for 8 h at 37°C with 5%
CO2. After 8 h, 10 μl CCK-8 reagents were added
per well and incubation was carried out for 2 h in a CO2
incubator. Finally, 100 μl supernatant from each well was
transferred into fresh 96-well plates and the absorbance was
measured at 450 nm using a spectrophotometer to calculate cell
proliferation ability. Inhibition ratio (%) = (OD ratio in the
control group − OD ratio in the experimental group)/OD ratio in the
control group × 100%. The experiment was repeated 3 times.
Lactic concentration examination
Lactic concentrations in the different groups were
examined using a lactate assay kit (BioVision) according to the
manufacturer's instructions.
Statistical analysis
At least 3 separate experiments were performed for
each measurement. All quantitative data are expressed as mean ± SD
and differences for comparisons were analyzed using t-test and
one-way ANOVA followed by Tukey's post hoc test. All the analyses
were performed using SPSS 19.0 software (SPSS, Inc., Chicago, IL,
USA) and P<0.05 was considered to indicate a statistically
significant result.
Results
Activation of HUVECs is detected in the
co-culture condition
In order to detect the HUVEC activity in different
conditions, immunofluorescence assay was conducted to detect CD31
and CD105 expression, which has been recognized as activity factors
for ECs (16). As shown in Fig. 2A, CD31 and CD105 showed the
strongest fluorescence intensity in the 3D co-culture group and the
weakest fluorescence intensity in the control group, and the
expression levels of CD31 and CD105 in the 2D co-cultured group
were higher than these levels in the control group and lower than
those in the 3D co-culture group. Consistently, the results of
western blotting showed similar expression levels, which reflected
gradually increased expression of CD31 and CD105 from the control
group, 2D co-culture to the 3D co-culture group (Fig. 2B).
Quercetin inhibits the activity of
MCT1
Quercetin exists in flowers, leafs and fruits of
many plants. As a natural inhibitor of MCT1, quercetin can inhibit
MCT1 expression and then influence the transfer of monocarboxylic
acid, which can affect the metabolism and activity of cells
(10). The expression levels of
PFKFB3 and MCT1 were downregulated by their targeting siRNAs, while
quercetin treatment only inhibited MCT1 expression but did not
affect PFKFB3 expression (Fig. 3A and
B).
Knockdown of MCT1 and PFKFB3 increases
the apoptosis rate of HUVECs under single-culture and co-culture
situations
Under the 3D co-culture condition, the apoptosis
rate was slightly lower than that under the single-cultured
condition, while the overall trend was similar and without
statistical differences (Fig. 4).
The apoptosis rate in cells treated with quercetin was
significantly increased when compared with that in the control
group (P<0.05). Meanwhile, no significant differences in the
apoptosis rate were found among the quercetin, MCT1KD
and PFKFB3KD groups (P>0.05). In addition, the
apoptosis rate was higher in the MKD + PKD
group than that in the single targeting MCT1 or PFKFB3 group
(P<0.05).
 | Figure 4Downregulation of the expression of
MCT1 and PFKFB3 increases the apoptosis rate of HUVECs. (A) A
chamber was planted with endothelial cells and the cell number was
~300–400/chamber. Cells were divided into 5 groups, namely the
control, quercetin (cells were treated with 5 μl 0.02
nmol/μl quercetin), MCT1KD (MCT1 was knocked down
with 5 μl 0.02 nmol/μl siMCT1), PFKFB3KD
(PFKFB3 was knocked down with 5 μl 0.02 nmol/μl
siPFKFB3) and MKD + PKD group (MCT1 and
PFKFB3 were both knocked down with 2.5 μl 0.02
nmol/μl siMCT1 and 2.5 μl 0.02 nmol/μl
siPFKFB3). Control group was treated with the same amount of DMEM.
The cells in the different treatment groups were stained with 2
μmol/l calcein and 10 μmol/l propidium iodide, and
then were observed under a microscope (magnification, ×200). (B)
Statistical results of the apoptosis rate and mortality ratio of
HUVECs in the different treatment groups. One-way ANOVA followed by
Tukey's post hoc test was used to analyze the differences among
groups. Columns, mean (n=3); bars, SD; *P<0.05,
**P<0.01 vs. the control group in the single-culture
cells; #P<0.05, ##P<0.01 vs. the
control group in the co-culture cells. |
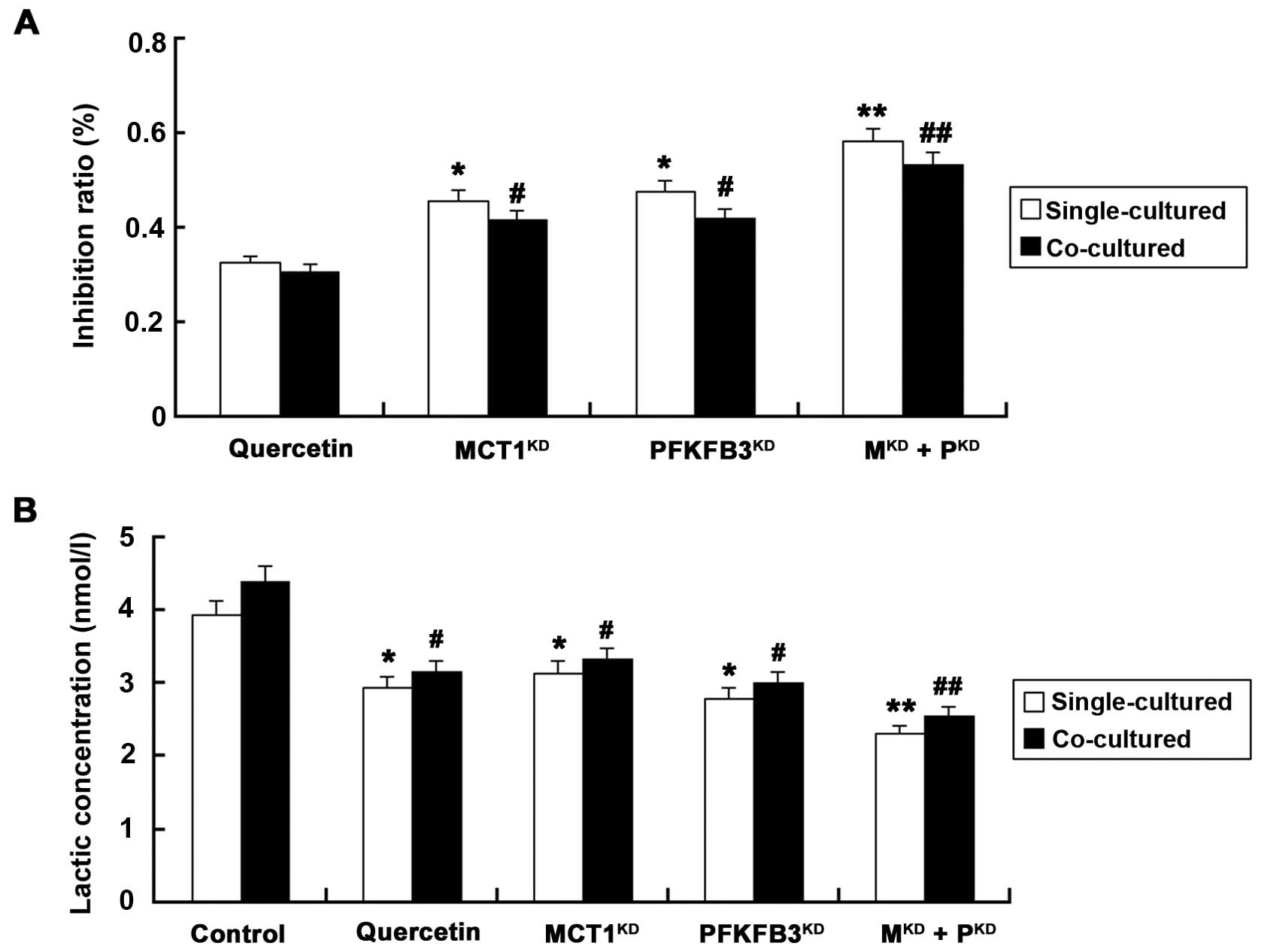
Knockdown of MCT1 and PFKFB3 increases
the inhibition ratio and decreases the lactate concentration
We next evaluated the cell proliferation and lactate
concentration in the co-cultured HUVECs and T24 cells following the
different treatments (Fig. 5). The
inhibition ratios were similar whether silencing of MCT1 or PFKFB3
was carried out by siRNAs (P>0.05), and the ratios were all
higher than that in cells treated with the same amount of quercetin
(P<0.05). Additionally, the suppression rate was highest in the
MKD + PKD group. Furthermore, the lactic
concentration was significantly reduced in cells following
silencing of MCT1 or/and PFKFB3, as compared with that in the
control group (P<0.05).
Discussion
Endothelial cells (ECs) are usually considered as
ideal targets for suppression of tumor angiogenesis owing to
various factors. i) Many important receptors on EC membranes take
part in angiogenesis; ii) EC structure is stable and it is
difficult to form drug resistance; iii) ECs have similar
characteristics in almost all solid tumor, thus, one target can be
used in different tumor treatments; iv) direct contact between ECs
and drugs in blood can reduce drug concentrations (17). ECs tend to form tip cell phenotype
to bud blood vessels under a high lactic acid concentration. At the
same time, another part of ECs translate into a stalk cell
phenotype, obtaining strong proliferation ability leading to new
sprout growth into vessel branches (18). All the experiments in the present
study were conducted in a simulated tumor microenvironment by
co-culturing HUVECs and T24 cells with a microfluidic device.
Recently, several studies have found that CD31 and
CD105 are specific sensitive microvessel (MV) markers in various
types of cancer, such as colon, cervix, endometrium and breast
(16,19–21).
We analyzed the fluorescence intensity of CD31 and CD105 in
single-, 2D- and 3D-cultured HUVECs, and strong positive expression
of CD31 and CD105 was found in the 3D co-cultured cells (Fig. 2). This occurred since the cells were
embedded into Matrigel in a 3D condition which was most similar to
a real in vivo situation and thus the variety of cellular
interactions were most frequent. This result also illustrated that
tumor cells could enhance EC activity.
MCT1 is an important transporter for lactic acid
entering ECs and plays an important role in the presence of lactic
acid (22). PFKFB3, acting as a
PFK1 allosteric activator, plays an important role in glycolysis.
Carmeliet et al confirmed that glycolysis is the important
source for ECs to gain energy and build new blood vessels (23). Meanwhile, research indicates that
blood vessel growth is inhibited by blocking glycolysis and
removing the energy source of vascular ECs. Targeting PFKFB3 can
effectively reduce the motility, proliferation, invasion and
angiogenesis of ECs (24). In the
present study, the apoptosis rate of the HUVECs was significantly
increased after silencing of MCT1 or/and PFKFB3 (Fig. 4). The possible reason may be that
silencing of MCT1 or/and PFKFB blocked the main energy metabolic
pathway, which accounts for ~85% of the energy of ECs (25).
Rivera and Bergers found that PFKFB3 promoted EC
proliferation by reducing Notch pathway activity (26). Végran et al reported that
lactate could enter ECs through MCT-1 to trigger the
phosphorylation and degradation of IκBα, and then stimulate the
NF-κB pathway to drive cell migration and tube formation in
colorectal adenocarcinoma and breast cancer (27). CCK-8 assay verified that blocking
MCT1 or PFKFB3 reduced the proliferation activity of HUVECs and the
combined blockage of the targets provided a better result (Fig. 5). Single blockage of PFKFB3 was
found to only partly and transitorily reduce EC activity, since the
lactic acid from other cells in the microenvironment can resist
this effect (28). Multipoint
target blocking is considered as the most effective method for
inhibiting tumor cell proliferation (29). The effects on apoptosis and
proliferation were much stronger following the combination of
blocking MCT1 and PFKFB3 when compared with the effects following
either blocking MCT1 or PFKFB3. Thus, we speculated that the energy
metabolic pathway and lactic acid effects may be involved.
As the end-product of metabolism, lactate acid
remains stable and high concentrations in the tumor
microenvironment stimulate angiogenesis (30). It has been reported that high serum
lactate dehydrogenase levels are considered as a poor prognostic
indicator in most types of cancers, including pancreatic carcinoma,
malignant lymphoma and colorectal cancers (31–33).
It has been reported that lactic acid exists as the substrate of
many types of enzymes in tumor ECs, and an increase in its
concentration could promote angiogenesis by promoting the
activities of NF-κB and IL-8/CXCL8 signaling pathways by inhibiting
PDH2 (6). In the present study, the
lactate concentration was significantly decreased by silencing MCT1
or/and PFKFB3. However, the underlying mechanisms involved in the
effects of MCT1 and PFKFB3 on lactate concentration in BC warrant
further study.
Research has demonstrated that angiogenesis,
proliferation and metastatic activity of cancers could be
effectively inhibited by targeting MCT1 and PFKFB3 (34–36).
Quercetin can be used as an anti-cough drug and is a good
expectorant. Recently, quercetin was found to have an inhibitory
effect on MCT1 activity, thus promoting cell apoptosis (37). According to our results, quercetin
effectively suppressed cell proliferation and promoted apoptosis
via a significant decrease in the activity of MCT1 and
downregulation of lactic acid concentration may be involved in
these effects in BC. Considering that no obvious differences in
effects were found between quercetin and siMCT1, we can conclude
that quercetin has potential antitumor effects by targeting
MCT1.
In conclusion, the present study demonstrated that
tumor cells enhance EC activity under a simulated microenvironment
by 3D co-culture of HUVECs and T24 cells. Meanwhile, cell apoptosis
increased and proliferation decreased following the blocking of
MCT1 or/and PFKFB3 via influencing the energy metabolic pathway and
lactic acid concentration, which are critical for angiogenesis.
Quercetin may be developed as a potential antitumor drug by
downregulating MCT1. However, further studies should be carried out
to investigate the molecular mechanisms of quercetin in regards to
the effect on MCT1.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (nos. 30901481,
81372752 and 81472411), the Wu Jieping Medical Foundation
(320.6750.13261), and the Natural Science Foundation of Shandong
Province, China (ZR2014HM088). We thank Professor Chang Gui Li
(Qingdao University, China) for providing the Gout Laboratory. We
also thank Professor Zhen Liu and Ling Ling Cui for providing
technical guidance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sleeckx N, Van Brantegem L, Van den Eynden
G, Fransen E, Casteleyn C, Van Cruchten S, Veldhuis Kroeze E and
Van Ginneken C: Angiogenesis in canine mammary tumours: A
morphometric and prognostic study. J Comp Pathol. 150:175–183.
2014. View Article : Google Scholar
|
|
3
|
Müller K, Ellenberger C, Hoppen HO and
Schoon HA: Immunohistochemical study of angiogenesis and angiogenic
factors in equine granulosa cell tumours. Res Vet Sci. 92:471–477.
2012. View Article : Google Scholar
|
|
4
|
Hoelder S, Clarke PA and Workman P:
Discovery of small molecule cancer drugs: Successes, challenges and
opportunities. Mol Oncol. 6:155–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ngo H, Tortorella SM, Ververis K and
Karagiannis TC: The Warburg effect: Molecular aspects and
therapeutic possibilities. Mol Biol Rep. 42:825–834. 2015.
View Article : Google Scholar
|
|
6
|
Bonavia R, Inda MM, Vandenberg S, Cheng
SY, Nagane M, Hadwiger P, Tan P, Sah DW, Cavenee WK and Furnari FB:
EGFRvIII promotes glioma angiogenesis and growth through the NF-κB,
interleukin-8 pathway. Oncogene. 31:4054–4066. 2012. View Article : Google Scholar
|
|
7
|
Philip B, Ito K, Moreno-Sánchez R and
Ralph SJ: HIF expression and the role of hypoxic microenvironments
within primary tumours as protective sites driving cancer stem cell
renewal and metastatic progression. Carcinogenesis. 34:1699–1707.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Bock K, Georgiadou M, Schoors S,
Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B,
Cauwenberghs S, Eelen G, et al: Role of PFKFB3-driven glycolysis in
vessel sprouting. Cell. 154:651–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Izumi H, Takahashi M, Uramoto H, Nakayama
Y, Oyama T, Wang KY, Sasaguri Y, Nishizawa S and Kohno K:
Monocarboxylate transporters 1 and 4 are involved in the invasion
activity of human lung cancer cells. Cancer Sci. 102:1007–1013.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ge X, Lyu P, Cao Z, Li J, Guo G, Xia W and
Gu Y: Overexpression of miR-206 suppresses glycolysis,
proliferation and migration in breast cancer cells via PFKFB3
targeting. Biochem Biophys Res Commun. 463:1115–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bokhari M, Carnachan RJ, Cameron NR and
Przyborski SA: Culture of HepG2 liver cells on three dimensional
polystyrene scaffolds enhances cell structure and function during
toxicological challenge. J Anat. 211:567–576. 2007.PubMed/NCBI
|
|
13
|
Knight E and Przyborski S: Advances in 3D
cell culture technologies enabling tissue-like structures to be
created in vitro. J Anat. 227:746–756. 2015. View Article : Google Scholar
|
|
14
|
Huh D, Matthews BD, Mammoto A,
Montoya-Zavala M, Hsin HY and Ingber DE: Reconstituting organ-level
lung functions on a chip. Science. 328:1662–1668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi H, Jiang H, Wang L, Cao Y, Liu P, Xu
X, Wang Y, Sun L and Niu H: Overexpression of monocarboxylate anion
transporter 1 and 4 in T24-induced cancer-associated fibroblasts
regulates the progression of bladder cancer cells in a 3D
microfluidic device. Cell Cycle. 14:3058–3065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saad RS, El-Gohary Y, Memari E, Liu YL and
Silverman JF: Endoglin (CD105) and vascular endothelial growth
factor as prognostic markers in esophageal adenocarcinoma. Hum
Pathol. 36:955–961. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim C, Yang H, Fukushima Y, Saw PE, Lee J,
Park JS, Park I, Jung J, Kataoka H, Lee D, et al: Vascular RhoJ is
an effective and selective target for tumor angiogenesis and
vascular disruption. Cancer Cell. 25:102–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eelen G, Cruys B, Welti J, De Bock K and
Carmeliet P: Control of vessel sprouting by genetic and metabolic
determinants. Trends Endocrinol Metab. 24:589–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akagi K, Ikeda Y, Sumiyoshi Y, Kimura Y,
Kinoshita J, Miyazaki M and Abe T: Estimation of angiogenesis with
anti-CD105 immunostaining in the process of colorectal cancer
development. Surgery. 131(Suppl): S109–S113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saad R and Dabbs D: Endoglin, CD31, and
CD34 expression in the breast cancer. Laboratory Investigation.
Nature Publishing Group; New York, NY, USA: 2001
|
|
21
|
Saad RS, Jasnosz KM, Tung MY and Silverman
JF: Endoglin (CD105) expression in endometrial carcinoma. Int J
Gynecol Pathol. 22:248–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su J, Chen X and Kanekura T: A
CD147-targeting siRNA inhibits the proliferation, invasiveness, and
VEGF production of human malignant melanoma cells by downregulating
glycolysis. Cancer Lett. 273:140–147. 2009. View Article : Google Scholar
|
|
23
|
Carmeliet P, De Smet F, Loges S and
Mazzone M: Branching morphogenesis and antiangiogenesis candidates:
Tip cells lead the way. Nat Rev Clin Oncol. 6:315–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, An X, Guo X, Habtetsion TG, Wang Y,
Xu X, Kandala S, Li Q, Li H, Zhang C, et al: Endothelial PFKFB3
plays a critical role in angiogenesis. Arterioscler Thromb Vasc
Biol. 34:1231–1239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stapor P, Wang X, Goveia J, Moens S and
Carmeliet P: Angiogenesis revisited - role and therapeutic
potential of targeting endothelial metabolism. J Cell Sci.
127:4331–4341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rivera LB and Bergers G: Angiogenesis.
Targeting vascular sprouts. Science. 344:1449–1450. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Végran F, Boidot R, Michiels C, Sonveaux P
and Feron O: Lactate influx through the endothelial cell
monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway
that drives tumor angiogenesis. Cancer Res. 71:2550–2560. 2011.
View Article : Google Scholar
|
|
28
|
Schoors S, Cantelmo AR, Georgiadou M,
Stapor P, Wang X, Quaegebeur A, Cauwenberghs S, Wong BW, Bifari F,
Decimo I, et al: Incomplete and transitory decrease of glycolysis:
A new paradigm for anti-angiogenic therapy? Cell Cycle. 13:16–22.
2014. View
Article : Google Scholar :
|
|
29
|
Niu H, Jiang H, Cheng B, Li X, Dong Q,
Shao L, Liu S and Wang X: Stromal proteome expression profile and
muscle-invasive bladder cancer research. Cancer Cell Int.
12:392012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tennant DA, Durán RV and Gottlieb E:
Targeting metabolic transformation for cancer therapy. Nat Rev
Cancer. 10:267–277. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yi JH, Kim JH, Baek KK, Lim T, Lee DJ, Ahn
YC, Kim K, Kim SJ, Ko YH and Kim WS: Elevated LDH and paranasal
sinus involvement are risk factors for central nervous system
involvement in patients with peripheral T-cell lymphoma. Ann Oncol.
22:1636–1643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fahmueller YN, Nagel D, Hoffmann RT,
Tatsch K, Jakobs T, Stieber P and Holdenrieder S: Predictive and
prognostic value of circulating nucleosomes and serum biomarkers in
patients with metastasized colorectal cancer undergoing Selective
Internal Radiation Therapy. BMC Cancer. 12:52012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tas F, Aykan F, Alici S, Kaytan E, Aydiner
A and Topuz E: Prognostic factors in pancreatic carcinoma: Serum
LDH levels predict survival in metastatic disease. Am J Clin oncol.
24:547–550. 2001. View Article : Google Scholar
|
|
34
|
Murray CM, Hutchinson R, Bantick JR,
Belfield GP, Benjamin AD, Brazma D, Bundick RV, Cook ID, Craggs RI,
Edwards S, et al: Monocarboxylate transporter MCT1 is a target for
immunosuppression. Nat Chem Biol. 1:371–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vander Heiden MG: Targeting cancer
metabolism: A therapeutic window opens. Nat Rev Drug Discov.
10:671–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Le Floch R, Chiche J, Marchiq I, Naiken T,
Ilc K, Murray CM, Critchlow SE, Roux D, Simon M-P and Pouysségur J:
CD147 subunit of lactate/H+ symporters MCT1 and
hypoxia-inducible MCT4 is critical for energetics and growth of
glycolytic tumors. Proc Natl Acad Sci USA. 108:16663–16668. 2011.
View Article : Google Scholar
|
|
37
|
Chao JI, Su WC and Liu HF: Baicalein
induces cancer cell death and proliferation retardation by the
inhibition of CDC2 kinase and survivin associated with opposite
role of p38 mitogen-activated protein kinase and AKT. Mol Cancer
Ther. 6:3039–3048. 2007. View Article : Google Scholar : PubMed/NCBI
|