Introduction
Cancer is the leading cause of death worldwide,
accounting for 8.2 billion deaths in 2012 alone (1). Multimodal treatment, including
chemotherapy, surgery, and radiotherapy, has dramatically reduced
cancer mortality and improved the quality of life of individuals
with cancer (1–3). However, not all patients respond
positively to currently available therapies, and relapse is common
in patients who initially respond to chemotherapy. The
epithelial-mesenchymal transition (EMT) is an essential mechanism
involved in tumor progression and metastasis, and the tumor
microenvironment, including the extracellular matrix and numerous
stromal cell types, has been shown to induce EMT (4,5).
Therefore, the development of novel treatments targeting the EMT
process may provide effective therapies for patients who do not
respond to current treatments or who experience chemoresistant
relapse.
Platelets, the smallest anucleate hematopoietic
cells, are now recognized as key regulators of tumor progression
and metastasis (6–8). In the circulation, platelet
aggregation protects cancer cells from shear stress and immune
surveillance through the formation of a platelet cloak. Platelets
also facilitate cancer cell adherence to vascular endothelial
cells, which leads to extravasation into the stroma and the
formation of secondary tumors (9).
However, the presence and role of platelets in primary tumors are
not well understood.
Platelets contain numerous platelet-derived growth
mediators and cytokines related to EMT, such as transforming growth
factor-β (TGF-β), vascular endothelial growth factor-A (VEGF-A),
and plasminogen activator inhibitor-1 (PAI-1). Labelle et al
reported that direct signaling between platelets and breast cancer
cells in the vasculature induces the latter to undergo EMT
(10). Furthermore, investigations
have demonstrated that tumors undergoing EMT show increased
resistance to chemotherapy (11,12).
Moreover, in a study targeting chemoresistant breast cancer cells
following neo-adjuvant chemotherapy, we found that some patients
achieved pathological complete response (pCR; defined as no
residual invasive cancer in the breast and lymph nodes), which
would be expected to be associated with a more favorable prognosis
than that in patients who did not achieve pCR (13). Chemoresistance involves numerous
complex mechanisms, including gene pathways associated with
apoptosis/senescence and DNA repair, which are often influenced by
communication between host and tumor cells (14). Furthermore, EMT, anti-apoptotic
mechanisms, and stemness induced by the cancer microenvironment
have been shown to play important roles in chemoresistance
(15).
Therefore, we hypothesized that platelets
surrounding tumor cells could also be detected in primary sites and
could be associated with EMT and chemoresistance. The aims of this
study were as follows: i) to confirm the presence of platelets
surrounding primary tumor cells in breast cancer; ii) to explore
the associations between tumor cells associated with platelets and
EMT; and iii) to evaluate the association between the presence of
platelets surrounding tumor cells and chemoresistance, and
survival.
Materials and methods
Patients and clinical specimens
We retrospectively analyzed data from 74 patients
with human epidermal growth factor receptor 2 (HER2)-negative
breast cancer who had undergone neo-adjuvant chemotherapy at
Kanazawa University Hospital between 2006 and 2013. Patients were
selected according to the following inclusion criteria: women,
histologically confirmed invasive ductal carcinoma of the breast
with no evidence of metastatic disease and defined as clinical
stage I to IIIC (any T, N3, M0) with the same neo-adjuvant
chemotherapy regimen [four cycles of docetaxel (Taxotere) 75
mg/m2 followed by four cycles of fluorouracil,
epirubicin 100 mg/m2, and cyclophosphamide (FEC-100)].
Additionally, patients were excluded from the analysis if they met
any of the following criteria: ⅰ) invasive lobular carcinoma; ⅱ)
ductal or lobular carcinoma in situ; and ⅲ) HER2-positive
breast cancer, defined as immunohistochemistry (IHC) 3+ or
fluorescence in situ hybridization (FISH)/dual in
situ hybridization (DISH) positive. All study procedures were
approved by the Ethics Committee of the Kanazawa University
Hospital. Written informed consent was obtained from each patient
enrolled in the study.
The tumors were staged according to the
International Union against Cancer tumor-node-metastasis (TNM)
classification 7th edition (16).
Histological subtype and grade were classified on the basis of the
World Health Organization (WHO) guidelines for the Pathology and
Genetics of Tumors of the Breast and Female Genital Organs
(17). ER status, progesterone
receptor (PR) status, Ki-67 index, histology, and nuclear grade
were evaluated in biopsy specimens analyzed prior to neo-adjuvant
chemotherapy. Biopsies were performed by taking 3–5 extra cores in
a needle biopsy with a 14-gauge needle or by vacuum-assisted biopsy
with an 11-gauge needle. Biopsy samples were obtained uniformly
from various regions of the entire tumor.
The pathological response to neo-adjuvant
chemotherapy, including anthracycline and/or taxanes, was evaluated
in surgical specimens after therapy. pCR was defined as the
complete eradication of all invasive cancer in both the breast and
axillary nodes. Any other response was considered to be
non-pCR.
Associations between clinicopathological parameters,
including CD42b expression and pCR, were investigated with
univariable/multivariable logistic regression. Odds ratios (ORs)
and 95% confidence intervals (CIs) with two-sided p-values were
used. p<0.05 was considered statistically significant. Overall
survival (OS) was defined as the time between the first day of
chemotherapy and the date of breast cancer-related death; patients
still alive were censored at the last date of follow-up.
Recurrence-free survival (RFS) was defined as the interval between
the first day of chemotherapy and the date of disease relapse or
death from related causes; patients still alive were censored at
the last date of follow-up.
Immunohistochemical examination
The Dako Envision system, with dextran polymers
conjugated to horseradish peroxidase (Dako, Carpinteria, CA, USA),
was used for immunohistochemical staining to avoid any endogenous
biotin contamination. Formalin-fixed, paraffin-embedded tissues
were cut into sections (4 µm thick). The sections were
deparaffinized with xylene and rehydrated in increasing dilutions
of ethanol. Endogenous peroxidase was blocked by immersing sections
in 3% H2O2 and 100% methanol for 20 min at
room temperature. Antigen retrieval was achieved by microwaving
sections at 95°C for 10 min in 0.001 M citrate buffer (pH 6.7).
After blocking the endogenous peroxidase, the sections were
incubated with Protein Block Serum-Free (Dako) at room temperature
for 10 min to prevent non-specific staining. Sections were then
incubated with primary antibodies [anti-glycoprotein Ib (CD42b;
Abcam, Cambridge, UK) at a 1:100 dilution for platelet
identification; anti-E-cadherin (clone 4A2C7; Zymed) at a 1:50
dilution; anti-vimentin (ab92547; Abcam) at a 1:250 dilution; and
anti-β-catenin (ab16051; Abcam) at a 1:1,000 dilution as a marker
for EMT] followed by quenching of the endogenous peroxidase
activity. Peroxidase activity was detected using the enzyme
substrate 3-amino-9-ethylcarbazole. Sections were incubated in
Tris-buffered saline without the primary antibodies as negative
controls, counterstained with Mayer's hematoxylin, and mounted with
mounting medium.
All biopsy specimens were fixed with 10% formalin
and embedded in paraffin. The percentage of stained cells was
recorded in at least five fields at ×400 magnification in randomly
selected areas. Cases in which >10% of cancer cells were stained
were defined as positive. To eliminate sampling bias, we confirmed
that there was no difference between available resected specimens
and biopsy specimen for this evaluation method. Two observers who
were unaware of the clinical data independently reviewed all the
pathological slides.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software following the guidelines described by Bremer and
Doerge (18) and the GraphPad Prism
User Guide. Differences in categorical variables were tested for
significance using χ2 tests. Results were considered
significant when p<0.05. OS and RFS rates were estimated using
Kaplan-Meier method and compared using the log-rank test.
Results
Patient and clinicopathological
characteristics
Patient characteristics, including age, menopausal
status, and tumor stage are summarized in Table I. The median patient age was 52
years (range, 27–73 years), and 31 patients were premenopausal. The
tumor stages were as follows: stage I, n=22; stage II, n=32; and
stage III, n=20. ER status was positive in 48 tumors and negative
in 26 tumors. The median Ki-67 index was 40 (range, 0–90). With
respect to histological subtype, 72 tumors were invasive ductal
carcinoma, and two were of an unknown subtype. The nuclear grades
of the tumors were as follows: 1 in 14 cases, 2 in 11 cases, 3 in
34 cases, and unknown in 15 cases. The median follow-up time was 69
months (range, 28–117 months). There were 5 deaths (4 deaths in
CD42b-positive and 1 death in CD42b-negative groups) and 9
recurrences (7 recurrences in the CD42b-positive and 2 recurrences
in the CD42b-negative group). The median RFS and OS values were not
reached. The 5-year RFS and OS rates were 93.2 and 98.6%,
respectively.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Data |
|---|
| Age (years), median
(range) | 52 (27–73) |
| Menopause, n (%) | |
| Premenopause | 31 (48) |
| Postmenopause | 33 (52) |
| Stage, n (%) | |
| I | 22 (30) |
| IIA | 18 (24) |
| IIB | 14 (19) |
| IIIA | 7 (9) |
| IIIB | 4 (6) |
| IIIC | 9 (12) |
| ER status, n (%) | |
| Positive | 48 (65) |
| Negative | 26 (35) |
| Ki-67 index, %
(range) | 40 (0–90) |
| Histology, n (%) | |
| Scirrhous
carcinoma | 55 (74) |
| Papillotubular
carcinoma | 8 (11) |
| Solid-tubular
carcinoma | 9 (12) |
| Unknown | 2 (3) |
| Nuclear grade, n
(%) | |
| 1 | 14 (19) |
| 2 | 11 (15) |
| 3 | 34 (46) |
| Unknown | 15 (20) |
Platelets surrounding primary tumor
cells
All tumors were evaluated for CD42b expression, a
platelet-specific marker. CD42b expression was observed in 44 of 74
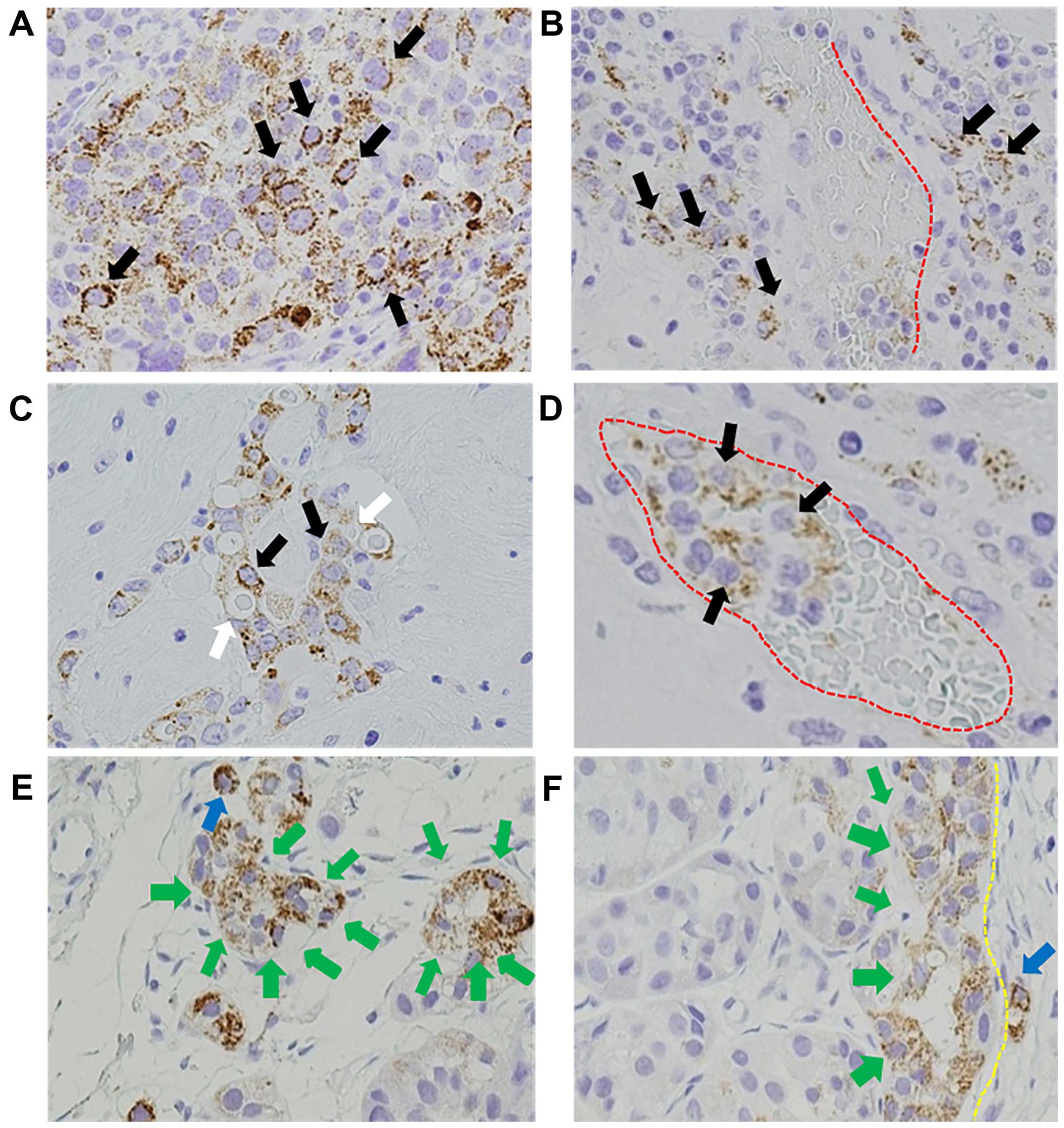
(59%) primary breast tumors (Fig.
1A), with particularly strong staining at the invasive front,
which was observed in 37 of 74 (84%) specimens (Fig. 1F), and migratory tumor cells in the
perivascular tissue, which was observed in 30 of 44 (68%) specimens
(Fig. 1B–D).
Relationship between platelets
surrounding primary tumor cells and clinicopathological
features
The relationships between CD42b expression and
clinicopathological features, including stage, nuclear grade,
histology, ER status, and pathological responce are summarized in
Table II. A statistically
significant association was noted between CD42b expression and
pathological response (p<0.0001). There were no significant
associations between CD42b expression and stage, nuclear grade,
histology, or ER status.
 | Table IIRelationship between CD42b expression
and the clinicopathological characteristics of the primary breast
cancer cases. |
Table II
Relationship between CD42b expression
and the clinicopathological characteristics of the primary breast
cancer cases.
| Clinicopathological
parameters | CD42b expression
| t or χ2
test
(p-value) |
|---|
Positive
(≥10%)
n (%) | Negative
(<10%)
n (%) |
|---|
| Patients | 44 (59) | 30 (41) | |
| Stage | | | 0.2280 |
| I | 11 (25) | 11 (36) | |
| II | 18 (41) | 14 (46) | |
| III | 15 (34) | 5 (18) | |
| Nuclear grade | | | 0.1539 |
| G1 | 6 (14) | 8 (26) | |
| G2 | 6 (14) | 5 (18) | |
| G3 | 25 (56) | 10 (33) | |
| Unknown | 7 (16) | 7 (23) | |
| Histology | | | 0.4172 |
| Scirrhous
carcinoma | 33 (75) | 22 (73) | |
| Papillotubular
carcinoma | 3 (7) | 5 (16) | |
| Solid-tubular
carcinoma | 6 (13) | 3 (11) | |
| Unknown | 2 (5) | 0 (0) | |
| ER status | | | 0.6115 |
| Positive | 29 (66) | 22 (73) | |
| Negative | 15 (34) | 8 (27) | |
| Chemotherapy
response | | | 0.0001 |
| pCR | 4 (9) | 15 (50) | |
| Non-pCR | 40 (91) | 15 (50) | |
Expression of EMT markers in primary
tumor cells associated with platelets
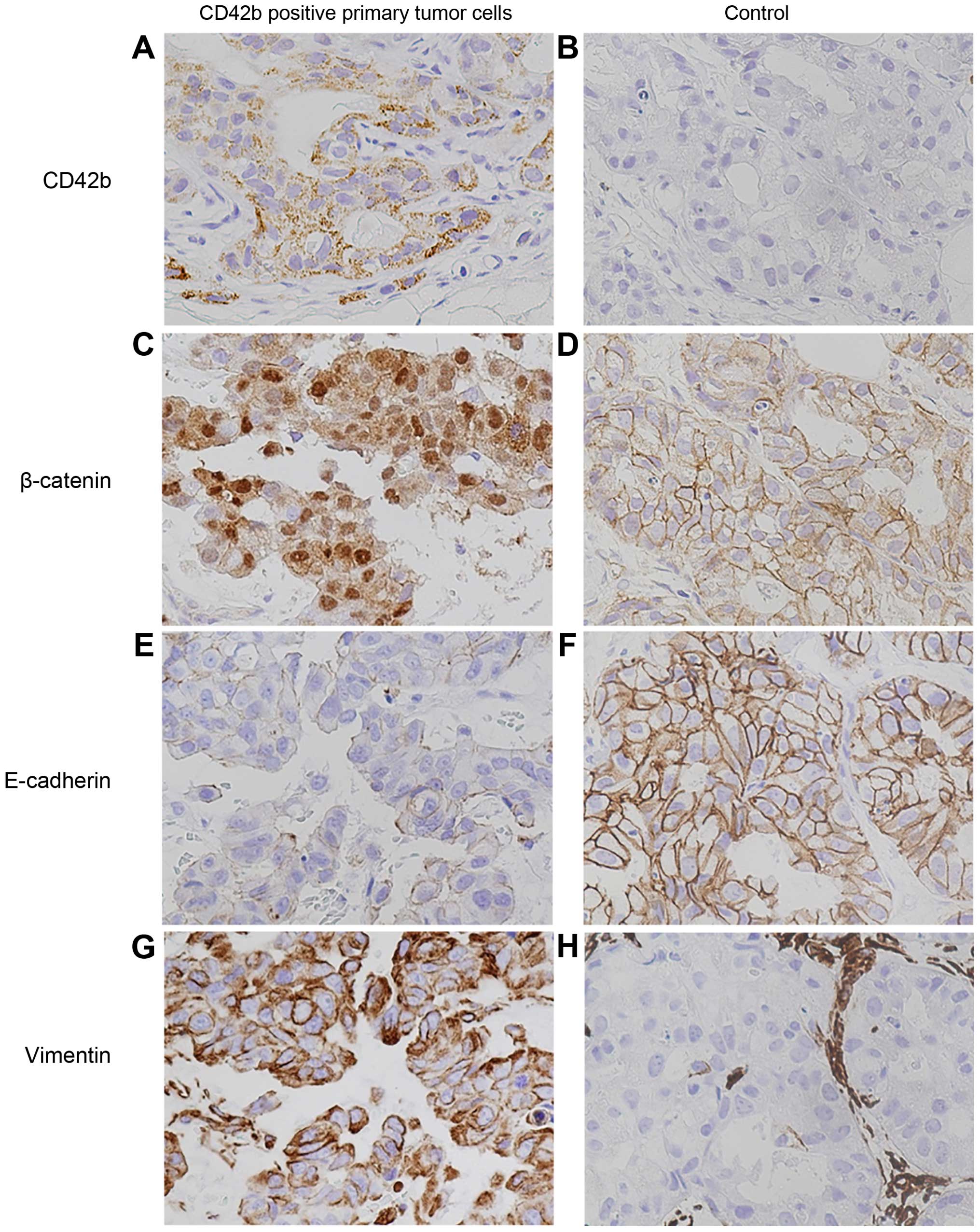
Tumor cells associated with CD42b immunore-activity
showed EMT-like morphological changes, including loss of
apical-basal polarity and detachment from the basement membrane at
the invasive front (Fig. 1E and F).
In order to investigate the expression of EMT markers in
CD42b-positive tumor cells, immunohistochemistry was performed on
biopsy specimens. All tumors were evaluated for E-cadherin,
vimentin, and β-catenin expression. We found nuclear staining of
β-catenin in CD42b-positive tumor cells (Fig. 2A and C), while CD42b-negative tumor
cells showed a membranous pattern of β-catenin staining (Fig. 2B and D). CD42b-positive tumor cells
also showed loss of E-cadherin expression and gain of vimentin
expression (Fig. 2E and G). In
contrast, CD42b-negative tumor cells showed membranous expression
of E-cadherin and β-catenin, but loss of vimentin expression
(Fig. 2F and H).
Relationship between platelets
surrounding primary tumor cells in biopsy specimens and
pathological response to neo-adjuvant chemotherapy
Analysis of the relationship between CD42b
expression and pathological response to neo-adjuvant chemotherapy
showed that pCR differed significantly with respect to CD42b
expression. When compared to patients with CD42b-positive tumors,
those with CD42b-negative tumors achieved a pCR far more frequently
(10 vs. 50%, respectively; p=0.0001) (Table II).
Univariate analysis of clinicopathological
parameters showed that CD42b expression (p<0.0001) was
significantly associated with pCR rate. Multivariate analysis
identified CD42b expression (p<0.0001), ER status (p=0.03), and
nuclear grade (p=0.02) as independent predictors of pCR rate
(Table III).
 | Table IIIUnivariable and multivariable
analysis of clinicopathological parameters including CD42b
expression for prediction of pCR. |
Table III
Univariable and multivariable
analysis of clinicopathological parameters including CD42b
expression for prediction of pCR.
| Clinicopathological
parameters | Univariable
analysis
| Multivariable
analysis
|
|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| CD42b expression
(≥10 vs. <10%) | 0.1 | 0.02–0.34 | <0.0001 | 0.03 | 0.003–0.15 | <0.0001 |
| ER status (positive
vs. negative) | 0.49 | 0.17–1.44 | NS | 0.21 | 0.04–0.9 | 0.03 |
| Clinical stage (III
vs. I–II) | 0.45 | 0.11–1.78 | NS | 1.08 | 0.18–5.76 | NS |
| Nuclear grade (G3
vs. G1-2) | 1.77 | 0.61–5.1 | NS | 5.31 | 1.27–29.4 | 0.02 |
Relationship between CD42b expression and
survival outcomes
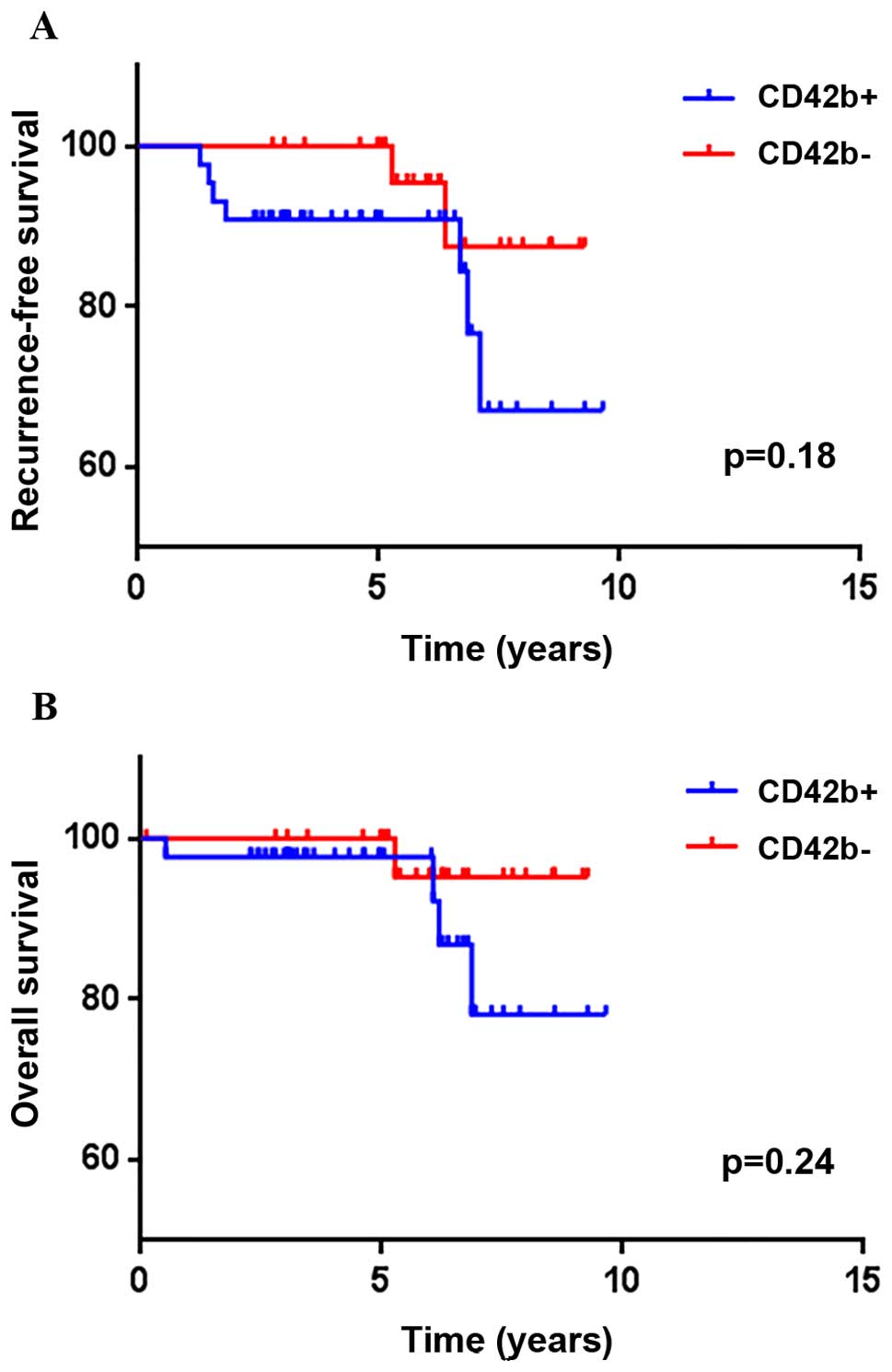
RFS and OS between tumors with CD42b exp ression and
those without CD42b expression are shown in Fig. 3. RFS and OS displayed no significant
differences, regardless of CD42b expression (p=0.18 and 0.24)
(Fig. 3).
Discussion
In cancer progression and metastasis, platelets play
an essential role in the host tumor microenvironment and have been
shown to interact with cancer cells. In this study, we demonstrated
that platelets aggregated around primary tumor cells in 59% of the
breast cancer specimens. Moreover, we showed that primary tumor
cells surrounded by platelets were found in sites in which EMT was
occurring based on molecular and morphological changes. Finally, we
also found that primary tumor cells associated with platelets
exhibited chemoresistance to common anticancer drugs (including
anthracycline and taxanes). Therefore, our data provide important
insights into the mechanisms of breast cancer progression.
In patients with platelets surrounding primary tumor
cells, wherein platelets were present at numbers >10% of the
total number of tumor cells, significant residual invasive
carcinoma cells were observed in surgical specimens resected after
chemotherapy. These results indicated that platelet aggregation
around primary tumor cells may play a crucial role in inducing EMT
and chemoresistance. Thus, platelet aggregation around primary
tumor cells may be an effective predictor of chemoresistance and a
novel therapeutic target for overcoming chemoresistance, one of the
major complications of cancer therapies.
Platelets, the smallest anucleate hematopoietic
cells, cannot be detected by traditional hematoxylin and eosin
staining. Therefore, the presence of platelet aggregation around
primary tumor cells is difficult to recognize. Cancer cells were
shown to have the ability to interact with platelets in
vitro several decades ago (19–22).
Furthermore, the interaction between circulating tumor cells (CTCs)
and circulating platelets is now recognized as a hallmark of the
metastatic potential of cancer (6,8).
Recent studies have demonstrated that the presence of platelet
aggregation around tumor cells can be detected both in the
circulation and in primary tumor cells in patients with pancreatic
cancer (23). Here, we used
immunohistochemistry to demonstrate that platelets aggregated
around primary tumors in about half of the breast cancer patients.
Therefore, these data further support that the metastatic potential
of platelets in primary sites, in addition to those in circulation,
should also be analyzed.
Platelets were detected in HER2-negative breast
cancer, regardless of stage, nuclear grade, ER status, and Ki-67
index. Other studies have reported the interaction between
intrinsic subtype and tumor cell-platelet interactions.
Luminal-type breast cancer cells have been reported to induce
greater aggregation of platelets than other types of breast cancer
cells in vitro (19,24). Moreover, in addition to facilitating
tumor invasiveness, migration, tumor growth, cell survival, and
angiogenesis, circulating platelets also play a crucial role in
inducing EMT in malignancy (10).
Circulating platelets are supported by chemical mediators, such as
TGF-β, VEGF-A, and PDGF, released from activated platelets
(10,25). We demonstrated that primary tumor
cells associated with platelet aggregation showed morphological and
molecular characteristics of EMT in breast cancer. In particular,
we observed nuclear translocation of β-catenin, which reflects the
down-regulation of E-cadherin and may lead to activation of the Wnt
pathway, thus inducing transcriptional enhancement of c-Myc and
cyclin-D (26). These events could
promote tumor cell migration from the primary site. Miyashita et
al reported that primary tumor cells surrounded by platelets
exhibited characteristics of EMT in pancreatic cancer (23). Thus, these findings support our
hypothesis that induction of EMT by platelet aggregation may occur
during early processes of metastasis, even at primary tumor
sites.
In this study, we also showed that primary tumor
cells associated with platelet aggregation were less responsive to
chemotherapy. Patients whose pre-treatment biopsy specimens
contained platelets surrounding tumor cells at a rate >10% of
the total number of tumor cells showed significant residual cancer
cells in surgical specimens following chemotherapy. Recent reports
have demonstrated that human platelets increase cancer cell
survival, proliferation, and chemoresistance to 5-fluorouracil and
paclitaxel in colon and ovarian cancer in vivo (27). Moreover, chemoresistance could be
induced by platelets throughout EMT (28), PAI-1-mediated anti-apoptotic
pathways, direct protection, or immunosuppression mediated by
downregulation of NKG2-D (29,30).
These results indicate that platelet aggregation surrounding
primary tumor cells may be a predictive factor for chemotherapeutic
success. Additionally, if platelet-mediated EMT and chemoresistance
could be modulated, we may be able to achieve enhanced
chemotherapeutic efficacy. As such, it is imperative to elucidate
the mechanisms of tumor cell-platelet interactions in primary tumor
sites.
In the present study, we were unable to demonstrate
a significant relationship between CD42b expression and survival
outcomes. The prognostic impact of pathological complete response
(pCR) varies dependent on the intrinsic subtype of breast cancer.
pCR is a suitable surrogate end point for luminal B/HER2-negative,
HER2-positive, and triple-negative disease, but not in luminal
breast cancer. Because our study consisted of these different
subtypes, there is necessity to evaluate a greater number of
samples. Moreover, duration of follow-up limited the ability to
evaluate the recurrence and death of breast cancer; increased
follow-up time is required.
This study had several limitations, including its
retrospective nature, small sample size, potential selection bias,
and heterogeneity of tumor characteristics. With respect to the
heterogeneity of tumor characteristics, we performed preliminary
experiments on the expression of CD42b in available resected
specimens and biopsies as consistently as possible to reduce the
effects of tumor heterogeneity. We confirmed that there was no
difference between available resected specimens and biopsy specimen
for this evaluation method.
We concluded that platelets may have tremendous
potential to induce tumor progression and metastasis, even when
found within the primary tumor site. This phenomenon may represent
a novel predictive factor for chemoresistance, and our results may
provide important insights into new therapeutic targets in breast
cancer. To discover and validate novel therapeutic targets, we are
now conducting research to elucidate the mechanisms of the
chemoresistance caused by platelets in breast cancer cells.
References
|
1
|
Halsted WS: The results of operations for
the cure of cancer of the breast performed at the Johns Hopkins
Hospital from June, 1889, to January, 1894. Ann Surg. 20:497–555.
1894. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darby S, McGale P, Correa C, Taylor C,
Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et
al: Early Breast Cancer Trialists' Collaborative Group (EBCTCG):
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Veronesi U, Zucali R and Luini A: Local
control and survival in early breast cancer: The Milan trial. Int J
Radiat Oncol Biol Phys. 12:717–720. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ansieau S, Bastid J, Doreau A, Morel AP,
Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S,
et al: Induction of EMT by twist proteins as a collateral effect of
tumor-promoting inactivation of premature senescence. Cancer Cell.
14:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lal I, Dittus K and Holmes CE: Platelets,
coagulation and fibri-nolysis in breast cancer progression. Breast
Cancer Res. 15:2072013. View
Article : Google Scholar
|
|
7
|
Takagi S, Takemoto A, Takami M, Oh-Hara T
and Fujita N: Platelets promote osteosarcoma cell growth through
activation of the platelet-derived growth factor receptor-Akt
signaling axis. Cancer Sci. 105:983–988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar
|
|
9
|
Ludwig RJ, Boehme B, Podda M, Henschler R,
Jager E, Tandi C, Boehncke WH, Zollner TM, Kaufmann R and Gille J:
Endothelial P-selectin as a target of heparin action in
experimental melanoma lung metastasis. Cancer Res. 64:2743–2750.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
13
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lønning PE and Knappskog S: Mapping
genetic alterations causing chemoresistance in cancer: Identifying
the roads by tracking the drivers. Oncogene. 32:5315–5330. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Castells M, Thibault B, Delord JP and
Couderc B: Implication of tumor microenvironment in
chemoresistance: Tumor-associated stromal cells protect tumor cells
from cell death. Int J Mol Sci. 13:9545–9571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; Hoboken, NJ: 2009
|
|
17
|
Devitee FATP: Pathology and Genetics
Tumours of the Breast and Female Genital Organs. World Health
Organization (WHO) guidelines; 2002
|
|
18
|
Bremer M and Doerge RW: Statistics at the
Bench: A Step-by-Step Handbook for Biologists. Cold Spring Harbor
Laboratory Press; Long Island, NY: 2010
|
|
19
|
Lian L, Li W, Li ZY, Mao YX, Zhang YT,
Zhao YM, Chen K, Duan WM and Tao M: Inhibition of MCF-7 breast
cancer cell-induced platelet aggregation using a combination of
anti-platelet drugs. Oncol Lett. 5:675–680. 2013.PubMed/NCBI
|
|
20
|
Heinmöller E, Weinel RJ, Heidtmann HH,
Salge U, Seitz R, Schmitz I, Müller KM and Zirngibl H: Studies on
tumor-cell-induced platelet aggregation in human lung cancer cell
lines. J Cancer Res Clin Oncol. 122:735–744. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bazou D, Santos-Martinez MJ, Medina C and
Radomski MW: Elucidation of flow-mediated tumour cell-induced
platelet aggregation using an ultrasound standing wave trap. Br J
Pharmacol. 162:1577–1589. 2011. View Article : Google Scholar :
|
|
22
|
Medina C, Jurasz P, Santos-Martinez MJ,
Jeong SS, Mitsky T, Chen R and Radomski MW: Platelet
aggregation-induced by caco-2 cells: Regulation by matrix
metalloproteinase-2 and adenosine diphosphate. J Pharmacol Exp
Ther. 317:739–745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyashita T, Tajima H, Makino I,
Nakagawara H, Kitagawa H, Fushida S, Harmon JW and Ohta T:
Metastasis-promoting role of extravasated platelet activation in
tumor. J Surg Res. 193:289–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuznetsov HS, Marsh T, Markens BA, Castaño
Z, Greene-Colozzi A, Hay SA, Brown VE, Richardson AL, Signoretti S,
Battinelli EM, et al: Identification of luminal breast cancers that
establish a tumor-supportive macroenvironment defined by
proangiogenic platelets and bone marrow-derived cells. Cancer
Discov. 2:1150–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Devarajan E, Song YH, Krishnappa S and Alt
E: Epithelial- mesenchymal transition in breast cancer lines is
mediated through PDGF-D released by tissue-resident stem cells. Int
J Cancer. 131:1023–1031. 2012. View Article : Google Scholar
|
|
26
|
Eger A, Stockinger A, Park J, Langkopf E,
Mikula M, Gotzmann J, Mikulits W, Beug H and Foisner R:
Beta-catenin and TGFbeta signalling cooperate to maintain a
mesenchymal phenotype after FosER-induced epithelial to mesenchymal
transition. Oncogene. 23:2672–2680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Radziwon-Balicka A, Medina C, O'Driscoll
L, Treumann A, Bazou D, Inkielewicz-Stepniak I, Radomski A, Jow H
and Radomski MW: Platelets increase survival of adenocarcinoma
cells challenged with anticancer drugs: Mechanisms and implications
for chemoresistance. Br J Pharmacol. 167:787–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sayan AE: Tumour-promoting role of
EMT-inducing transcription factor ZEB1 in mantle cell lymphoma.
Cell Death Differ. 21:194–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang H, Placencio VR and DeClerck YA:
Protumorigenic activity of plasminogen activator inhibitor-1
through an antiapoptotic function. J Natl Cancer Inst.
104:1470–1484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kopp HG, Placke T and Salih HR:
Platelet-derived transforming growth factor-beta down-regulates
NKG2D thereby inhibiting natural killer cell antitumor reactivity.
Cancer Res. 69:7775–7783. 2009. View Article : Google Scholar : PubMed/NCBI
|