Introduction
Lung cancer is considered as the leading cause of
cancer-related mortality among men worldwide and the second among
women (1). Almost as many patients
die of lung cancer every year than die of prostate, breast and
colon cancer combined in the USA (2). According to the differences in
clinical behavior and the aims of treatment, lung cancers can be
divided into two broad categories; small cell lung cancer (SCLC)
which accounts for 15% of all lung cancer cases and non-small cell
lung cancer (NSCLC) which accounts for 85% of cases (3).
A number of studies have documented smoking as the
leading risk factor for lung cancers. Since the first Surgeon
General's Report on the association of smoking with lung cancer in
1964, increased public awareness of the health risks associated
with smoking has gradually decreased the number of smokers over the
past five decades in developed countries (4). However, contrary to expectations, the
significant decrease in smokers has not yielded a correlating
decrease in the incidence and mortality of lung cancer (5); this finding strongly suggests that
factors other than smoking play significant roles in the
development, progression and responsiveness to therapeutics of lung
cancer. Interestingly, increasing evidence suggests that chronic
psychological stress has been recognized as an important risk
factor in promoting the genesis and development of cancers
including lung cancer in recent years (6). In response to stressors, activation of
the hypothalamic-pituitary-adrenal (HPA) axis leads to the release
of glucocorticoids, catecholamines, including norepinephrine and
epinephrine, and other stress hormones from the adrenal gland as
well as from the brain and sympathetic nerve terminals (7). The effects of catecholamines are
mainly mediated by β-adrenergic receptors (β-ARs) which are
expressed in almost all mammalian cell types. Recent findings have
indicated that β-ARs can regulate multiple cellular processes that
contribute to the initiation and progression of cancer, including
inflammation, angiogenesis, apoptosis, cell motility and
trafficking, and the activation of tumor-associated viruses
(8).
β-ARs, consisting of three subtypes
(β1-AR, β2-AR and β3-AR), are
members of the superfamily of G protein-coupled receptors (GPCRs).
β1-ARs and β2-ARs are expressed in the
majority of mammalian cells, while β3-ARs are almost
exclusively found in adipocytes (9). β2-ARs can associate with
heterotrimeric guanine nucleotide-binding proteins (G proteins),
and multiple cytosolic scaffold proteins including β-arrestin and
Src, to initiate various signaling pathways and modulate the
activity of intracellular effectors such as adenylyl cyclase and
mitogen-activated protein kinases (MAPKs) (10). Studies have shown that nitrosamine
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) binds to the
β1-AR-induced extracellular signal-regulated kinase 1/2
(ERK1/2) and cyclic adenosine monophosphate response
element-binding protein (CREB)/ATF-1 phosphorylation via
transactivation of the epithelial growth factor receptor (EGFR)
pathway in both human lung adenocarcinoma cell line NCI-H322 and
human peripheral airway cell line HPLD1 (11). Moreover, NNK also induced
ERK1/2 and CREB phosphorylation through β2-AR
in pulmonary adenocarcinoma in the hamster (12). However, the function of
β2-AR in human lung epithelial-derived A549 cells and
the underlyng mechanisms are not well understood.
To this end, the present study was designed to
investigate the function and mechanism of β2-AR-mediated
cell proliferation in A549 cells. Our results demonstrated that
activation of the β2-AR can significantly enhance the
proliferation of lung cancer cells via activation of the
ERK1/2/CREB pathway. In addition, matrix
metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth
factor (VEGF) may be involved in isoproterenol (ISO)-induced A549
cell proliferation.
Materials and methods
Antibodies and reagents
Antibodies for western blotting, including
phospho-ERK1/2 (Thr202/Tyr204) (cat. 9101, polyclonal,
raised in rabbit, 1:2,000), ERK1/2 (cat. 9102,
polyclonal, raised in rabbit, 1:2,000), phospho-CREB (Ser133) (cat.
9198, monoclonal, rabbit anti-human, 1:2,000), CREB (cat. 9197,
monoclonal, rabbit anti-human, 1:2,000), β-actin (cat. 4970,
monoclonal, rabbit anti-human, 1:4,000), anti-rabbit IgG HRP-linked
antibody (cat. 7074, goat anti-rabbit, 1:20,000) as well as U0126
(specific ERK1/2 inhibitor) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). ISO (a
broad-spectrum β-adrenergic agonist) and ICI-118,551 (ICI) (a
selective β2-AR antagonist) were purchased from Tocris
Cookson, Inc. (Bristol, UK). Fetal bovine serum (FBS), culture
medium and other solutions used for cell culture were from
Invitrogen (Shanghai, China). MTT was purchased from Carl Roth GmbH
& Co., KG (Karlsruhe, Germany). SYBR® Premix Ex Taq™
II was obtained from Takara Bio, Inc. (Otsu, Japan). Lipofectamine
2000 was procured from Thermo Fisher Scientific (Shanghai, China).
The siRNAs against CREB and control siRNA were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture and transfection
Human lung adenocarcinoma A549 cells were purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and cultured according to the relevant specifications. The cells
were grown in Dulbecco's minimal essential medium (DMEM) containing
4.5 g/l glucose supplemented with 10% FBS and 1% glutamine. All
cells were maintained at 37°C in a humidified atmosphere containing
5% CO2. A549 cells were transfected using Lipofectamine
2000 according to the manufacturer's instructions, using siRNAs
against CREB for 48 h.
Reverse transcription PCR
Total RNA was isolated from the A549 cells using
TRIzol reagent, and reverse transcription was carried out according
to the manufacturer's instructions from Invitrogen. PCR
amplification was performed on 5 µl cDNA, 12.5 µl 2X
Taq PCR Master Mix, 0.5 µl of 10 µM forward primer,
0.5 µl of 10 µM reverse primer and 6.5 µl
ddH2O under the following conditions: 94°C for 5 min, 30
cycles of denaturation for 1 min at 95°C, 1 min of annealing at
55°C, elongation at 72°C for 1 min, and extension at 72°C for 1
min. PCR analysis was performed using the following sense and
antisense primers: β1-AR forward,
5′-GGGAGAAGCATTAGGAGGG-3′ and reverse, 5′-CAAGGAAAGCAAGGTGGG-3′
which amplify a 270-bp fragment; β2-AR forward,
5′-CAGCAAAGGGACGAGGTG-3′ and reverse, 5′-AAGTAATGGCAAAGTAGCG-3′
which amplify a 334-bp fragment; β-actin forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse, 5′-GCCATCACGCCACAGTTT
C-3′ which amplify a 101-bp fragment; MMP-2 forward,
5′-CCGTCGCCCATCATCAAGTTC-3′ and reverse,
5′-GCAGCCATAGAAGGTGTTCAGG-3′ which amplify a 90-bp fragment; MMP-9
forward, 5′-TGGTCCTGGTGCTCCTGGTG-3′ and reverse,
5′-GCTGCCTGTCGGTGAGATTGG-3′ which amplify a 111-bp fragment; VEGF
forward, 5′-CTGGGCTGTTCTCGCTTCG-3′ and reverse,
5′-CTCTCCTCTTCCTTCTCTTCTTCC-3′ which amplify a 140-bp fragment.
Quantitative real-time polymerase chain
reaction (RT-qPCR)
The cDNAs were obtained and primers were used as
previously described (13). The
analysis using SYBR® Premix Ex Taq™ II from Takara Bio,
Inc., the 20 µl PCR system was composed of 10 µl
SYBR® Premix Ex Taq™ II, 0.4 µl ROX Reference Dye
II, 6 µl ddH2O, 0.8 µl of 10 µM
forward primer, 0.8 µl of 10 µM reverse primer and 2
µl cDNA. RT-qPCR amplification was performed under the
following conditions: 95°C for 30 sec, 40 cycles of denaturation
for 5 sec at 95°C, 34 sec of annealing at 60°C, elongation at 95°C
for 15 sec, and extension at 60°C for 1 min. At least three
independent experiments were conducted and samples were assessed in
triplicate in each experiment.
Western blotting
Lysates from the cultured cells were sonicated and
protein concentrations were determined using the Bradford reagent
from Bio-Rad Laboratories. Equal amounts of protein (20 µg)
were resolved by sodium dodecyl sulphate-polyacrylamide gel
electrophoresis. The proteins were transferred to nitrocellulose
membranes (Millipore, Bedford, MA, USA), which were incubated in
blocking buffer (5% non-fat dry milk in Tris-buffered saline and
0.1% Tween-20) for 1 h, followed by incubation with the primary
antibodies overnight at 4°C and a 2-h incubation with
HRP-conjugated secondary antibodies (1:20,000). Signals were
visualised by enhanced chemiluminescence (Pierce, Rockford, IL,
USA) associated fluorography, and their quantification was
conducted by volume densitometry using ImageJ software (National
Institutes of Health, Bethesda, MD, USA) and total protein
normalization.
MTT assay
Cell proliferation was investigated by MTT assay.
Briefly, the cells (5×104/well) were plated into
flat-bottom 24-well plates (Costar, Corning, NY, USA). After 24 h,
the cells were serum-starved overnight and incubated with different
concentrations of ISO and U0126 with or without ICI for different
times. Following incubation, 20 µl MTT (5 mg/ml) was added
to each well, and the cells were grown in complete media at 37°C
for 3 h. The supernatant was removed, and then 500 µl DMSO
was added to each well of the 24-well plate and oscillated for 10
min. Subsequently, the absorbance was read at 570 nm using an
enzyme-linked immunosorbent assay reader.
Statistical analysis
Data are presented as means ± SEM from at least
three independent experiments. Statistical analysis of the data was
performed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
β1-AR and β2-AR
expression in lung cancer cells
We first detected whether lung cancer cells express
β-ARs. The results of reverse transcription-PCR analysis indicated
that both β1-AR and β2-AR were expressed in
the A549 cells (Fig. 1A). This was
confirmed by RT-qPCR analysis (Fig.
1B). Interestingly, the levels of β2-AR mRNA were
significantly higher as compared to β1-AR. This result
suggests that β2-AR may be the predominant β-AR in A549
cells and this finding encouraged us to elucidate the role of
β2-AR in these cells.
β2-AR activation promotes the
proliferation of human A549 cells
We then investigated the effect of β-AR agonist ISO
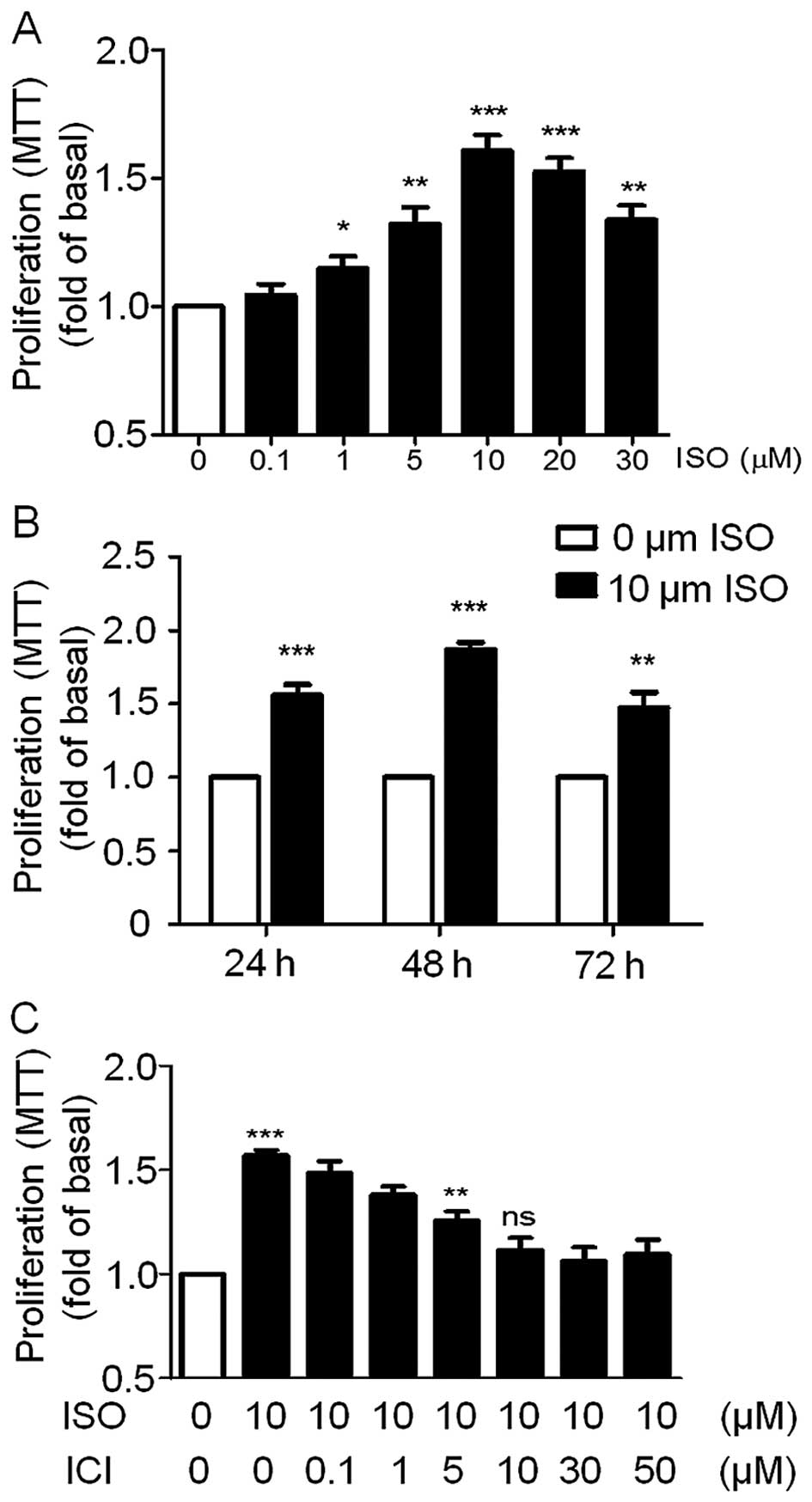
on the A549 cell proliferation. We first analyzed the
dose-dependent effect of ISO on A549 cell viability. The A549 cells
were incubated with 0.1, 1, 5, 10, 20 and 30 µM ISO for 24 h
and then cell viability was detected by MTT assay. As shown in
Fig. 2A, ISO significantly enhanced
the cell viability of the A549 cells maximally by a single
treatment with 10 µM ISO while cell viability decreased
dramatically with higher doses of ISO. To demonstrate the effects
of ISO at different treatment times on A549 cell viability, the
cells were incubated with 10 µM ISO for 24, 48 or 72 h. Cell
viability assays revealed that ISO significantly increased A549
cell growth at these three time points with the strongest effect at
48 h (Fig. 2B). Based on these
results, we chose 10 µM ISO as the reference concentration
and a 48-h treatment time for the subsequent studies unless
specifically indicated. Importantly, pre-treatment of the A549
cells with the β2-AR-specific antagonist ICI to block
endogenous β2-ARs blocked ISO-induced cell growth in a
dose-dependent manner and was completely blocked following a 10
µM dose (Fig. 2C). These
results suggest that ISO was able to promote A549 cell
proliferation through activation of β2-AR. This is
consistent with our previous data showing that β2-AR is
highly expressed in A549 cells (Fig.
1).
β2-AR activation induces
ERK1/2 and CREB phosphorylation in A549 cells
We demonstrated that ISO significantly increased
A549 cell growth. MAPK pathways constitute a large modular network
that regulates a variety of physiological processes, such as cell
growth, differentiation, and apoptotic cell death. To this end, we
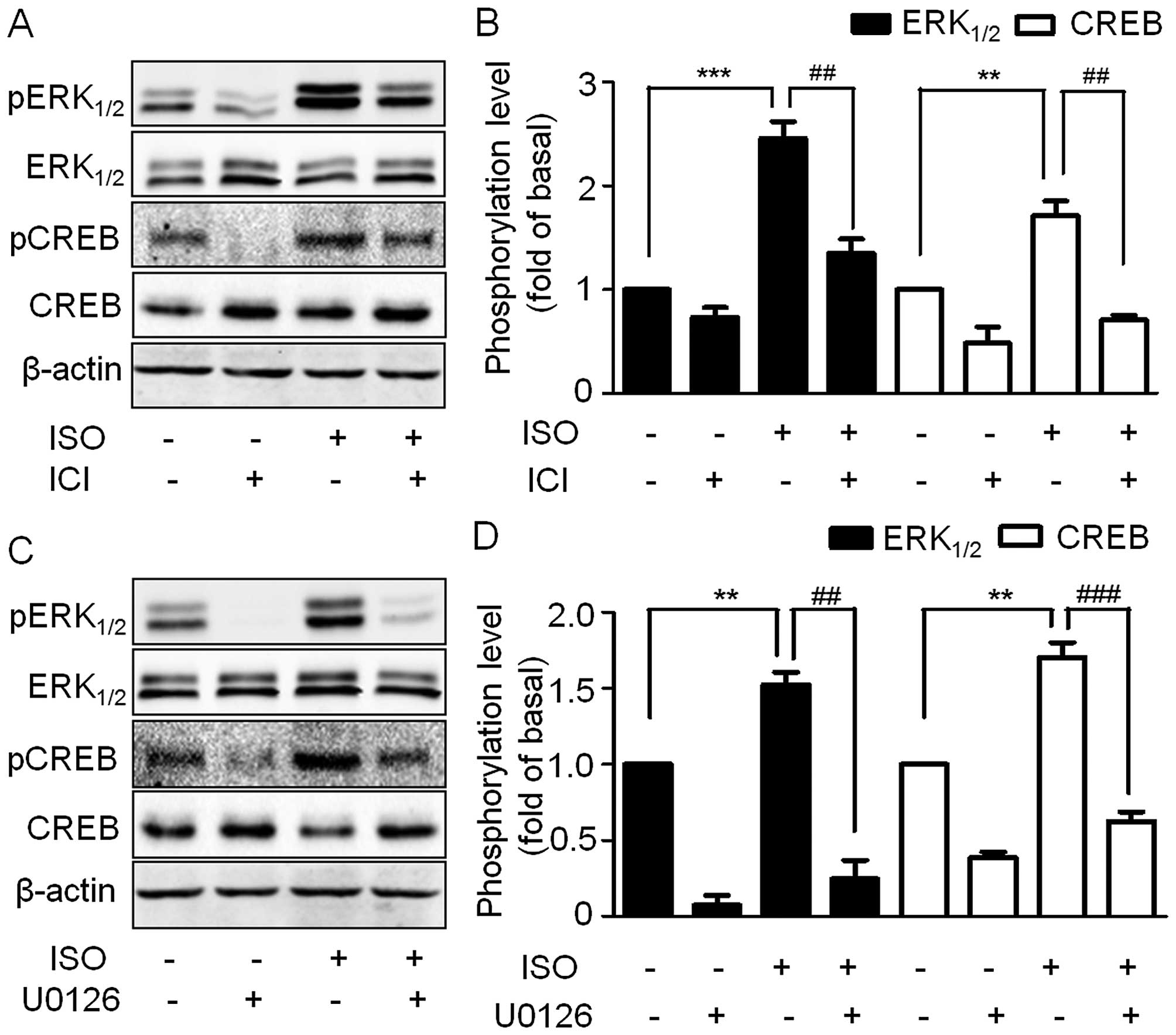
firstly monitored ERK1/2 phosphorylation levels
following ISO treatment. ISO caused a rapid and transient increase
in ERK1/2 phosphorylation with no changes in
ERK1/2 expression levels (Fig. 3A and B). ERK1/2
phosphorylation peaked at 10 min and then decreased. Similarly
CREB, an important transcription factor that plays important roles
in cell proliferation, was also phosphorylated in a transient
manner and also peaked at 10 min (Fig.
3A and C).
 | Figure 3ISO increases ERK1/2 and
CREB phosphorylation in A549 cells. (A) Cells were cultured in
6-well plates. After starvation with serum-free medium for 24 h,
the cells were treated with 10 µM ISO for the indicated
times. Cell lysates were subjected to immunoblotting with
antibodies against phospho-ERK1/2, ERK1/2,
phospho-CREB, CREB and β-actin. The blots shown are representative
of three independent experiments. (B and C) Quantitation of the
western blotting results as shown in (A). Data from at least three
independent experiments are expressed as means ± SEM vs. control.
*P<0.05, **P<0.01,
***P<0.001, ns vs. control. ISO, isoproterenol;
ERK1/2, extracellular signal-regulated kinase 1/2; CREB,
cyclic adenosine monophosphate response element-binding protein;
ns, not significant. |
To further confirm the ERK1/2 and CREB
phosphorylation mediated by β2-AR, the A549 cells were
pre-treated with ICI and then stimulated by ISO. ERK1/2
and CREB phosphorylation was blocked by ICI (Fig. 4A and B). As CREB is an important
downstream target of ERK1/2, we therefore tested whether
ISO-induced CREB phosphorylation is mediated through
ERK1/2. We treated A549 cells with the selective
MEK1/2 inhibitor U0126. Consistent with our hypothesis,
inhibition of the MAPK pathway led to a strong inhibition of
ISO-induced ERK1/2 and CREB phosphorylation (Fig. 4C and D), thus suggesting that the
activation of β2-AR by ISO induces ERK1/2
phosphorylation which in turn activates CREB.
MEK1/2 and CREB inhibition
suppresses ISO-mediated proliferation in A549 cells
Given the ability of ISO to significantly promote
the proliferation of A549 cells and induce ERK1/2 and
CREB phosphorylation, in order to further illustrate whether
ISO-mediated ERK1/2 and CREB activation are involved in
cell proliferation, A549 cells were pre-treated with U0126, and
then treated with 10 µM ISO. The proliferative effects of
ISO were suppressed by U0126 (Fig.
5A). Furthermore, knockdown of CREB expression by specific
siRNA significantly suppressed the A549 cell proliferation in
comparison with control siRNA (Fig. 5B
and C). All the above results indicate that ERK1/2
is involved in ISO-mediated proliferation through CREB
phosphorylation in A549 cells.
Knockdown of CREB inhibits ISO-mediated
increases in MMP-2, MMP-9 and VEGF levels in A549 cells
MMPs, the gelatinases MMP-2 and MMP-9 in particular,
and VEGF have been documented as contributing to the aggressiveness
of highly metastatic tumors (14).
We then examined the effect of ISO on the expression of MMP-2,
MMP-9 and VEGF in the A549 cells. The RT-qPCR results showed that
expression levels of the MMP-2, MMP-9 and VEGF genes were
significantly upregulated after treatment with 10 µM ISO for
48 h (Fig. 6A). Interestingly,
these effects were blocked by knockdown of CREB before ISO
treatment (Fig. 6B–D), indicating
that the action of ISO on A549 cell invasiveness is through
β2-AR-mediated CREB phosphorylation.
 | Figure 6Effects of ISO on the expression of
MMP-2, MMP-9, VEGF at the mRNA level. (A) Cells were treated with
10 µM ISO for 48 h, RT-qPCR was performed to detect the mRNA
level of MMP-2, MMP-9, VEGF. **P<0.01,
***P<0.001 vs. control. (B-D) Effects of siRNA
against CREB on ISO-induced MMP-2, MMP-9, VEGF expression. Data are
shown as means ± SEM of three triplicate experiments.
***P<0.001 vs. basal with control siRNA.
#P<0.05, ##P<0.01 vs. ISO-treated cells
transfected with control siRNA. ISO, isoproterenol; MMP, matrix
metalloproteinase; VEGF, vascular endothelial growth factor;
RT-qPCR, quantitative real-time polymerase chain reaction; CREB,
cyclic adenosine monophosphate response element-binding
protein. |
Discussion
Neurotransmitters are signaling substances that
traditionally play important roles in both the central and
peripheral nervous systems. However, accumulating studies have
demonstrated that the stress neurotransmitters adrenaline and
noradrenaline have a direct influence on the migration and
invasiveness of multiple tumor cells, including cancers of the
lung, prostate, colon, stomach, breast and ovary (15–19).
Although several studies have preliminarily investigated the role
of β-ARs in lung cancer cell lines such as human lung
adenocarcinoma cell line NCI-H322 and human peripheral airway cell
line HPLD1 (11), the function of
β-ARs especially β2-AR on human lung epithelial-derived
A549 cells and the underlying mechanisms have not been well
studied. In the present study, we investigated the molecular
mechanisms of β2-AR involved in lung cancer cell
proliferation. Our results showed that activation of the
β2-AR by ISO significantly enhanced the proliferation of
A549 cells. These effects were found to be mediated by the
MAPK/CREB pathway as blocking the MAPK pathway or knockdown of CREB
expression was able to suppress ISO-induced A549 cell
proliferation.
β-ARs are constitutively expressed in most mammalian
cells. However, the distribution and the expression level of these
subtypes may vary from tissue to tissue or even from species to
species in a given tissue (20). A
much higher level of β2-AR mRNA was detected in
hepatocellular carcinoma (HCC) tumor cells, while β1-AR
mRNA was almost undetectable (21,22).
Moreover, the β2-AR density in HCC cellular membranes
was much higher than the β2-AR density in non-adjacent
non-tumor liver cell membranes (23). β1-ARs predominated over
β2-ARs in NCI-H322 and NCI-H441 cell lines derived from
a human pulmonary adenocarcinoma with Clara cell phenotype
(11,24). In the present study, both reverse
transcription PCR and RT-qPCR results revealed that the expression
level of β2-ARs was much higher as compared to
β1-ARs in the A549 lung cancer cells. The presence of
mRNA does not ensure that the protein is present in the cells in
some cases. Nevertheless, none of the commercial antibodies
targeting β-ARs work (25) and even
much of what is known by using these antibodies has been thrown
into doubt (26). However, this has
been confirmed by the following functional assay showing that ISO
treatment can promote A549 cell proliferation. Moreover, this
effect can be completely blocked by ICI, a selective antagonist of
β2-AR. Thus, this confirmed that β2-ARs are
the predominant β-ARs in A549 cells.
As demonstrated in several studies, agonists binding
to β-ARs activate the heterotrimeric Gαs proteins to stimulate
adenylyl cyclase synthesis of cAMP. The transient cAMP flux can
initiate multiple cellular processes via two major downstream
effector systems including cAMP-dependent protein kinase (PKA) and
guanine nucleotide exchange protein activated by adenylyl cyclase
(EPAC) (8), both of which play
important roles in cell morphology and motility. Previous studies
suggest that β2-AR exerts an effect on carcinogenesis
mainly through activating signaling via adenylyl cyclase and its
downstream effectors cAMP, PKA, CREB and STAT3 as well as
transactivation of the EGFR pathway. In NCI-H322 and HPLD1 lung
cancer cell lines, NNK, an agonist for β-ARs, has been shown to
upregulate ERK1/2 and CREB/ATF-1 phosphorylation
(11). However, it is not known
whether they are also the effectors downstream of β2-AR
in A549 cells. In our study, we found that ISO treatment
significantly induced ERK1/2 and CREB phosphorylation.
The MEK1/2 inhibitor U0126 used to block
ERK1/2 activity inhibited CREB activation. However,
whether the EGFR transactivation pathway was involved in this
process was not investigated. Moreover, in addition to
heterotrimeric G proteins, more and more biochemical and cellular
studies indicate that β2-ARs may induce
ERK1/2 activation through a wide variety of
intracellular proteins that are independent of heterotrimeric G
protein activation (27). Among
these intracellular proteins, β-arrestins and Src have been shown
to initiate a broad variety of signaling events such as the
activatation of ERK1/2 signaling upon β2-AR
activation (28). These are also
the possible candidate proteins that may be involved in
β2-AR-induced ERK1/2 activaton in A549
cells.
The ability of tumor cells to invade the
extracellular matrix plays an important role in invasion and
metastasis. MMPs are key factors in the degradation of the
components of the extracellular matrix and therefore have been
regarded as major critical molecules assisting tumor cells during
metastasis (29–31). Studies have shown that the
catecholamine hormones may influence cancer progression by
modulating the expression of MMPs and VEGF in ovarian cancer cells
(19,32). In the present study, we found that
ISO had a role in modulating the expression of MMP-2, MMP-9 and
VEGF in the A549 cells, which may contribute to the aggressiveness
of the highly metastatic types of lung cancer cells. Notably, the
ISO-mediated upregulation of MMP-2, MMP-9 and VEGF was inhibited by
the knockdown of CREB expression.
Acknowledgments
The present study was supported by grants from the
Science Foundation of Nanchang University (nos. 06301133 and
06301132), and the Scientific Foundation for Young Scientists of
Jiangxi Provincial Science and Technology Department (no.
20122BAB215020).
Abbreviations:
|
β2-AR
|
β2-adrenergic receptor
|
|
ISO
|
isoproterenol
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
CREB
|
cyclic adenosine monophosphate
response element-binding protein
|
|
MMP
|
matrix metalloproteinase
|
|
VEGF
|
vascular endothelial growth factor
|
|
SCLC
|
small cell lung cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
HPA
|
hypothalamic-pituitary-adrenal
|
|
ICI
|
ICI-118,551
|
|
EGFR
|
epithelial growth factor receptor
|
|
HCC
|
hepatocellular carcinoma
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PKA
|
cAMP-dependent protein kinase
|
References
|
1
|
Kelly A, Blair N and Pechacek TF: Women
and smoking: Issues and opportunities. J Womens Health Gend Based
Med. 10:515–518. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakashita S, Sakashita M and Sound Tsao M:
Genes and pathology of non-small cell lung carcinoma. Semin Oncol.
41:28–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wakelee HA, Chang ET, Gomez SL, Keegan TH,
Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK,
et al: Lung cancer incidence in never smokers. J Clin Oncol.
25:472–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yano T, Haro A, Shikada Y, Maruyama R and
Maehara Y: Non-small cell lung cancer in never smokers as a
representative 'non-smoking-associated lung cancer': Epidemiology
and clinical features. Int J Clin Oncol. 16:287–293. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robinson JD and Cinciripini PM: The
effects of stress and smoking on catecholaminergic and
cardiovascular response. Behav Med. 32:13–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Timmermans W, Xiong H, Hoogenraad CC and
Krugers HJ: Stress and excitatory synapses: From health to disease.
Neuroscience. 248:626–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cole SW and Sood AK: Molecular pathways:
Beta-adrenergic signaling in cancer. Clin Cancer Res. 18:1201–1206.
2012. View Article : Google Scholar :
|
|
9
|
Schuller HM and Al-Wadei HAN:
Neurotransmitter receptors as central regulators of pancreatic
cancer. Future Oncol. 6:221–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Audet M and Bouvier M: Insights into
signaling from the beta2-adrenergic receptor structure. Nat Chem
Biol. 4:397–403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laag E, Majidi M, Cekanova M, Masi T,
Takahashi T and Schuller HM: NNK activates ERK1/2 and CREB/ATF-1
via beta-1-AR and EGFR signaling in human lung adenocarcinoma and
small airway epithelial cells. Int J Cancer. 119:1547–1552. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schuller HM and Cekanova M: NNK-induced
hamster lung adenocarcinomas over-express beta2-adrenergic and EGFR
signaling pathways. Lung Cancer. 49:35–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Xu C, Tu H, Wang Y, Sun Q, Hu P,
Hu Y, Rondard P and Liu J: GABAB receptor upregulates fragile X
mental retardation protein expression in neurons. Sci Rep.
5:104682015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Entschladen F, Drell TL IV, Lang K, Joseph
J and Zaenker KS: Tumour-cell migration, invasion, and metastasis:
Navigation by neurotransmitters. Lancet Oncol. 5:254–258. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Wadei HA, Takahashi T and Schuller HM:
Caffeine stimulates the proliferation of human lung adenocarcinoma
cells and small airway epithelial cells via activation of PKA, CREB
and ERK1/2. Oncol Rep. 15:431–435. 2006.PubMed/NCBI
|
|
17
|
Palm D, Lang K, Niggemann B, Drell TL IV,
Masur K, Zaenker KS and Entschladen F: The norepinephrine-driven
metastasis development of PC-3 human prostate cancer cells in
BALB/c nude mice is inhibited by beta-blockers. Int J Cancer.
118:2744–2749. 2006. View Article : Google Scholar
|
|
18
|
Drell TL IV, Joseph J, Lang K, Niggemann
B, Zaenker KS and Entschladen F: Effects of neurotransmitters on
the chemokinesis and chemotaxis of MDA-MB-468 human breast
carcinoma cells. Breast Cancer Res Treat. 80:63–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sood AK, Bhatty R, Kamat AA, Landen CN,
Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S and Cole SW:
Stress hormone-mediated invasion of ovarian cancer cells. Clin
Cancer Res. 12:369–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mersmann HJ: Overview of the effects of
beta-adrenergic receptor agonists on animal growth including
mechanisms of action. J Anim Sci. 76:160–172. 1998.PubMed/NCBI
|
|
21
|
Yuan A, Li Z, Li X, Yi S, Wang S, Cai Y
and Cao H: The mitogenic effectors of isoproterenol in human
hepatocellular carcinoma cells. Oncol Rep. 23:151–157. 2010.
|
|
22
|
Bevilacqua M, Norbiato G, Chebat E, Baldi
G, Bertora P, Regalia E, Colella G, Gennari L and Vago T: Changes
in alpha-1 and beta-2 adrenoceptor density in human hepatocellular
carcinoma. Cancer. 67:2543–2551. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kassahun WT, Guenl B, Ungemach FR, Jonas S
and Abraham G: Expression and functional coupling of liver
β2-adrenoceptors in the human hepatocellular carcinoma.
Pharmacology. 89:313–320. 2012. View Article : Google Scholar
|
|
24
|
Schuller HM, Tithof PK, Williams M and
Plummer H III: The tobacco-specific carcinogen
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic
agonist and stimulates DNA synthesis in lung adenocarcinoma via
beta-adrenergic receptor-mediated release of arachidonic acid.
Cancer Res. 59:4510–4515. 1999.PubMed/NCBI
|
|
25
|
Pradidarcheep W, Stallen J, Labruyère WT,
Dabhoiwala NF, Michel MC and Lamers WH: Lack of specificity of
commercially available antisera against muscarinergic and
adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol.
379:397–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daly CJ and McGrath JC: Previously
unsuspected widespread cellular and tissue distribution of
β-adrenoceptors and its relevance to drug action. Trends Pharmacol
Sci. 32:219–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shenoy SK and Lefkowitz RJ:
β-arrestin-mediated receptor trafficking and signal transduction.
Trends Pharmacol Sci. 32:521–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, McGarrigle D and Huang X-Y: When a
G protein-coupled receptor does not couple to a G protein. Mol
Biosyst. 3:849–854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fingleton B: Matrix metalloproteinases:
Roles in cancer and metastasis. Front Biosci. 11:479–491. 2006.
View Article : Google Scholar
|
|
31
|
Curran S and Murray GI: Matrix
metalloproteinases: Molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lutgendorf SK, Cole S, Costanzo E, Bradley
S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M and Sood
AK: Stress-related mediators stimulate vascular endothelial growth
factor secretion by two ovarian cancer cell lines. Clin Cancer Res.
9:4514–4521. 2003.PubMed/NCBI
|