Introduction
Triple-negative breast cancers (TNBCs) account for
approximately 15–20% of all diagnosed breast cancer types and are
more prevalent in younger women (1–3). TNBC
tumors predominantly present as invasive ductal carcinomas
characterized by large tumor size, poor differentiation, high
proliferation and high histologic grade (4,5).
Although TNBCs respond to chemotherapy better than other breast
cancer subtypes, patients with TNBCs have the worst clinical
outcomes because of the higher risk of distant metastasis and
significantly shorter overall survival (6,7).
Treatment options for TNBC patients are currently limited to
cytotoxic chemotherapy because of the absence of the well-defined
molecular targets of the estrogen receptor (ER), progesterone
receptor (PR) and HER2 (8).
Therefore, new therapeutic targets for treating TNBC patients are
urgently needed.
Stanniocalcin-1 (STC-1), a hypocalcemic glycoprotein
hormone, is known to regulate plasma Ca2+ homeostasis
(9). Mammalian STC-1 is expressed
ubiquitously and is involved in numerous developmental and
pathophysiological processes, including cancer, pregnancy and
organogenesis (10–13). Aberrant STC-1 expression is
associated with cancer development and poor prognosis in colorectal
and gastric cancer (14–17). The expression of STC-1 is regulated
by transcription factors NF-κB and p53 in colon and nasopharyngeal
cancer cells (18,19). Transcription factor HIF-1α is also
involved in the activation of STC-1 expression in colon,
nasopharyngeal and ovarian cancer cells (20). However, the clinical significance of
STC-1 expression in breast cancer has not been fully elucidated,
and little is known about regulation of this protein in breast
cancer cells.
In the present study, we investigated the clinical
significance of STC-1 in TNBC patients and its regulation in TNBC
cells. Elevated STC-1 expression was associated with poor prognosis
in TNBC patients, and increased the invasiveness of TNBC cells.
Furthermore, STC-1 expression was significantly higher in TNBC
cells than in non-TNBC cells. Basal levels of STC-1 expression in
TNBC cells were completely suppressed by blockage of PI-3K/Akt or
NF-κB signaling pathways. In contrast, CA-Akt or NF-κB
overexpression increased transcript levels of STC-1 in TNBC cells
as well as secretion of this protein. Notably, we observed
crosstalk between Akt and NF-κB with regard to STC-1 expression.
CA-Akt overexpression increased the levels of NF-κB phosphorylation
in TNBC cells. Taken together, we demonstrated that the
PI-3K/Akt/NF-κB signaling axis directly regulates STC-1 expression
in TNBC cells. STC-1 may be an important therapeutic target in TNBC
patients.
Materials and methods
Reagents
Cell culture media and antibiotics were purchased
from Life Technologies (Rockville, MD, USA). Fetal bovine serum
(FBS) was purchased from HyClone Laboratories (Logan, UT, USA).
Rabbit monoclonal anti-total and phospho-Akt, NF-κB, p38, IκBα and
STC-1 antibodies were purchased from Epitomics (Burlingame, CA,
USA). β-actin antibody was purchased from AbFrontier Co., Ltd.
(Seoul, Korea). Secondary horseradish peroxidase (HRP)-conjugated
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). LY294002, Bay11–7085 and SB253580 were purchased
from Tocris Bioscience (Ellisville, MO, USA). Recombinant human
STC-1 was purchased from ProSpec HOR-259 (ProSpec, Tel Aviv,
Israel). Mouse IgG and anti-STC-1 monoclonal antibodies were
purchased from R&D Systems (Minneapolis, MN, USA).
Cell cultures and drug treatment
MCF7, MDA-MB-231 and Hs578T breast cancer cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% FBS, 100 IU/ml penicillin and 100 µg/ml
streptomycin. T47D breast cancer cells were cultured in RPMI-1640
supplemented with 10% FBS, 100 IU/ml penicillin and 100
µg/ml streptomycin. Cells were grown in a humidified
atmosphere with 5% CO2 at 37°C. In the drug treatment
experiment, after serum starvation for 24 h, cells were treated
with LY294002, Bay11–7085 or SB253580 for 24 h.
Analysis of public expression
database
Expression data were downloaded from a public
database [Kaplan-Meier plotter database (http://kmplot.com/breast)]. The clinical value of
STC-1 in triple-negative breast cancer patients was analyzed by
Kaplan-Meier analysis. The hazard ratio with 95% confidence
intervals and log-rank P-values were calculated.
Western blotting
Cell lysates were prepared to detect anti-total and
phospho-Akt, NF-κB, IκBα, STC-1 and β-actin expression. Equal
amounts of proteins (50 µg) were boiled for 5 min in Laemmli
sample buffer and then electrophoresed in 10% sodium dodecyl
sulfate polyacrylamide (SDS-PAGE) gels. Separated proteins were
transferred to polyvinylidene fluoride (PVDF) membranes, and the
membranes were blocked with 10% skim milk in Tris-buffered saline
(TBS) containing 0.01% Tween-20 (TBS/T) for 15 min. Blots were
washed three times in TBS/T and then incubated with anti-total or
phospho-Akt, NF-κB, IκBα, STC-1 and β-actin antibodies in TBS/T
buffer at 4°C overnight. Blots were washed three times in TBS/T and
subsequently incubated with secondary HRP-conjugated antibodies
(1:2,000 dilution) in TBS/T buffer. After an 1-h incubation at room
temperature (RT), blots were washed three times in TBS/T. ECL™
prime reagent (GE Healthcare, Buckinghamshire, UK) was used for
development.
Real-time polymerase chain reaction
(PCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's protocol. Isolated RNA samples were then used for
RT-PCR. Samples of total RNA (1 µg) were reverse-transcribed
into cDNA in 20 µl reaction volumes using a First-Strand
cDNA Synthesis kit for RT-PCR, according to the manufacturer's
instructions (MBI Fermentas, Hanover, MD, USA). Gene expression
levels were quantified by real-time PCR using a SensiMix SYBR kit
(Bioline, Ltd., London, UK) and 100 ng of cDNA per reaction. The
primer sequences used for this analysis were as follows: human
ACTB: forward, 5′-TCA CCA TTG GCA ATG AGC GGT T-3′ and reverse,
5′-AGT TTC GTG GAT GCC ACA GGA CT-3′; human STC-1: forward, 5′-CAC
ACC CAC GAG CTG ACT TC-3′ and reverse, 5′-TCT CCC TGG TTA TGC ACT
CTC-3′. An annealing temperature of 60°C was used for all primers.
PCRs were performed in a standard 384-well plate format with an ABI
7900HT real-time PCR detection system (Applied Biosystems, Foster
City, CA, USA). For data analysis, the raw threshold cycle
(CT) value was first normalized to the
housekeeping gene for each sample to obtain a
ΔCT. The normalized ΔCT was
then calibrated to control cell samples to obtain
ΔΔCT values.
Plasmid DNA transfection
Empty vector and constitutively active-Akt (CA-Akt)
plasmid DNA were a generous gift from Dr Shin Incheol (Hanyang
University, Seoul, Korea). NF-κB p65 plasmid DNA was a generous
gift from Dr Lee Myung-Shik (Yonsei University, Seoul, Korea).
Hs578T cells were seeded in a 6-well plate. Cell transfection was
performed using Lipofectamine 2000 (Invitrogen) according to the
manufacturer's protocol. Cells were maintained in culture media
without FBS and antibiotics for 24 h while Lipofectamine
transfection, and then further incubated in fresh culture media
with 10% FBS for 24 h.
Enzyme-linked immunosorbent assay
ELISA assay was performed on culture media (200
µl) collected from Hs578T breast cancer cells. Secreted
protein levels of STC-1 were measured using an ELISA kit (R&D
Systems) according to the manufacturer's instructions. Secreted
protein levels were analyzed at the wavelength of 450 nm on a
spectrophotometer (SpectraMax 190; Molecular Devices, Sunnyvale,
CA, USA).
Cell invasion assay
Cell invasive capacity was analyzed by a Boyden
chamber assay, as previously described (21). Twenty-four-well Boyden chambers with
Matrigel-coated filters (8 µm pore size) were purchased from
Becton-Dickinson (San Diego, CA, USA). Hs578T and MDA-MB-231 cells
were resuspended in culture media (5×104 cells/well) and
then added to the Matrigel-coated upper compartment of invasion
chambers in the presence or absence of 50 ng/ml STC-1 and/or 2
µg/ml IgG or STC-1 antibody. Fresh culture media with 5% FBS
was added to the lower compartment of the invasion chamber. After a
24 or 48 h of incubation, the cells on the upper side of the filter
were removed using cotton swabs. The underside of the filter was
fixed in 100% methanol, washed in 1X PBS and stained using
hematoxylin and eosin (H&E). Cells that had invaded through the
matrigel were located on the underside of the filter. These cells
were analyzed using a ScanScopeXT apparatus (Aperio Technologies,
Vista, CA, USA).
Statistical analysis
Statistical significance was determined using
Student's t-test. Results are presented as means ± SEM. All quoted
P-values were two-tailed and differences were considered
statistically significant when the P-value was <0.05.
Statistical analyses were performed using Microsoft Excel.
Results
STC-1 expression in breast cancer cells
and its clinical significance in TNBC patients
To evaluate the clinical significance of STC-1
expression, we examined the levels of STC-1 expression in breast
cancer cells. As shown in Fig. 1A,
STC-1 protein expression was higher in TNBC cells than in non-TNBC
cells. STC-1 protein expression was particularly high in Hs578T
TNBC cells (Fig. 1A). Under the
same conditions, STC-1 mRNA expression was also increased in TNBC
cells (Fig. 1B). STC-1 transcript
levels were increased to 2.34±0.21-fold (MDA-MB-231 cells) and
8.97±0.29-fold (Hs578T cells) of the control cells (MCF7 cells)
(Fig. 1B).
In addition, we investigated the co-relation between
STC-1 expression and clinical outcomes in breast cancer patients
and the prognostic value of STC-1 in breast cancer patients using a
Kaplan-Meier plotter database (http://kmplot.com/breast). We found that induction of
STC-1 was involved with a poor prognosis in TNBC patients (Fig. 1C). Patients with high STC-1 levels
showed poorer relapse-free survival than patients with low STC-1
levels (P=0.00011) (Fig. 1C).
However, STC-1 expression level did not affect the relapse-free
survival of luminal-type or HER2-type breast cancer patients (data
not shown).
We also investigated the role of STC-1 in the
invasiveness of TNBC cells. As shown in Fig. 1D, invasive rates of Hs578T and
MDA-MB-231 TNBC cells were significantly increased by STC-1
treatment. In contrast, STC-1-induced cell invasiveness was
completely blocked by STC-1 antibody treatment in Hs578T and
MDA-MB-231 TNBC cells. Based on these results, we concluded that
elevated STC-1 expression is associated with poor prognosis in TNBC
patients and that it enhances the invasiveness of TNBC cells.
Basal levels of STC-1 expression are
decreased by LY294002 or Bay11–7085 treatment in TNBC cells
Next, we compared the phosphorylation levels of
signaling molecules such as Akt, NF-κB and p38 between MCF7
non-TNBC and Hs578T TNBC cells. As shown in Fig. 2A, the phosphorylation levels of Akt
and NF-κB were significantly higher in Hs578T TNBC cells than MCF7
non-TNBC cells. Furthermore, we investigated regulation of STC-1
expression using MDA-MB-231 and Hs578T TNBC cells. Each cell type
was treated with various specific inhibitors for 24 h. After 24 h,
we harvested cell lysates to detect the levels of STC-1 mRNA
expression. Basal STC-1 mRNA expression in TNBC cells was decreased
by LY (a specific PI-3K inhibitor) or Bay (a specific NF-κB
inhibitor), but not by SB (a specific p38 inhibitor) (Fig. 2B). LY and Bay decreased STC-1 mRNA
expression to 0.60±0.03-fold and 0.55±0.03-fold of the control
level, respectively, in Hs578T TNBC cells (Fig. 2B, left panel). In addition, STC-1
levels were decreased to 0.35±0.01-fold (LY) and 0.57±0.01-fold
(Bay) of the control level in MDA-MB-231 TNBC cells (Fig. 2B, right panel). Under the same
conditions, we examined the effect of specific inhibitors on the
phosphorylation levels of signaling molecules. As expected,
phosphorylation levels of Akt, NF-κB and p38 were significantly
decreased by LY, Bay and SB, respectively, in Hs578T and MDA-MB-231
TNBC cells (Fig. 2C). These results
demonstrated that STC-1 expression is regulated by PI-3K/Akt and/or
NF-κB signaling pathways in TNBC cells.
 | Figure 2Basal levels of STC-1 expression are
decreased by LY294002 or Bay11-7085 treatment in TNBC cells. (A)
After starvation for 24 h, cells were harvested and expression of
p-Akt, t-Akt, p-NF-κB, t-NF-κB and β-actin was assessed by western
blotting. (B) After serum starvation for 24 h, Hs578T (left panel)
and MDA-MB-231 (right panel) TNBC cells were treated with 5
µM LY, Bay, or SB for 24 h. STC-1 transcript levels were
determined by real-time PCR. (C) Under the serum-free conditions,
alterations of Akt, NF-κB and p38 expression in response to
specific inhibitors were analyzed by western blotting. Results are
representative of three independent experiments. Values shown are
means ± SEM. **P<0.01 vs. control. LY; LY294002, Bay;
Bay11-7085, SB; SB253580. |
STC-1 expression is increased by CA-Akt
overexpression
Next, we investigated whether Akt directly regulates
STC-1 expression in Hs578T TNBC cells. Constitutively active-Akt
(CA-Akt) was transfected into Hs578T TNBC cells for 48 h. We
confirmed the levels of phospho and total-Akt expression in Hs578T
cells (Fig. 3A). Under the same
conditions, STC-1 mRNA expression was significantly increased by
CA-Akt overexpression in Hs578T cells (Fig. 3B). Transcript levels of STC-1
increased to 7.49±0.83-fold of the control level in CA-Akt
overexpressing cells (Fig. 3B). In
addition, we observed that the amount of secreted STC-1 was
increased by CA-Akt overexpression (Fig. 3C). The amount of CA-Akt-induced
STC-1 secreted protein was 3.79±0.40-fold that of the control
(Fig. 3C). Together, these results
indicate that the PI-3K/Akt signaling pathway plays an important
role in STC-1 expression in TNBC cells.
STC-1 expression is increased by NF-κB
overexpression
To further investigate the role of NF-κB in STC-1
expression, we overexpressed NF-κB in Hs578T cells for 48 h. As
shown in Fig. 4A, we confirmed the
levels of phospho and total-NF-κB expression in Hs578T cells. STC-1
mRNA expression was significantly increased by NF-κB overexpression
in Hs578T cells (Fig. 4B). STC-1
mRNA expression was 3.71±0.48-fold of the control level in NF-κB
overexpressing cells (Fig. 4B). In
addition, levels of secreted STC-1 were increased by NF-κB
overexpression (Fig. 4C). Level of
NF-κB-induced STC-1 secreted protein was 1.44±0.03-fold of the
control level (Fig. 4C). These
results demonstrated that STC-1 expression is upregulated through
an NF-κB-dependent pathway in TNBC cells.
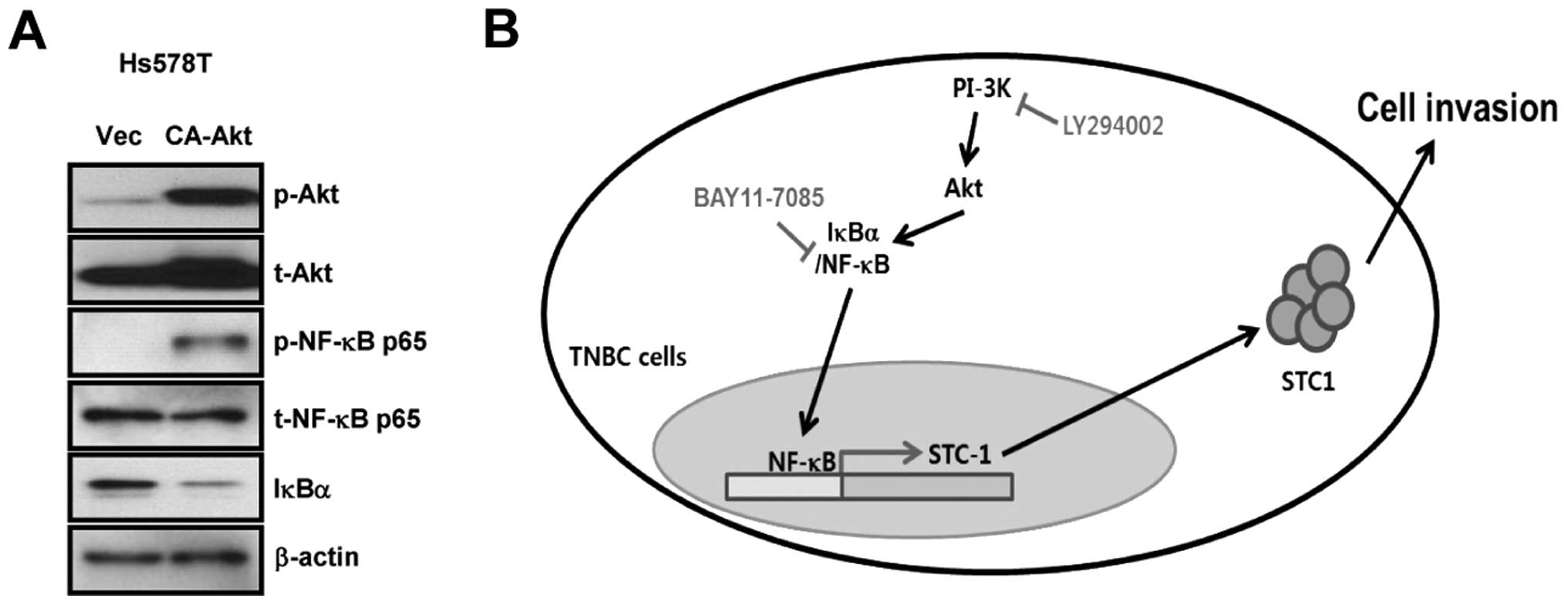
CA-Akt enhances NF-κB activity and the
degradation of IκBα in Hs578T TNBC cells
As shown in Figs. 3
and 4, basal STC-1 expression was
upregulated by CA-Akt or NF-κB over-expression in Hs578T TNBC
cells. We therefore investigated if cross-talk between the Akt and
NF-κB pathways affected STC-1 expression in Hs578T TNBC cells.
First, we transfected Hs578T TNBC cells with CA-Akt for 48 h and
then harvested whole cell lysates to measure Akt, NF-κB and IκBα
expression. CA-Akt overexpression in Hs578T cells significantly
increased phosphorylation of NF-κB (Fig. 5A). Degradation of IκBα was also
increased in CA-Akt overexpressing cells (Fig. 5A). However, the phosphorylation
level of Akt was not altered by NF-κB overexpression (data not
shown). Akt activity therefore appears to trigger the
phosphorylation of NF-κB and the degradation of IκBα, resulting in
upregulation of STC-1 expression in TNBC cells.
Discussion
Aberrant STC-1 expression is correlated with poor
clinical outcomes in colorectal, lung and gastric cancer, and has
been reported to be a promising target in various human
malignancies (14,15,22).
STC-1 expression level is directly related to tumor growth,
angiogenesis, and metastasis in gastric, breast and ovarian cancers
(15,23,24).
In the present study, TNBC patients with high STC-1 levels had a
poorer prognosis than those with low STC-1 levels with regard to
relapse-free survival. However, the level of STC-1 expression did
not affect the relapse-free survival of patients with other breast
cancer types, such as luminal- and HER2-type breast cancer (data
not shown). We also observed that STC-1 augmented the invasiveness
of TNBC cells, while STC-1-induced cell invasiveness was completely
suppressed by treatment of cells with an STC-1 monoclonal antibody.
These findings indicate that STC-1 expression is associated with
survival in TNBC patients and is directly involved in the
invasiveness of TNBC cells.
STC-1 expression has been reported to be higher in
hepatocellular carcinoma and colorectal cancer tissues than
cancer-free tissues (16). STC-1
expression can be upregulated by various extracellular stimuli such
as vascular endothelial growth factor (VEGF), hypoxia, and glial
cell line-derived neurotrophic factor (GDNF), based on studies in
several tumor tissues and cell lines (16,20,25).
Moreover, STC-1 expression can be regulated by a variety of
transcription factors, including NF-κB, p53 and HIF-1α, in colon
and nasopharyngeal cancer cells (18,19,26).
To date, the regulatory mechanism of STC-1 expression in breast
cancer has not been elucidated. Here, we observed that STC-1 mRNA
and protein expression were significantly increased in TNBC cells
compared with non-TNBC cells. In addition, the activities of Akt
and NF-κB were also higher in Hs578T TNBC cells than control cells.
Thus, we investigated the roles of Akt and NF-κB in STC-1
expression. LY (a specific PI-3K inhibitor) or Bay (a specific
NF-κB inhibitor) decreased expression of STC-1 mRNA and protein in
TNBC cells. In contrast, CA-Akt or NF-κB overexpression increased
STC-1 expression in TNBC cells. Based on these data, we suggest
that STC-1 expression is upregulated through PI-3K/Akt and/or
NF-κB-dependent pathways in TNBC cells.
In a previous study, Ozes et al (27) reported that Akt triggers TNF-induced
NF-κB activation through phosphorylation of IKK-α. IL-1-induced
PI-3K activation leads to phosphorylation and activation of the
NF-κB p65/RelA subunit (28).
Therefore, the PI-3K/Akt signaling pathway is regulated by multiple
mechanisms, and there is often crosstalk with other signaling
pathways. As shown in Fig. 5B, we
also found crosstalk between Akt and NF-κB in TNBC cells. When
CA-Akt was overexpressed in Hs578T TNBC cells, phosphorylation of
NF-κB and degradation of IκBα significantly increased. In addition,
Akt and NF-κB activities were suppressed by LY294002 treatment of
Hs578T TNBC cells. However, Bay11–7085 only suppressed the activity
of NF-κB, but not Akt. These results demonstrated that Akt is
located upstream of NF-κB and that it directly upregulates NF-κB
activity through degradation of IκBα in TNBC cells.
The aim of the present study was to investigate the
regulatory mechanism of STC-1 expression in TNBC cells. Elevated
STC-1 expression was associated with a poor prognosis in TNBC
patients, and also increased the invasiveness of TNBC cells. STC-1
expression was higher in TNBC cells than non-TNBC cells. In
addition, basal levels of STC-1 expression in TNBC cells were
completely suppressed by LY294002 or Bay11–7085 treatment. In
contrast, STC-1 expression was increased by CA-Akt or NF-κB
overexpression in Hs578T TNBC cells. Interestingly, we found that
CA-Akt overexpression also triggered the phosphorylation of NF-κB
and the degradation of IκBα in Hs578T TNBC cells. We conclusively
demonstrated that the PI-3K/Akt/NF-κB signaling pathway plays a
pivotal role in STC-1 expression in TNBC cells. Our results also
suggest that STC-1 may be a promising therapeutic target in TNBC
patients.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Education (2015R1D1A1A01057585)
and by a grant of the Korea Health Technology R&D Project
through the Korea Health Industry Development Institute (KHIDI),
funded by the Ministry of Health & Welfare, Republic of Korea
(HI14C3418).
References
|
1
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carey L, Winer E, Viale G, Cameron D and
Gianni L: Triple-negative breast cancer: Disease entity or title of
convenience? Nat Rev Clin Oncol. 7:683–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fulford LG, Reis-Filho JS, Ryder K, Jones
C, Gillett CE, Hanby A, Easton D and Lakhani SR: Basal-like grade
III invasive ductal carcinoma of the breast: Patterns of metastasis
and long-term survival. Breast Cancer Res. 9:R42007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dent R, Hanna WM, Trudeau M, Rawlinson E,
Sun P and Narod SA: Pattern of metastatic spread in triple-negative
breast cancer. Breast Cancer Res Treat. 115:423–428. 2009.
View Article : Google Scholar
|
|
6
|
Ismail-Khan R and Bui MM: A review of
triple-negative breast cancer. Cancer Control. 17:173–176.
2010.PubMed/NCBI
|
|
7
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshiko Y and Aubin JE: Stanniocalcin 1 as
a pleiotropic factor in mammals. Peptides. 25:1663–1669. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang AC, Jellinek DA and Reddel RR:
Mammalian stanniocalcins and cancer. Endocr Relat Cancer.
10:359–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olsen HS, Cepeda MA, Zhang QQ, Rosen CA,
Vozzolo BL and Wagner GF: Human stanniocalcin: A possible hormonal
regulator of mineral metabolism. Proc Natl Acad Sci USA.
93:1792–1796. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stasko SE and Wagner GF: Stanniocalcin
gene expression during mouse urogenital development: A possible
role in mesenchymal-epithelial signalling. Dev Dyn. 220:49–59.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang KZ, Westberg JA, Paetau A, von
Boguslawsky K, Lindsberg P, Erlander M, Guo H, Su J, Olsen HS and
Andersson LC: High expression of stanniocalcin in differentiated
brain neurons. Am J Pathol. 153:439–445. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamura S, Oshima T, Yoshihara K, Kanazawa
A, Yamada T, Inagaki D, Sato T, Yamamoto N, Shiozawa M, Morinaga S,
et al: Clinical significance of STC1 gene expression in patients
with colorectal cancer. Anticancer Res. 31:325–329. 2011.PubMed/NCBI
|
|
15
|
Fang Z, Tian Z, Luo K, Song H and Yi J:
Clinical significance of stanniocalcin expression in tissue and
serum of gastric cancer patients. Chin J Cancer Res. 26:602–610.
2014.PubMed/NCBI
|
|
16
|
Fujiwara Y, Sugita Y, Nakamori S, Miyamoto
A, Shiozaki K, Nagano H, Sakon M and Monden M: Assessment of
Stanniocalcin-1 mRNA as a molecular marker for micrometastases of
various human cancers. Int J Oncol. 16:799–804. 2000.PubMed/NCBI
|
|
17
|
Paulitschke V, Kunstfeld R, Mohr T, Slany
A, Micksche M, Drach J, Zielinski C, Pehamberger H and Gerner C:
Entering a new era of rational biomarker discovery for early
detection of melanoma metastases: Secretome analysis of associated
stroma cells. J Proteome Res. 8:2501–2510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Law AY, Lai KP, Lui WC, Wan HT and Wong
CK: Histone deacetylase inhibitor-induced cellular apoptosis
involves stan-niocalcin-1 activation. Exp Cell Res. 314:2975–2984.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai KP, Law AY, Yeung HY, Lee LS, Wagner
GF and Wong CK: Induction of stanniocalcin-1 expression in
apoptotic human nasopharyngeal cancer cells by p53. Biochem Biophys
Res Commun. 356:968–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeung HY, Lai KP, Chan HY, Mak NK, Wagner
GF and Wong CK: Hypoxia-inducible factor-1-mediated activation of
stanniocalcin-1 in human cancer cells. Endocrinology.
146:4951–4960. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon M, Lee J, Nam SJ, Shin I, Lee JE and
Kim S: Induction of fibronectin by HER2 overexpression triggers
adhesion and invasion of breast cancer cells. Exp Cell Res.
333:116–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du YZ, Gu XH, Li L and Gao F: The
diagnostic value of circulating stanniocalcin-1 mRNA in non-small
cell lung cancer. J Surg Oncol. 104:836–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang AC, Doherty J, Huschtscha LI,
Redvers R, Restall C, Reddel RR and Anderson RL: STC1 expression is
associated with tumor growth and metastasis in breast cancer. Clin
Exp Metastasis. 32:15–27. 2015. View Article : Google Scholar
|
|
24
|
Liu G, Yang G, Chang B, Mercado-Uribe I,
Huang M, Zheng J, Bast RC, Lin SH and Liu J: Stanniocalcin 1 and
ovarian tumorigenesis. J Natl Cancer Inst. 102:812–827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ismail RS, Baldwin RL, Fang J, Browning D,
Karlan BY, Gasson JC and Chang DD: Differential gene expression
between normal and tumor-derived ovarian epithelial cells. Cancer
Res. 60:6744–6749. 2000.PubMed/NCBI
|
|
26
|
Law AY, Ching LY, Lai KP and Wong CK:
Identification and characterization of the hypoxia-responsive
element in human stanniocalcin-1 gene. Mol Cell Endocrinol.
314:118–127. 2010. View Article : Google Scholar
|
|
27
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sizemore N, Leung S and Stark GR:
Activation of phosphatidylinositol 3-kinase in response to
interleukin-1 leads to phosphorylation and activation of the
NF-kappaB p65/RelA subunit. Mol Cell Biol. 19:4798–4805. 1999.
View Article : Google Scholar : PubMed/NCBI
|