Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common malignancy and the second leading cause of cancer-related
death (1). In China, the incidence
of liver cancer accounts for 50% of the cases worldwide, and
mortality caused by liver cancer is second only to lung cancer
(2). Although there has been
noticeable progress in the treatment of HCC and surgical resection
have greatly improved survival in patients at very early stage,
long-term survival remains unsatisfactory and many patients may
develop a tumor recurrence, which is the primary cause of treatment
failure. Current therapeutic strategies have failed to solve the
high recurrence rate and high mortality of HCC. Therefore, it is
urgent to seek new effective therapy strategies to combat HCC.
Sorafenib, a multi-kinase inhibitor, has been
approved for the clinical treatment of advanced HCC and it prolongs
the overall survival of HCC patients nearly 3 months (3). A number of studies have shown that
sorafenib blocks tumor angiogenesis and proliferation.
Mechanistically, sorafenib inhibits multiple signaling pathways
including Raf-1 (or C-Raf) and B-Raf, vascular endothelial growth
factor (VEGF) receptors 2 and 3, platelet-derived growth factor
(PDGF) receptor, c-KIT and FMS-like tyrosine kinase 3 (FLT3)
(4). In addition, sorafenib may be
the only choice of systemic therapy and also represent the new
first-line treatment standard for advanced HCC patients. However,
clinical trials seem disappointing as a large number of patients
with advanced HCC are unresponsive or acquire resistance to
sorafenib. Hence, it is necessary to evaluate the effect of
sorafenib in combination with other antitumor agents on HCC.
Cancer stem cells (CSCs), also termed as cancer
initiating cells, is a subset of cells within tumors that are
believed to initiate and maintain cancers. There is an increasing
interest among researchers on the CSCs concept. To date, CSCs have
been confirmed to exist in many tumors including leukemia, breast
and brain cancer, glioma, pancreatic and liver cancer (5–11). The
critical role of CSCs in tumorigenesis, tumor metastasis and
resistance to anticancer therapy has also been demonstrated in HCC
(12–14). Hence, targeting CSCs may be
potential treatments for HCC.
8-Bromo-7-methoxychrysin (BrMC) is a novel chrysin
analogue, synthesized by our team, inhibiting the growth of a
variety of tumor cells. BrMC strongly inhibited the proliferation
of human colon carcinoma cell line (HT-29) and human gastric cancer
cell line (SGC-7901) (15,16). It was also found that BrMC led to
HCC cell apoptosis by stimulating the ROS production and activation
of JNK (17). Our previous study
demonstrated that BrMC significantly inhibited the self-renewal and
tumorigenicity in nude mice of CD133-positive sphere-forming cells
from hepatoma cell line SMMC-7721 in a dose-dependent manner
(18). The evidence confirmed that
BrMC had treatment effect to HCC and had litter impact on human
embryonic liver cell line L-02 (17).
Nuclear factor-κB (NF-κB) pathway plays an important
role in many physiological and pathological processes including
immunity, inflammation, cell proliferation and differentiation
(19). The abnormal activation of
NF-κB pathway can be found in many cancer cells and is also
responsible for tumorigenesis and chemoresistance (20,21).
Furthermore, NF-κB can be activated by a number of chemotherapeutic
agents including sorafenib (22)
and apoptosis inducing factor such as TNF-related apoptosis
inducing ligand. Thus, this may suggest that the sorafenib-induced
NF-κB activation may partially contribute to the resistance to
sorafenib.
Our previous study revealed that, BrMC inhibited
liver cancer stem cells (LCSCs) properties in SMMC-7721 cell lines
by downregulation of Twist expression (18). The EMT-related protein twist1 has
been associated with early signs of metastasis following tumor
hypoxia and NF-κB activity (23,24).
It has been reported that chrysin (the lead compound of BrMC)
effectively can downregulate the tumor hypoxia inducible factor-1α
(HIF-1α) protein (25). Based on
the above evidence, we first looked forward with a new idea for the
combination of sorafenib and BrMC. In the present study, we
evaluated the inhibition of sorafenib alone and combined with BrMC
on the characteristics of liver cancer stem-like cells (LCSLCs) and
their potential mechanism.
Materials and methods
Chemicals
BrMC was synthesized as previously described
(15). The purity was analyzed as
99.5% by HPLC. Sorafenib was purchased from the Jinan Kaien
Pharmaceutical Technology Co., Ltd. (Jinan, Shandong, China).
Cell culture and reagents
Human HCC SMMC-7721 cells were purchased from the
Chinese Academy Cell Bank (Shanghai, China), and were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; Thermo Scientific, Waltham, MA, USA), 100
IU/ml penicillin G and 100 μg/ml streptomycin (Invitrogen
Life Technologies, Carlsbad, CA, USA) in a humidified atmosphere
containing 5% CO2 at 37°C. Trypsin and dimethyl
sulfoxide (DMSO) were purchased from Amersco Co. (Solon, OH,
USA).
Sphere formation and self-renewal
assay
Parental cells were collected and washed to remove
serum, and then suspended in serum-free stem cell conditional
medium containing DMEM/F12 (Gibco-Invitrogen, Carlsbad, CA, USA)
supplemented with 100 IU/ml penicillin G, 100 μg/ml
streptomycin, 20 ng/ml EGF, 10 ng/ml bFGF (both from Peprotech
Inc., Rocky Hill, NJ, USA), and 1X B27 (Invitrogen). The cells were
next plated in ultra-low adherence culture plates (6-wells) at a
density of 5,000 cells/well kept under a humidified incubator
containing 5% CO2 at 37°C. After 5 days of culture,
sphere-forming cells (SFCs) were obtained after trypsin-EDTA
digestion, and visualized in a microscope followed by cell
counting. For comparing the sphere formation capability of
different generation, the first generation SFCs were plated at a
density of 1,000 cells/ml in ultra-low adhesion 6-well culture
plates, and then to obtain the second, third and fourth generation
SFCs after continuous culture.
To investigate the effects of BrMC and sorafenib on
the self-renewal of SFCs, single cell suspension of the third
generation SFCs was plated at a density of 1,000 cells/ml in
ultra-low adhesion 24-well culture plates. The different
concentrations of sorafenib (5, 15 and 45 μmol/l) and BrMC
(5 μmol/l) + different concentrations of sorafenib (5, 15
and 45 μmol/l) were added to the stem cell conditioned
medium of SFCs with the control groups treated with 0.1% DMSO and
BrMC (5 μmol/l), respectively. The number of tumor spheroids
under the Olympus IX51 inverted fluorescence microscope was counted
after culturing for 5 days.
Transwell invasion assay in vitro
To detect the invasive ability of SFCs by Transwell
chamber system with 8.0-μm pore size polycarbonate membrane,
and the lower side of the filter coated with 10 μl of
gelatin and the upper side coated with 10 μl of
Matrigel.
DMEM medium (1.0 ml) supplemented with 10% FBS as a
chemical inducer was added to the 24-well cell culture plate, and
then embedded in the Transwell chamber. A total of 5,000 parent
cells or SFCs were plated in the top chamber of the Transwell
coated with Matrigel and treated with different concentrations of
sorafenib (5, 15 and 45 μmol/l) and BrMC (5 μmol/l) +
different concentrations of sorafenib (5, 15 and 45 μmol/l)
for 24 h. The cells that had not invaded through the pores of the
insert were eliminated with a sterile cotton swab and discarded.
Cells invaded to the lower chamber were fixed with methanol,
stained with crystal violet and counted under the optical
microscope.
Scratch assay
The SFCs were treated with different concentrations
of sorafenib (5, 15 and 45 μmol/l) and BrMC (5
μmol/l) + different concentrations of sorafenib (5, 15 and
45 μmol/l) for 24 h. Then, were seeded in 6-well plates at a
density of 4×105/well in DMEM complete medium
supplemented with 10% FBS. When the cells grew to 85% confluency, a
wound was generated by scratching the surface of the plates in the
central region with a 200 μl pipette tip. Phosphate-buffered
saline (PBS) was used for washing 2 times to remove floating cells
and debris. Cells were incubated for 48 h, and were photographed at
0, 24 and 48 h in the same location of the wound, respectively. The
number of cells in the scratch area was counted.
Apoptosis analysis by flow cytometry
Cells were stained with Annexin V-FITC apoptosis
detection kit (BD Biosciences, San Jose, CA, USA). According to the
manufacturer's instructions, the cells were incubated with 5 ml of
Annexin V and 5 ml of propidium iodide (PI) for 15 min at room
temperature, and then the stained cells were analyzed on a FACS
flow cytometer.
Western blot analysis
Cells were washed with pre-cold PBS, and lysed in 1
ml lysis enzyme buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.2
mM EDTA, 0.2% NP-40, 10% glycerol, 1Mβ-Me, 1 μg/ml Trasylol,
0.5 μg/ml leupeptin, 0.1 mM 0.1 mM
Na3VO4, 0.5 mM 4-NPP, 0.5 mM NaF and protease
inhibitors]. The cells were scraped and collected after incubated
for 20 min at 4°C. Lysates were centrifuged at 1,2000 rpm for 15
min at 4°C to prepare whole cell extracts. Protein was separated by
10% SDS-PAGE gel after electrophoresis, and transferred to a
polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica,
MA, USA). The membranes were detected by rabbit antibodies against
Twist, HIF-1α and NF-κB (p65) or mouse antibodies against CD133,
CD44, ALDH1, N-cadherin, E-cadherin and β-actin, respectively.
NF-κB binding activity assay
The NF-κB activity in the nuclear protein (20
μg) of treated or control LCSLCs was measured using a
DNA-binding ELISA kit (TransAM™ NF-κB p65 assay; Active Motif,
Carlsbad, CA, USA) according to the manufacturer's instructions.
Absorbance at 405 nm wavelength (A405) was measured by means of an
enzyme-labeling instrument (ELX-800 type; Bio-Tek, Shanghai,
China).
Statistical analysis
Data are presented as the mean ± SE and were
analyzed by SPSS 17.0 statistical software. Multiple comparison
were performed by one-way ANOVA and pair-wise comparison was
performed by LSD-t method. P<0.05 were considered to indicate a
statistically significant result.
Results
Cultivation and amplification of LCSLCs
from the SMMC-7721 cell line
Human HCC SMMC-7721 cell line cultured in normal
condition remained as monolayer, with anchorage-dependent growth
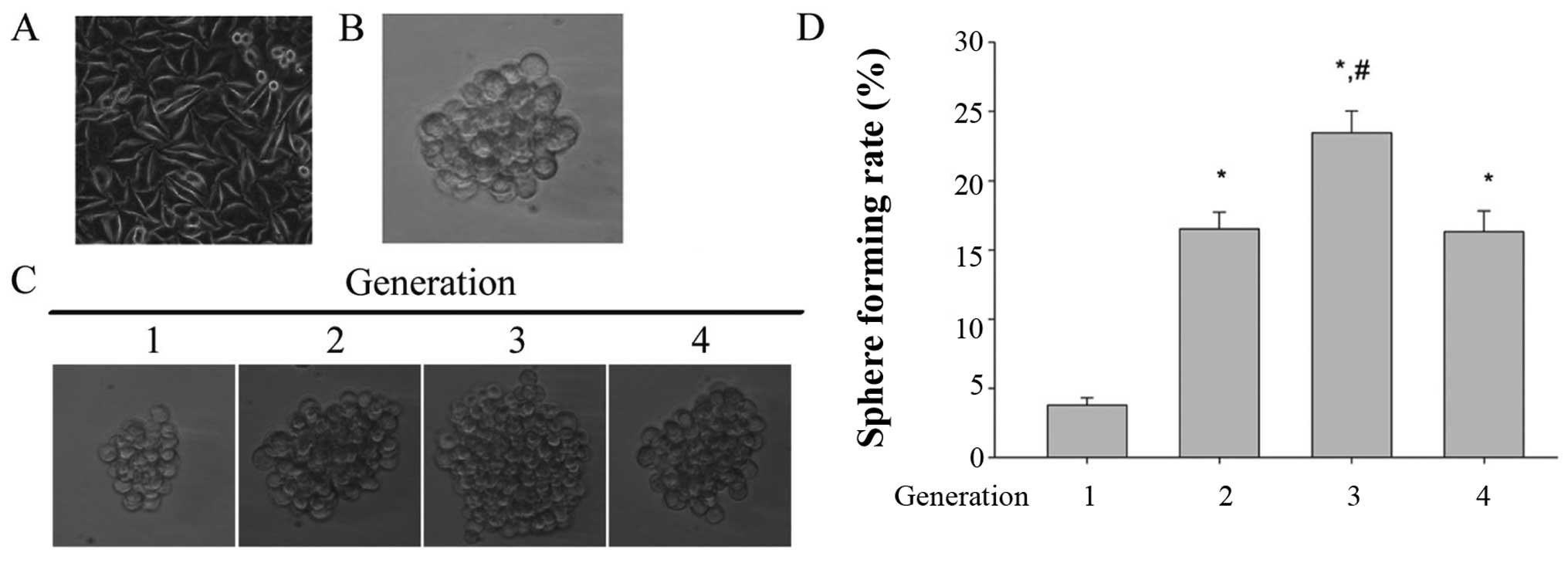
(Fig. 1A, parental cells). Numerous
studies confirmed that the spheres cultured in serum-free culture
medium (SFM) are highly enriched for cancer stem cells (CSCs). In
order to enrich LCSLCs from SMMC-7721 cell line, stem cell
conditioned medium suspension culture method was used. Under these
conditions, the cells grew as non-adherent, 3-dimensional sphere
clusters. After 5 days of incubation, the anchorage-independent
colonies were found that formed in the SMMC-7721 cell line in the
case of inoculation of 2,000 cells/well (Fig. 1B).
Self-renewal is one of most important properties of
cancer stem cells. To test the self-renewal ability, the tumor
spheres were disperse into single-cell suspension, and then applied
to multiple continuous subculture at the 1,000/ml of the
inoculation density in the stem cell conditioned medium. Fig. 1C and D show the size of the tumor
spheres and the sphere formation rate of different generations,
respectively. The result showed that the tumor spheres derived from
SMMC-7721 possess the ability to form spheroids, and the third
generation of tumor spheroids has the maximum size of tumor
spheroids and the highest sphere forming rate. These data indicated
that the tumor spheroids derived from SMMC-7721 has the capacity of
self-renewal and the third generation was given the strongest
self-renewal capability. Therefore, the third generation tumor
spheroids were regarded as the LCSLCs or the SFCs.
Characterization of LCSLCs from the
SMMC-7721 cell line
To characterize the SFCs, western blot analysis was
performed to test the protein expression level of these biomarkers.
The results showed the higher expression level of CSC biomarkers
(CD133, CD44 and ALDH1) in SFCs compared with the parental cells
(PCs) (Fig. 2A).
CSCs have higher migratory and invasion capacity,
which are thought to contribute to the metastasis and growth. The
migration and invasion capabilities of SFCs and PCs were evaluated
by scratch method and Transwell chamber invasion assay in
vitro, respectively. The results demonstrated that SFCs showed
stronger migration and invasion capabilities than PCs (Fig. 2B and C). CSCs are also deemed to
promote metastasis through EMT characteristics correlating with the
mobility of cancer cells. We evaluated the protein expression of
well-known mesenchymal phenotype cell biomarkers (N-cadherin) and
epithelial phenotype cell biomarker (E-cadherin) by western blot
analysis. The results showed that the relative protein levels of
N-cadherin was highly expressed in SFCs, while that of E-cadherin
was low (Fig. 2D). In addition, the
spontaneous apoptosis levels of SFCs and PCs were detected using
Annexin V-FITC/PI. Both the spontaneous apoptosis of SFCs and PCs
were <0.5%, indicating both of them have good cell activity
(Fig. 2E). These results indicated
that the SFCs from SMMC-7721 cells possess LCSLCs properties.
The combination of BrMC and sorafenib
enhances the inhibition of self-renewal and expression of
biomarkers of LCSLCs
Our previous study revealed that BrMC can inhibit
the characteristic of LCSCs including proliferation, self-renewal
and invasion (17). In the present
study, we investigated whether BrMC in combination with sorafenib
synergistically inhibits the characteristic of LCSLCs. As shown in
Fig. 3A, the treatment with BrMC
(15 μmol/l) and sorafenib (5, 15 and 45 μmol/l) alone
inhibited the self-renewal, while the combination treatment group
showed an augmented inhibition of self-renewal. We performed
western blot analyses to evaluate the expression of CD133, CD44 and
ALDH1. Combined treatment with BrMC and sorafenib showed a higher
reduction of the expression level of CD133, CD44 and ALDH1 than
treatment with both of them alone in the LCSLCs in a dose-dependent
manner (Fig. 3B).
The combination of BrMC and sorafenib
enhances the inhibition of migration, invasion and the expression
of EMT biomarkers
Migration and invasion properties are important
characteristics of CSCs, and they are highly correlated with tumor
metastasis and growth. The scratch method was used to evaluate
whether the combination of BrMC and sorafenib have a stronger
inhibition on the migration of LCSLCs than both of them alone. The
results showed that the migration was significantly suppressed in a
dose-dependent manner after treatment with sorafenib for 24 and 48
h (Fig. 4A), and this inhibitory
effect was potentiated by the combination with BrMC.
We next performed a Transwell assay to demonstrate
whether the sorafenib and the combination groups affects invasion
of LCSLCs. As shown in Fig. 4B,
both sorafenib (5, 15 and 45 μmol/l) and BrMC (5
μmol/l) inhibited the invasion of LCSLCs, and the
combination group enhanced the invasion ability.
Western blot analysis was also performed to check
the variety of EMT biomarkers (E-cadherin and N-cadherin) in LCSLCs
treated with sorafenib (5, 15 and 45 μmol/l) and combined
with BrMC (5 μmol/l). We found that sorafenib upregulated
the E-cadherin, and the different concentrations of sorafenib (5,
15 and 45 μmol/l) combined with BrMC (5 μmol/l)
displayed a stronger effect on this upregulation (Fig. 4C). While the treatment of sorafenib
(5, 15 and 45 μmol/l) and combination with BrMC (5
μmol/l) led to the downregulation of N-cadherin in LCSLCs,
and the combination group also showed a stronger effect (Fig. 4D). Together, these data suggested
that sorafenib (5, 15 and 45 μmol/l) can effectively inhibit
EMT in LCSLCs, and BrMC can cooperate with sorafenib to enhance the
inhibition of EMT in a dose-dependent manner.
Synergistic apoptosis induction in LCSLCs
by sorafenib and BrMC
To study whether sorafenib and BrMC synergistically
induce apoptosis of LCSLCs, Annexin V-FITC/PI and flow cytometric
analysis were used. The results showed that BrMC (5 μmol/l)
with the different concentrations of sorafenib (5, 15 and 45
μmol/l) has a synergistic induction to cell apoptosis
activity of LCSLCs compared with the corresponding concentration of
sorafenib (5, 15 and 45 μmol/l) group (Fig. 5).
Synergistic downregulation of the
expression of HIF-1α, Twist1 and NF-κB in LCSLCs by sorafenib and
BrMC
Hypoxia is one of the fundamental biological
phenomena that plays a key role in the development and
aggressiveness of a wide variety of cancers including HCC (26,27).
The homeostatic response to hypoxia is predominantly mediated by
the transcription factor HIF-1α which has been widely accepted to
be associated with tumor invasion, metastasis and treatment
resistance. Transcription factor Twist1 was proved to be a critical
EMT molecule.
To evaluate the effect on the protein expression of
HIF-1α, Twist1 and NF-κB in LCSLCs and PCs, western blot analysis
were performed. Fig. 6A shows the
higher expression of HIF-1α in LCSLCs than PCs, and sorafenib
downregulated the expression of HIF-1α and BrMC collaborated with
sorafenib to reduce the HIF-1α protein level in LCSLCs. The same
function on the expression of Twist1 was observed (Fig. 6B).
 | Figure 6The expression levels of HIF-1α,
Twist1 and NF-κB in SFCs. (A) The expression levels of HIF-1α,
Twist1 and NF-κB in SFCs compared with that in PCs. (B) The
downregulation of HIF-1α by sorafenib (0, 5, 15 and 45
μmol/l) alone and combined with BrMC (5 μmol/l). (C)
The downregulation of Twist1 by sorafenib (0, 5, 15 and 45
μmol/l) alone and combined with BrMC (5 μmol/l). (D)
The effects of sorafenib (0, 5, 15 and 45 μmol/l) alone and
combined with BrMC (5 μmol/l) on the expression levels of
NF-κB in SFCs. *P<0.05 compared with 0 h;
#P<0.05 compared with sorafenib alone. (E) The
effects of sorafenib (0, 5, 15 and 45 μmol/l) alone and
combined with BrMC (5 μmol/l) on the NF-κB DNA binding
activity measured using a ELISA kit. *P<0.05 compared
with 0.1% DMSO; #P<0.05 compared with sorafenib
alone. |
The expression level of NF-κB (p65) in LCSLCs was
higher than that in PCs as shown in Fig. 6C. Previous study reported that
sorafenib can strongly induce the activation of NF-κB signaling
pathway (22), which was also
verified in the present study. The increased expression level of
NF-κB was observed in LCSLCs treated with different concentrations
of sorafenib (5, 15 and 45 μmol/l). Instead, BrMC showed
strongly antagonistic action on the upregulation of NF-κB caused by
sorafenib (Fig. 6D). To further
examine the effect of sorafenib alone and the combination group on
the NF-κB activation, the NF-κB activity assay was performed by use
of a DNA-binding ELISA kit. The results showed that sorafenib
significantly increased the DNA binding activity for NF-κB in a
dose-depentent manner, while remarkably decreased NF-κB activation
was observed in the combination group (Fig. 6E).
Discussion
Recent findings support the concept that cancer stem
cells (CSCs) possess the ability to initiate tumor formation,
self-renewal and resist chemotherapeutic drugs, thereby causing
relapse of the tumor. Targeted therapy of CSCs recommend new
strategies and approaches for cancer treatment. Accumulating
evidence has demonstrated the existence of CSCs in hepatocellular
carcinoma (HCC) (11,28–30).
Although a number of studies have reported the
separation of CSCs, the enrichment, isolation and identification of
CSCs remain priorities for development of novel cancer therapeutic
strategies. In the present study, we employed suspension culture
method in ultra-low adherence plates with serum-free stem cell
conditional medium to obtain SFCs. CD133 and CD44 have been widely
applied to isolate and characterize the CSCs in many solid tumors
(31,32). ALDH1 was also recommended as marker
in several types of cancers including HCC (33). The combination of multiple markers
was used to improve the specificity of LCSC markers by many
researches. Our findings clearly showed that the expression levels
of CD133, CD44 and ALDH1 in SFCs were significantly higher than
that in PCs. It was also found that SFCs from the SMCC-7721 cells
exhibited superactive self-renewal capacity, higher migration and
invasion capacity and EMT. Based on above-mentioned data, SFCs from
the SMCC-7721 cells was identified as LCSLCs.
Recently, the unstable efficacy of sorafenib has
raised concern among researchers, and 'sorafenib resistance̓ has
become a hot topic to describe the impaired efficacy of sorafenib,
particularly for patients with advanced HCC. EMT process and
microenvironment in HCC were reported responsible for the drug
resistance of sorafenib. It is also believed that resistance of HCC
to sorafenib may be explained by LCSCs. In order to improve the
impaired efficacy caused by sorafenib and resistance in advanced
HCC, drugs combination is a promising direction. In the present
study, we found that sorafenib suppressed the properties of LCSLCs
including self-renewal, EMT, cell migration and invasion in
vitro, while sorafenib combined with BrMC achieve efficient
inhibition on these properties. Regarding the molecular mechanism,
the combination of sorafenib and BrMC dose-dependently suppressed
the expression of CD133, CD44 and ALDH1, which was related to LCSCs
characteristics, and also reduced expression level of the
EMT-associated key protein twist1.
The hypoxic tumor microenvironment has been shown to
be associated with cancer progression. Hypoxia can promote the
stem-like properties (34). HIF-1α
is a key hypoxia-regulatory protein involved in the regulation of
several genes such as erythropoietin (EPO) and VEGF which
contribute to restoration of oxygen homeostasis. HIF-1α also plays
an important role in the acquirement of drug-resistance against
chemotherapeutic agents and correlates with the reduced rate of the
patients survival (35–37). In the present study, we found that
sorafenib in combination with BrMC synergistically inhibited the
expression of HIF-1α protein. Rausch et al (22) reported that the drug resistance of
sorafenib on CSCs may result from the activation of NF-κB signal
pathway induced by sorafenib. We also found BrMC strongly
antagonized the upregulation of NF-κB protein and the increase of
DNA binding activity of NF-κB caused by sorafenib. Moreover, the
combination of sorafenib and BrMC enhanced the apoptosis induced by
sorafenib.
Collectively, we report for the first time the
synergistic action of sorafenib and BrMC on HCC and its potential
mechanism. Our results provide evidence that the combination of
sorafenib and BrMC may be an attractive alternative for the
treatment of HCC.
Acknowledgments
The present study was financially supported by
grants from the National Natural Science Foundation of China (nos.
81172375 and 31400311), the Program for Excellent Talents of Hunan
Normal University (no. ET1508), the Key Project of Administration
of Traditional Chinese Medicine of Hunan Province (no. 201539), the
Project of Hunan Provincial Natural Science Foundation (no.
13JJ3061), the Science and Technology Planning Project of Hunan
Province (no. 2015SK20324) and the Construct Program of the Key
Discipline of Basic Medicine in Hunan Province.
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014. International Agency for Research on Cancer (IARC);
2014
|
|
2
|
Zuo TT, Zheng RS, Zhang SW, Zeng HM and
Chen WQ: Incidence and mortality of liver cancer in China in 2011.
Chin J Cancer. 34:508–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keating GM and Santoro A: Sorafenib: A
review of its use in advanced hepatocellular carcinoma. Drugs.
69:223–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim Y, Wu Q, Hamerlik P, Hitomi M, Sloan
AE, Barnett GH, Weil RJ, Leahy P, Hjelmeland AB and Rich JN:
Aptamer identification of brain tumor-initiating cells. Cancer Res.
73:4923–4936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ignatova TN, Kukekov VG, Laywell ED,
Suslov ON, Vrionis FD and Steindler DA: Human cortical glial tumors
contain neural stem-like cells expressing astroglial and neuronal
markers in vitro. Glia. 39:193–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH,
Yao XH, Gao L, Wang JM and Bian XW: Isolation and characterization
of cancer stem cells from a human glioblastoma cell line U87.
Cancer Lett. 265:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gedaly R, Galuppo R, Daily MF, Shah M,
Maynard E, Chen C, Zhang X, Esser KA, Cohen DA, Evers BM, et al:
Targeting the Wnt/β-catenin signaling pathway in liver cancer stem
cells and hepatocellular carcinoma cell lines with FH535. PLoS One.
9:e992722014. View Article : Google Scholar
|
|
12
|
Nagano H, Ishii H, Marubashi S, Haraguchi
N, Eguchi H, Doki Y and Mori M: Novel therapeutic target for cancer
stem cells in hepatocellular carcinoma. J Hepatobiliary Pancreat
Sci. 19:600–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li S and Li Q: Cancer stem cells and tumor
metastasis (Review). Int J Oncol. 44:1806–1812. 2014.PubMed/NCBI
|
|
14
|
Oishi N, Yamashita T and Kaneko S:
Molecular biology of liver cancer stem cells. Liver Cancer.
3:71–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng X, Meng WD, Xu YY, Cao JG and Qing
FL: Synthesis and anticancer effect of chrysin derivatives. Bioorg
Med Chem Lett. 13:881–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang HL, Zheng X and Cao JG: Induction of
apoptosis of human gastric carcinoma SGC-790 cell line by
8-bromo-7-mehoxychrysin. Zhongguo Yaolixue Tongbao. 24:1370–1372.
2008.
|
|
17
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren KQ, Cao XZ, Liu ZH, Guo H, Quan MF,
Liu F, Jiang L, Xiang HL, Deng XY and Cao JG:
8-bromo-5-hydroxy-7-methoxychrysin targeting for inhibition of the
properties of liver cancer stem cells by modulation of Twist
signaling. Int J Oncol. 43:1719–1729. 2013.PubMed/NCBI
|
|
19
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI
|
|
20
|
Chandler NM, Canete JJ and Callery MP:
Increased expression of NF-κB subunits in human pancreatic cancer
cells. J Surg Res. 118:9–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F and Sethi G: Targeting transcription
factor NF-kappaB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta. 1805:167–180. 2010.PubMed/NCBI
|
|
22
|
Rausch V, Liu L, Kallifatidis G, Baumann
B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zöller
M, et al: Synergistic activity of sorafenib and sulforaphane
abolishes pancreatic cancer stem cell characteristics. Cancer Res.
70:5004–5013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hill RP, Marie-Egyptienne DT and Hedley
DW: Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol.
19:106–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu B, Xue J, Li Z, Shi X, Jiang BH and
Fang J: Chrysin inhibits expression of hypoxia-inducible factor-1α
through reducing hypoxia-inducible factor-1α stability and
inhibiting its protein synthesis. Mol Cancer Ther. 6:220–226. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong CC, Kai AK and Ng IO: The impact of
hypoxia in hepatocellular carcinoma metastasis. Front Med. 8:33–41.
2014. View Article : Google Scholar
|
|
28
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mikhail S and He AR: Liver cancer stem
cells. Int J Hepatol. 2011:4869542011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji J and Wang XW: Clinical implications of
cancer stem cell biology in hepatocellular carcinoma. Semin Oncol.
39:461–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu CP, Wang AQ, Zhang HH, Wan XS, Yang
XB, Chen SG and Zhao HT: Research progress and prospects of markers
for liver cancer stem cells. World J Gastroenterol. 21:12190–12196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and
Li J: Cancer stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular
carcinoma. Int J Cancer. 126:2067–2078. 2010. View Article : Google Scholar
|
|
33
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY,
Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the
CD133 liver cancer stem cell populations. Mol Cancer Res.
6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu CP, Du HD, Gong HL, Li DW, Tao L, Tian
J and Zhou L: Hypoxia promotes stem-like properties of laryngeal
cancer cell lines by increasing the CD133+ stem cell
fraction. Int J Oncol. 44:1652–1660. 2014.PubMed/NCBI
|
|
35
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gruber G, Greiner RH, Hlushchuk R,
Aebersold DM, Altermatt HJ, Berclaz G and Djonov V:
Hypoxia-inducible factor 1 alpha in high-risk breast cancer: An
independent prognostic parameter? Breast Cancer Res. 6:R191–R198.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurokawa T, Miyamoto M, Kato K, Cho Y,
Kawarada Y, Hida Y, Shinohara T, Itoh T, Okushiba S, Kondo S, et
al: Overexpression of hypoxia-inducible-factor 1alpha(HIF-1alpha)
in oesophageal squamous cell carcinoma correlates with lymph node
metastasis and pathologic stage. Br J Cancer. 89:1042–1047. 2003.
View Article : Google Scholar : PubMed/NCBI
|