Introduction
Osteosarcoma (OS) is the most common primary solid
tumor of bone in children and adolescence among various types of
bone tumors (1). With the
introduction of chemotherapy in the 1970's, the 5-year survival
rate after surgery has increased to 50–70% in patients without
metastasis (2–4). However, there has been no further
improvement during the last three decades in terms of the survival
rate and it remains at 20–30% for patients with detectable
metastasis (4,5). The development of chemoresistance in
OS contributes to the plateau of the survival rate to a certain
extent. It is necessary to investigate the mechanisms of OS drug
resistance.
The cancer stem cell (CSC) model is one emerging
model for the development of drug resistance in malignancies. CSCs
markedly promote drug resistance in various cancers (6). It has been demonstrated that
CD133-positive (CD133+) cells in OS exhibit CSC
characteristics (7–9). However, the mechanisms of drug
resistance in CD133+ OS cells need to be further
elucidated.
DNA-dependent protein kinase catalytic subunit
(DNA-PKcs) is a member of the large phosphatidylinositol 3-kinase
(PI3K)-related kinase (PIKK) family. DNA-PKcs, along with accessory
heterodimeric complexes, Ku70 and Ku80, are involved in DNA damage
repair via non-homologous end joining (NHEJ). Our previous studies
revealed that inhibition of DNA-PKcs sensitized OS cells to
chemotherapeutic agents (10),
indicating that DNA-PKcs plays a significant role in
chemoresistance. Moreover, DNA-PKcs was found to be overexpressed
in OS CSCs (11), which might be
one of the causes of chemoresistance in OS.
P-glycoprotein (P-gp), a member of the ATP-binding
cassette (ABC) transporters, is encoded by the ABCB1 gene and plays
an important role in chemoresistance in tumors. Hence, it is
necessary to understand the mechanisms of the regulation of P-gp.
It has been demonstrated that P-gp expression is at a higher level
in OS CSCs compared with that in non-CSCs (9). Although DNA-PKcs and P-gp are involved
in chemoresistance and are overexpressed in OS CSCs, there has been
no study concerning the relationship between DNA-PKcs and P-gp in
OS CSCs to date. Previous studies have revealed that the expression
of P-gp is regulated by the PI3K/Akt/NF-κB pathway in other cancers
(12,13). This prompted us to investigate the
relationship between DNA-PKcs and P-gp in OS CSCs, as well as the
role of the Akt/NF-κB pathway in this relationship.
We hypothesize that DNA-PKcs regulates P-gp via the
Akt/NF-κB axis in CD133+ OS cells. The purpose of this
study was to investigate the role of DNA-PKcs in P-gp expression
and the underlying molecular mechanism in drug-resistant
CD133+ MG-63 cells. Compared with CD133-negative
(CD133−) MG-63 cells, CD133+ MG-63 cells
showed increased expressions of DNA-PKcs and P-gp, as well as
higher activity of the Akt/NF-κB pathway. Downregulation of
DNA-PKcs significantly decreased the P-gp expression and activity
of the Akt/NF-κB pathway, and inhibition of the Akt/NF-κB pathway
downregulated the P-gp expression. All of these results revealed
that DNA-PKcs regulates P-gp via the Akt/NF-κB pathway in
CD133+ OS cells.
Materials and methods
Cell culture
The human MG-63 cell line was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). Cells
were cultured in Dulbecco's minimal essential medium (DMEM)
supplemented with 10% fetal bovine serum (both from Gibco, Grand
Island, NY, USA) at 37°C, with 5% CO2 in a 95%
humidified atmosphere.
Magnetic activated cell sorting
(MACS)
MACS was performed using CD133 MicroBead kit
(Miltenyi Biotec, Auburn, CA, USA) following the manufacturer's
instructions. Briefly, a single-cell suspension was prepared in the
MACS separation buffer. Cells were incubated with FcR Blocking
Reagent and CD133 MicroBeads at 4°C for 15 min. After washing
steps, magnetic separation was performed using an LS Column and
MACS Separator (Miltenyi Biotec). The magnetically labeled
CD133+ cells and unlabeled CD133− cells were
collected, respectively.
Cell viability assay
Cells were seeded in 96-well plates at a density of
5,000 cells/well. Then cisplatin (CDDP; Qilu Pharmaceutical Co.,
Ltd., Shandong, China) was added at increasing concentrations.
Survival of the cells was measured 24 h post-treatment using the
Cell Counting Kit-8 (CCK-8; BestBio, Shanghai, China) according to
the manufacturer's instructions. The cell survival rate was
presented as the percentage of viable cells compared with the
corresponding viable cells in the drug-free controls. The half
maximal inhibitory concentration (IC50) was calculated
from the relative survival curve.
Transfection of small interfering RNA
(siRNA) and inhibitor treatment
The CD133+ MG-63 cells were seeded into
ultra-low attachment 6-well plates in serum-free medium. The
serum-free medium consisted of Dulbecco's modified Eagle's medium
(DMEM), 20 ng/ml epidermal growth factor (EGF), 20 ng/ml basic
fibroblast growth factor (bFGF) (both from PeproTech, Rocky Hill,
NJ, USA) and N-2 Supplement (Gibco). For siRNA transfection, the
siDNA-PKcs, siNF-κB/p65 or control siRNAs (GenePharma Co., Ltd.,
Shanghai, China) were transfected into the cells using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. After 24 or 48 h of transfection, the
cells were harvested for further experiments at the gene or protein
level, respectively. For the inhibition experiment, the
CD133+ MG-63 cells were treated with Akt inhibitor
MK-2206 2HCl (10 µM) (Selleck, Houston, TX, USA) for 24 h,
and subjected to gene and protein expression experiments.
Immunofluorescence
The cells were seeded on 24-well chamber slides.
After adherence, the cells were fixed with 4% paraformaldehyde for
15 min. The fixed cells were incubated in 0.3% Triton X-100 for 10
min to permeabilize and 10% normal goat serum for 1 h to block
non-specific protein-protein interactions. Then the cells were
incubated with the rabbit polyclonal anti-human P-gp (ab129450,
1:200) and mouse monoclonal anti-human DNA-PKcs primary antibodies
(ab1832, 1:100) (both from Abcam, Cambridge, MA, USA) overnight at
4°C. The Alexa Fluor 488 goat anti-rabbit (ZF-0511, 1:200) and
Alexa Fluor 594 goat anti-mouse (ZF-0513, 1:200) (both from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
secondary antibodies were used for detection.
4′,6-Diamidino-2-phenylindole was used to stain the cell nuclei for
5 min at room temperature. Slides were observed on an inverted
fluorescence microscope (BX52; Olympus Corp., Tokyo, Japan).
Quantitative real-time polymerase chain
reaction (qPCR)
Total RNA was extracted with TRIzol lysis buffer
(Toyobo, Osaka, Japan) according to the manufacturer's
instructions. First-strand complementary DNA (cDNA) synthesis was
carried out using ReverTra Ace qPCR RT kit (Toyobo). Briefly, 1
µg of total RNA was used in a total volume of 10 µl
containing 2 µl 5X RT buffer, 0.5 µl RT Enzyme Mix,
0.5 µl Primer Mix and nuclease-free water. The reverse
transcription was performed in a thermal cycler (TGradient 96;
Biometra GmbH, Göttingen, Germany) with a temperature cycling
program of 15 min at 37°C, 5 min at 98°C. The cDNAs were used as
templates for PCR amplification using SYBR® Green
Realtime PCR Master Mix kit (Toyobo). In brief, reaction mixtures
(20 µl) for PCR were assembled using 2 µl cDNA
template, 6.4 µl distilled water, 10 µl 2X
SYBR® Green Realtime PCR Master Mix, 0.8 µl
forward primers (10 µM) and 0.8 µl reverse primers
(10 µM). The cycle parameters were 95°C for 30 sec followed
by 40 cycles at 95°C for 5 sec, 55°C for 10 sec and 72°C for 15
sec. The human GAPDH PCR product was used as an internal control.
The results were standardized with the formula: ΔCT = CTtarget −
CTcontrol and further converted to the fold of the target gene over
the control gene (2−ΔCT). The primer sequences of the
genes used in this study are presented in Table I.
 | Table IPrimer sequences for qPCR. |
Table I
Primer sequences for qPCR.
| Primers
(5′-3′) |
|---|
| PRKDC | F
ACAGAGATCCAGAAAGTGAGACA |
| R
AGCAACCGGTCCAAGGTATT |
| ABCB1 | F
ACAGAGGGGATGGTCAGTGT |
| R
TCACGGCCATAGCGAATGTT |
| GAPDH | F
CAGGAGGCATTGCTGATGAT |
| R
GAAGGCTGGGGCTCATTT |
Western blot analysis
The cells were harvested and total proteins were
extracted with RIPA lysis buffer, and the protein concentrations
were quantified with the Enhanced BCA Protein Assay kit (both from
Beyotime, Shanghai, China). Equal amounts of protein were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electroblotted onto a polyvinylidene fluoride (PVDF)
membrane. Non-specific sites were blocked for 1.5 h with 5% non-fat
milk in Tris-buffered saline and Tween-20 (TBST) at room
temperature. The PVDF membranes were incubated at 4°C overnight
with primary antibodies. Primary antibodies included: rabbit
polyclonal anti-human γH2AX (S139) (ab2893, 1:1,000), rabbit
polyclonal anti-human DNA-PKcs (ab230, 1:2,000), rabbit polyclonal
anti-human P-gp (ab129450, 1:1,000) antibodies from Abcam; rabbit
polyclonal anti-human Akt (9272S, 1:1,000), rabbit polyclonal
anti-human phospho-Akt (S473) (9271S, 1:1,000), mouse monoclonal
anti-human phospho-IκB-α (9246S, 1:1,000), and rabbit monoclonal
anti-human phospho-NF-κB/p65 (3033S, 1:1,000) antibodies from Cell
Signaling Technology (Danvers, MA, USA); rabbit polyclonal
anti-human phospho-Akt (T308) (sc-16646-R, 1:100), rabbit
polyclonal anti-human inhibitor of κB (IκB)-α (sc-371, 1:100)
antibodies from Santa Cruz Biotechnology (Dallas, TX, USA); rabbit
polyclonal anti-human NF-κB/p65 (10745-1-AP, 1:500) antibody from
Proteintech (Chicago, IL, USA); mouse monoclonal anti-human β-actin
(TA-90, 1:500) antibody from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. After washing with TBST, membranes were
incubated with goat anti-rabbit secondary antibody (ZB-2301,
1:5,000) or goat anti-mouse secondary antibody (ZB-2305, 1:5,000)
(both from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
conjugated with horseradish peroxidase for 1.5 h at room
temperature. Immunoreactive bands were detected by enhanced
chemiluminescence substrate (EMD Millipore, Billerica, MA,
USA).
Statistical analysis
Each experiment was performed three times
independently. Data are expressed as means ± standard deviation
(SD). Student's t-test was used for comparisons. Differences were
considered statistically significant if P<0.05. Statistical
analysis was carried out using GraphPad Prism 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
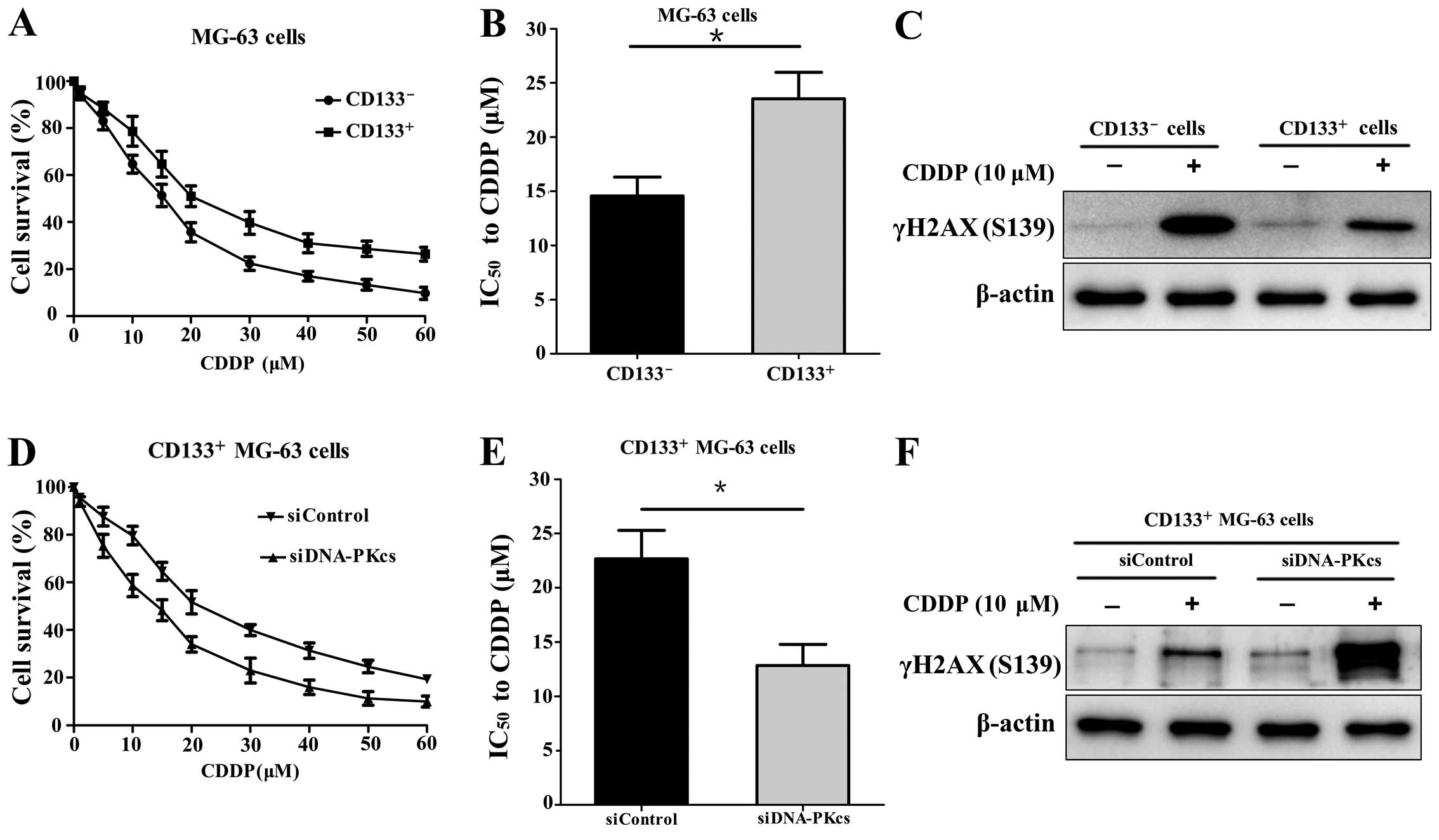
CD133+ MG-63 cells are more
resistant to CDDP compared with CD133- MG-63 cells
After MACS, the CD133+ and
CD133− cells were treated with CDDP at different
concentrations for 24 h, respectively. The cell viability was
measured and the result showed that the CD133+ cells
were more resistant to CDDP (Fig.
1A). The IC50 value of the CD133+ cells
was significantly higher than that of the CD133− cells
(23.55 vs. 14.57 µM; P<0.05) (Fig. 1B). In addition, the expression of
DNA double-strand break (DSB) marker γH2AX (S139) in the
CD133+ cells was lower than that in the
CD133− cells after CDDP (10 µM) treatment for 24
h (Fig. 1C).
Downregulation of DNA-PKcs sensitizes
CD133+ MG-63 cells to CDDP
After transfection with siDNA-PKcs or siControl, the
CD133+ MG-63 cells were treated with CDDP at different
concentrations for 24 h. It was shown that the CD133+
MG-63 cells with transfection of siDNA-PKcs were more sensitive to
CDDP compared with the CD133+ cells transfected with the
siControl (Fig. 1D). The
IC50 value of the siDNA-PKcs group was lower than that
of the siControl group (12.83 vs. 22.67 µM; P<0.05)
(Fig. 1E). The expression of γH2AX
(S139) was markedly elevated in the CD133+ MG-63 cells
with siDNA-PKcs transfection after CDDP (10 µM) treatment
(Fig. 1F). The results revealed
that downregulation of DNA-PKcs reduced the DNA damage repair and
increased the sensitivity to CDDP in the CD133+ MG-63
cells.
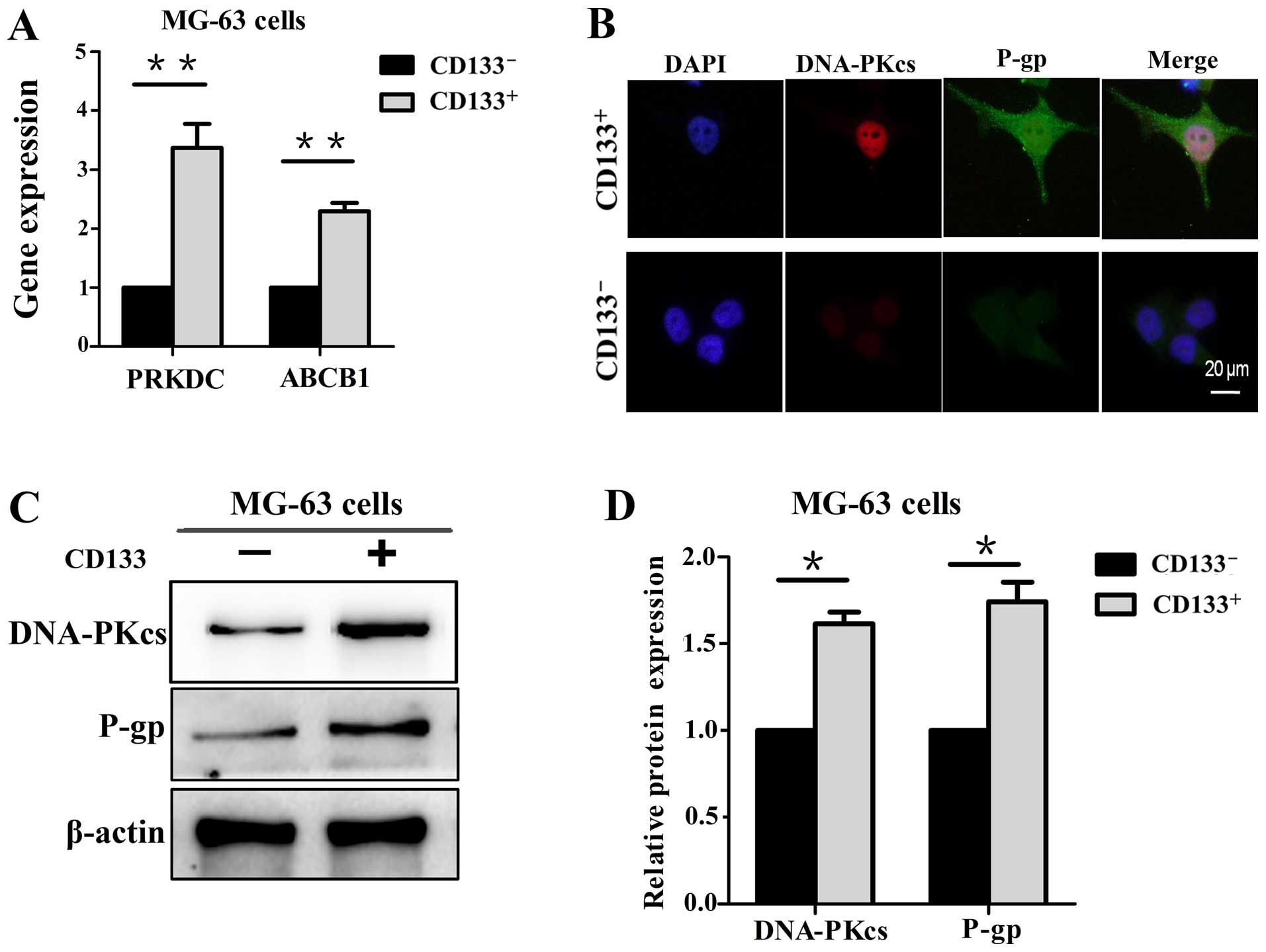
DNA-PKcs is involved in the expression of
P-gp
The expression level of DNA-PKcs and P-gp were first
investigated in the CD133+ and CD133− MG-63
cells, respectively. The results of qPCR revealed that the PRKDC
and ABCB1 genes were at higher levels in the CD133+
MG-63 cells (Fig. 2A). In addition,
immunofluorescence and western blot analysis showed that the
expression levels of DNA-PKcs and P-gp were elevated in the
CD133+ cells compared with levels in the
CD133− cells (Fig.
2B–D).
Then the CD133+ MG-63 cells were
transfected with siDNA-PKcs, and P-gp was examined at the gene and
protein levels. It was shown that the ABCB1 gene (Fig. 3A) and P-gp (Fig. 3B–D) expression were significantly
decreased following the downregulation of DNA-PKcs. Taken together,
the results indicate that DNA-PKcs is involved in P-gp expression,
and DNA-PKcs and P-gp are positively correlated with
chemoresistance to CDDP in CD133+ MG-63 cells.
The Akt/NF-κB pathway is implicated in
P-gp expression in CD133+ MG-63 cells
The expression levels of p-Akt (both S473 and T308)
and p-NF-κB/p65 were examined in both the CD133+ and
CD133− MG-63 cells. The results showed that p-Akt
(S473), p-Akt (T308) and p-NF-κB/p65 were expressed at higher
levels in the CD133+ MG-63 cells compared with these
levels in the CD133− MG-63 cells (Fig. 4A). Immunofluorescence showed that
NF-κB/p65 was mainly localized in the nuclei of CD133+
MG-63 cells (Fig. 4B). These
results indicate that CD133+ MG-63 cells display
hyperactivation of the Akt/NF-κB pathway.
Moreover, the results showed that P-gp and ABCB1
gene expression levels were decreased following the downregulation
of NF-κB/p65 via siNF-κB/p65 transfection (Fig. 4C and D). This suggests that
NF-κB/p65 is involved in P-gp expression. Following Akt inhibitor
MK-2206 2HCl (10 µM) treatment, the activity of NF-κB/p65
and expression levels of P-gp and ABCB1 genes in the
CD133+ MG-63 cells were examined. It was shown that the
expression levels of p-NF-κB/p65 and P-gp, as well as the ABCB1
gene were downregulated by inhibition of the activity of Akt
(Fig. 4D and E).
The results above demonstrated that the Akt/NF-κB
pathway is implicated in P-gp expression at the gene and protein
levels.
Downregulation of DNA-PKcs decreases the
activation of the Akt/NF-κB pathway in CD133+ MG-63
cells
The Akt/NF-κB pathway proteins were observed in the
CD133+ MG-63 cells after siDNA-PKcs transfection.
Inhibition of DNA-PKcs via siDNA-PKcs decreased the expression of
p-Akt (S473), p-IκB-α, p-NF-κB/p65, as well as P-gp (Fig. 5). It is worth noting that the
expression of p-Akt (T308) was consistent between the siDNA-PKcs
and siControl group. These results revealed that downregulation of
DNA-PKcs suppressed Akt/NF-κB pathway activation and P-gp
expression in the CD133+ MG-63 cells.
Taken together, all the results above revealed that
downregulation of DNA-PKcs decreased P-gp expression via
suppression of the Akt/NF-κB pathway in the CD133+ MG-63
cells.
Discussion
It is well known that a tumor is populated by
heterogeneous cell populations and drug-resistant clones exist
within the tumor (14,15). Targeting drug-resistant cells could
have significance in the treatment of OS. The CSC theory believes
that CSCs are relatively resistant to chemotherapeutic agents.
Therefore, if it was possible to target drug-resistant CSCs, this
would improve the therapeutic outcomes of OS. It has been well
established that CD133+ cells in OS display features of
CSCs (7–9), thus CD133+ MG-63 cells were
taken as the object of this study.
Some chemotherapeutic reagents lead to DNA DSBs
which are lethal for tumor cells. However, DSBs can be repaired by
two main pathways, homologous recombination and NHEJ. DNA-PKcs,
along with Ku70 and Ku80, are essential in DNA damage repair via
NHEJ. Overexpression of DNA-PKcs is found in various malignancies,
which is associated with poor prognosis (16,17).
However, inhibition of DNA-PKcs sensitizes cells to chemotherapy in
various tumor cells including OS (10,18,19).
This indicates that DNA-PKcs is correlated with chemoresistance in
tumors. Studies have revealed that DNA-PKcs is overexpressed in
CSCs (11,20). In this study, we found that
CD133+ MG-63 cells displayed overexpression of DNA-PKcs
and chemoresistance to CDDP, along with lower expression of γH2AX
(S139) after CDDP treatment, whereas downregulation of DNA-PKcs
increased DSBs after CDDP treatment and sensitivity to CDDP. This
demonstrates that DNA-PKcs overexpression leads to enhanced DNA
damage repair and is involved in increased chemoresistance in OS
CSCs.
The ABC family of drug transporters contributes to
resistance to chemotherapeutic agents when overexpressed in tumors.
P-gp is a well-characterized member of the ABC membrane
transporters which functions as a drug efflux pump and reduces
intracellular drug concentrations (21). Increased expression of P-gp is one
of the key causes of drug resistance in tumors. Studies have
reported that inhibition of P-gp reversed drug resistance in OS
(22–24). Previous data revealed that high
expression of P-gp is present in OS CSCs and is considered as one
of the mechanisms of drug resistance in OS (25,26).
The results of this study showed that DNA-PKcs and
P-gp were markedly elevated in CD133+ MG-63 cells, which
may explain the chemoresistance of these cells with a higher
IC50. In contrast, as the expression of DNA-PKcs was
downregulated by siRNA, P-gp and ABCB1 gene expression levels were
significantly decreased. This indicates that, besides DNA damage
repair, DNA-PKcs is involved in chemoresistance via the regulation
of P-gp expression. Therefore, the molecular mechanism through
which DNA-PKcs mediates P-gp expression needs to be further
investigated.
The PI3K/Akt signaling pathway is an important
mediator of cell growth, survival and motility. Dysregulation of
the PI3K/Akt pathway is implicated in resistance to chemotherapy in
a wide variety of neoplasias (27–29).
Activated Akt targets many proteins, including IκB kinase (IKK)
which is responsible for the phosphorylation and degradation of
IκB. Then NF-κB is released from the IκB-bound complex. With NF-κB
nuclear translocation and binding to its recognition sites, ABCB1
gene promoter activation is enhanced and gene expression is induced
(13,30). It has been demonstrated that
downregulation of NF-κB inhibits P-gp expression by blocking ABCB1
gene transcription (31–33). These findings suggest that the
Akt/NF-κB pathway may be able to mediate P-gp expression. Moreover,
DNA-PKcs is a member of the PIKK family and is involved in
Akt/NF-κB pathway activation (18).
Therefore, we postulate that DNA-PKcs is involved in P-gp
expression via the Akt/NF-κB pathway in CD133+ MG-63
cells.
To verify our hypothesis, the relationship between
the Akt/NF-κB pathway and P-gp expression was first investigated.
Our results revealed that p-Akt and p-NF-κB/p65 were highly
expressed and NF-κB/p65 was mainly localized in the nuclei in the
CD133+ cells compared with the CD133− cells,
which indicated that the Akt/NF-κB pathway was activated in these
cells. The results are consistent with previous reports (34,35).
However, inhibition of the Akt/NF-κB pathway via inhibition of Akt
activity or downregulation of NF-κB/p65 decreased P-gp and ABCB1
gene expression. These results demonstrated that the Akt/NF-κB
pathway was involved in P-gp expression in the CD133+
MG-63 cells.
Since P-gp expression was decreased following
downregulation of DNA-PKcs in the CD133+ MG-63 cells, we
downregulated the DNA-PKcs expression via siRNA and examined the
activation of the Akt/NF-κB pathway and P-gp expression. The
results showed that p-Akt (S473), p-IκB-α, p-NF-κB/p65, P-gp and
ABCB1 gene were decreased after transfection of siDNA-PKcs in the
CD133+ MG-63 cells, which demonstrated that
downregulation of DNA-PKcs decreased P-gp expression at the mRNA
and protein levels via suppression of the Akt/NF-κB pathway in
these cells. The results were supported by a previous report that
DNA-PKcs mediated Akt/NF-κB pathway activation followed by the
expression of P-gp in multidrug-resistant glioblastoma cells
(36).
In addition, we found that disruption of DNA-PKcs
decreased the expression of p-Akt (S473) rather than p-Akt (T308).
This suggests that, as a member of the PIKK family, DNA-PKcs
phosphorylates Akt at Ser473 specifically, which is consistent with
previous data reported in other studies (37–39).
This can be attributed to the fact that Ser473 is located in a
hydrophobic motif Phe-Xaa-Xaa-Phe-Ser-Tyr (Xaa is any amino acid)
in the C terminus, and DNA-PKcs has predisposition for
phosphorylation sites at the extreme terminus of its substrate,
which is critical for DNA-PK activity (38,40).
CD133+ OS cells which are well-known as
CSCs play important role in drug resistance. Our study presented
evidence that DNA-PKcs and P-gp were significantly elevated and the
Akt/NF-κB pathway was activated in the CD133+ MG-63
cells. Moreover, downregulation of DNA-PKcs decreased P-gp
expression and chemoresistance to CDDP via suppression of the
Akt/NF-κB pathway in these cells. We therefore propose that
combining DNA-PKcs inhibition targeting CSCs with conventional
chemotherapeutic agents may be considered as a strategy to improve
the treatment outcome of OS.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (81172551) and the Natural Science
Foundation of Shandong Province of China (ZR2011HM037).
References
|
1
|
Whelan J, McTiernan A, Cooper N, Wong YK,
Francis M, Vernon S and Strauss SJ: Incidence and survival of
malignant bone sarcomas in England 1979–2007. Int J Cancer.
131:E508–E517. 2012. View Article : Google Scholar
|
|
2
|
Sakamoto A and Iwamoto Y: Current status
and perspectives regarding the treatment of osteo-sarcoma:
Chemotherapy. Rev Recent Clin Trials. 3:228–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: Current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller BJ, Cram P, Lynch CF and Buckwalter
JA: Risk factors for metastatic disease at presentation with
osteosarcoma: An analysis of the SEER database. J Bone Joint Surg
Am. 95:e892013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu H, Fang X, Luo Q and Ouyang G: Cancer
stem cells: A potential target for cancer therapy. Cell Mol Life
Sci. 72:3411–3424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tirino V, Desiderio V, d'Aquino R, De
Francesco F, Pirozzi G, Graziano A, Galderisi U, Cavaliere C, De
Rosa A, Papaccio G, et al: Detection and characterization of
CD133+ cancer stem cells in human solid tumours. PLoS
One. 3:e34692008. View Article : Google Scholar
|
|
8
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, Fazioli F, Pirozzi G and Papaccio G: Human primary bone
sarcomas contain CD133+ cancer stem cells displaying
high tumorigenicity in vivo. FASEB J. 25:2022–2030. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Zhong XY, Li ZY, Cai JF, Zou L, Li
JM, Yang T and Liu W: CD133 expression in osteosarcoma and
derivation of CD133+ cells. Mol Med Rep. 7:577–584.
2013.
|
|
10
|
Li X, Tian J, Bo Q, Li K, Wang H, Liu T
and Li J: Targeting DNA-PKcs increased anticancer drug sensitivity
by suppressing DNA damage repair in osteosarcoma cell line MG63.
Tumour Biol. 36:9365–9372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian J, Li X, Si M, Liu T and Li J:
CD271+ osteosarcoma cells display stem-like properties.
PLoS One. 9:e985492014. View Article : Google Scholar
|
|
12
|
Choi BH, Kim CG, Lim Y, Shin SY and Lee
YH: Curcumin downregulates the multidrug-resistance mdr1b gene by
inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett.
259:111–118. 2008. View Article : Google Scholar
|
|
13
|
Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe
S, Mills GB and Unate H: Induction of human MDR1 gene expression by
2-acetylaminofluorene is mediated by effectors of the
phosphoinositide 3-kinase pathway that activate NF-kappaB
signaling. Oncogene. 21:1945–1954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooke SL, Ng CK, Melnyk N, Garcia MJ,
Hardcastle T, Temple J, Langdon S, Huntsman D and Brenton JD:
Genomic analysis of genetic heterogeneity and evolution in
high-grade serous ovarian carcinoma. Oncogene. 29:4905–4913. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xing J, Wu X, Vaporciyan AA, Spitz MR and
Gu J: Prognostic significance of ataxia-telangiectasia mutated,
DNA-dependent protein kinase catalytic subunit, and Ku
heterodimeric regulatory complex 86-kD subunit expression in
patients with nonsmall cell lung cancer. Cancer. 112:2756–2764.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willmore E, Elliott SL, Mainou-Fowler T,
Summerfield GP, Jackson GH, O'Neill F, Lowe C, Carter A, Harris R,
Pettitt AR, et al: DNA-dependent protein kinase is a therapeutic
target and an indicator of poor prognosis in B-cell chronic
lymphocytic leukemia. Clin Cancer Res. 14:3984–3992. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang Y, Chai Z, Wang D, Kuang T, Wu W and
Lou W: DNA-PKcs deficiency sensitizes the human hepatoma HepG2
cells to cisplatin and 5-fluorouracil through suppression of the
PI3K/Akt/NF-κB pathway. Mol Cell Biochem. 399:269–278. 2015.
View Article : Google Scholar
|
|
19
|
Ciszewski WM, Tavecchio M, Dastych J and
Curtin NJ: DNA-PK inhibition by NU7441 sensitizes breast cancer
cells to ionizing radiation and doxorubicin. Breast Cancer Res
Treat. 143:47–55. 2014. View Article : Google Scholar
|
|
20
|
Facchino S, Abdouh M, Chatoo W and Bernier
G: BMI1 confers radioresistance to normal and cancerous neural stem
cells through recruitment of the DNA damage response machinery. J
Neurosci. 30:10096–10111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mimeault M, Hauke R and Batra SK: Recent
advances on the molecular mechanisms involved in the drug
resistance of cancer cells and novel targeting therapies. Clin
Pharmacol Ther. 83:673–691. 2008. View Article : Google Scholar
|
|
22
|
Ye S, Zhang J, Shen J, Gao Y, Li Y, Choy
E, Cote G, Harmon D, Mankin H, Gray NS, et al: NVP-TAE684 reverses
multidrug resistance (MDR) in human osteosarcoma by inhibiting
P-glycoprotein (PGP1) function. Br J Pharmacol. 173:613–626. 2016.
View Article : Google Scholar
|
|
23
|
Yang X, Yang P, Shen J, Osaka E, Choy E,
Cote G, Harmon D, Zhang Z, Mankin H, Hornicek FJ, et al: Prevention
of multidrug resistance (MDR) in osteosarcoma by NSC23925. Br J
Cancer. 110:2896–2904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fanelli M, Hattinger CM, Vella S, Tavanti
E, Michelacci F, Gudeman B, Barnett D, Picci P and Serra M:
Targeting ABCB1 and ABCC1 with their specific inhibitor
CBT-1® can overcome drug resistance in osteosarcoma.
Curr Cancer Drug Targets. 16:261–274. 2016. View Article : Google Scholar
|
|
25
|
Martins-Neves SR, Lopes AO, do Carmo A,
Paiva AA, Simões PC, Abrunhosa AJ and Gomes CM: Therapeutic
implications of an enriched cancer stem-like cell population in a
human osteosarcoma cell line. BMC Cancer. 12:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gonçalves C, Martins-Neves SR,
Paiva-Oliveira D, Oliveira VE, Fontes-Ribeiro C and Gomes CM:
Sensitizing osteosarcoma stem cells to doxorubicin-induced
apoptosis through retention of doxorubicin and modulation of
apoptotic-related proteins. Life Sci. 130:47–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Fraser M, Moll UM, Basak A and
Tsang BK: Akt-mediated cisplatin resistance in ovarian cancer:
Modulation of p53 action on caspase-dependent mitochondrial death
pathway. Cancer Res. 66:3126–3136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Molina JR, Hayashi Y, Stephens C and
Georgescu MM: Invasive glioblastoma cells acquire stemness and
increased Akt activation. Neoplasia. 12:453–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wittig-Blaich SM, Kacprzyk LA, Eismann T,
Bewerunge-Hudler M, Kruse P, Winkler E, Strauss WS, Hibst R,
Steiner R, Schrader M, et al: Matrix-dependent regulation of AKT in
Hepsin-overexpressing PC3 prostate cancer cells. Neoplasia.
13:579–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou G and Kuo MT: NF-kappaB-mediated
induction of mdr1b expression by insulin in rat hepatoma cells. J
Biol Chem. 272:15174–15183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun J, Yeung CA, Co NN, Tsang TY, Yau E,
Luo K, Wu P, Wa JC, Fung KP, Kwok TT, et al: Clitocine reversal of
P-glycoprotein associated multi-drug resistance through
down-regulation of transcription factor NF-κB in R-HepG2 cell line.
PLoS One. 7:e407202012. View Article : Google Scholar
|
|
32
|
Zhao BX, Sun YB, Wang SQ, Duan L, Huo QL,
Ren F and Li GF: Grape seed procyanidin reversal of p-glycoprotein
associated multi-drug resistance via down-regulation of NF-κB and
MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS One.
8:e710712013. View Article : Google Scholar
|
|
33
|
Xia YZ, Ni K, Guo C, Zhang C, Geng YD,
Wang ZD, Yang L and Kong LY: Alopecurone B reverses
doxorubicin-resistant human osteosarcoma cell line by inhibiting
P-glycoprotein and NF-kappa B signaling. Phytomedicine. 22:344–351.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nomura A, Banerjee S, Chugh R, Dudeja V,
Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors,
induces epithelial-mesenchymal transition and increases metastasis
in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu Y, Yu J, Wang S, Lu R, Wu J and Jiang
B: Overexpression of CD133 enhances chemoresistance to
5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric
cancer cells. Oncol Rep. 32:2437–2444. 2014.PubMed/NCBI
|
|
36
|
Xi G, Hayes E, Lewis R, Ichi S,
Mania-Farnell B, Shim K, Takao T, Allender E, Mayanil CS and Tomita
T: CD133 and DNA-PK regulate MDR1 via the PI3K- or Akt-NF-κB
pathway in multidrug-resistant glioblastoma cells in vitro.
Oncogene. 35:241–250. 2016. View Article : Google Scholar
|
|
37
|
Stronach EA, Chen M, Maginn EN, Agarwal R,
Mills GB, Wasan H and Gabra H: DNA-PK mediates AKT activation and
apoptosis inhibition in clinically acquired platinum resistance.
Neoplasia. 13:1069–1080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng J, Park J, Cron P, Hess D and
Hemmings BA: Identification of a PKB/Akt hydrophobic motif Ser-473
kinase as DNA-dependent protein kinase. J Biol Chem.
279:41189–41196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rajagopalan S, Moyle MW, Joosten I and
Long EO: DNA-PKcs controls an endosomal signaling pathway for a
proinflammatory response by natural killer cells. Sci Signal.
3:ra142010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leslie NR, Biondi RM and Alessi DR:
Phosphoinositide-regulated kinases and phosphoinositide
phosphatases. Chem Rev. 101:2365–2380. 2001. View Article : Google Scholar : PubMed/NCBI
|