Introduction
Lung cancer remains the leading cancer killer in
developed countries with intricate etiologies and unfavorable
prognosis (1). According to the
statistical results of International Agency for Research on Cancer
(IARC), there were around 1.8 million new lung cancer cases
worldwide in 2012, accounting for 13% of all new cases (2). Surgery, radiotherapy and chemotherapy
are all potential suitable treatment methods for lung cancer
(3). Surgical resection is the most
preferred method for lung cancer treatment and a considerable
proportion of lung cancer patients could be cured by standardized
tumor resection. However, surgical treatment is only suitable for
the early stage (I–II period) or some III stage patients whose
tumors were confined to one side of the chest (4). In addition, surgery, radiotherapy and
chemotherapy can be used alone or together due to different tumor
types or tumor stages. Chemotherapy is most applicable for small
cell lung cancer (SCLC) while non-small cell lung cancer (NSCLC),
the most common type of lung cancer, can be treated with surgery,
chemotherapy, radiotherapy or a combination method depending on
different tumor progression. Despite the health status of lung
cancer patients have been much improved due to advanced surgical
techniques and adjuvant therapy, the overall survival (OS) status
of NSCLC is still unsatisfactory (5,6).
Targeted therapy, referring to design of appropriate treatment
according to definite protein or gene target, was considered as the
most effective treatment method for advanced-stage cancer patients
(7,8). Several lung cancer-related therapeutic
targets have been identified and the targeted drugs including
growth factor receptor inhibitors, angiogenesis inhibitors and
signal transduction inhibitors have been put into use for lung
cancer, especially for NSCLC patients (9–11).
However, the clinical efficacy of these drugs remains to be
validated and there is still an urgent need to investigate novel
molecular targets of NSCLC.
Forkhead box P3 (Foxp3), a key member of forkhead
box transcription factor family, has been considered as a
remarkable modulator of regulatory T cells (Treg) (12,13).
Previous studies had indicated that Foxp3 gene mutation can cause
severe autoimmune diseases (14).
Recently, the relationship between Foxp3 and tumoral diseases has
drawn much attention. Fleskens et al provided initial
evidence for a novel role of Foxp3 as a tumor suppressor in T-cell
acute lymphoblastic leukemia (T-ALL) (15). In breast cancer, Liu et al
revealed that miR-146a/b could induce cell apoptosis and
contributed to Foxp3-mediated tumor suppression during tumor
progress (16). These results
suggested that Foxp3 may function not only as the master regulator
in Tregs, but also as an X-linked tumor suppressor. However,
whether Foxp3 could inhibit the aggressive behavior, especially the
metastasis-associated biological process, of NSCLC is still
elusive. In the present work, we explored the clinical significance
of Foxp3 expression in NSCLC patients, the biological role of Foxp3
in NSCLC cell lines and the potential mechanisms.
Materials and methods
Clinical specimens
The clinical research protocol was approved by the
Ethics Committee of the First Affiliated Hospital of Zhengzhou
University, Zhengzhou University School of Medicine. Specimens for
construction of tissue microarrays were derived from lung cancer
patients who underwent curative resection at the First Affiliated
Hospital of Zhengzhou University, Zhengzhou University School of
Medicine. Inclusion criteria were: resectable lung cancers; without
neoadjuvant chemoradiotherapy; without evidences of primary tumors
of other organs; patients able to be interviewed during the
follow-up. Fresh tissues were cut in wedge shapes and stored in
liquid nitrogen immediately or fixed with formaldehyde for
immunohistochemistry assay. Patients in prognostic group (n=99)
were followed until October 2011, and follow-up range was 1–69
months. OS was defined as interval between surgery and death or
between surgery and the last observation point.
Immunohistochemistry and scoring
A tissue microarray block containing tumor samples
and pared normal tissues was constructed. Immunostaining scores
were independently evaluated by two pathologists who were blinded
to clinical outcome. Integrated optical density (IOD) was counted
and measured by using Image-Pro Plus v6.0 software (Media
Cybernetics, Inc., Bethesda, MD, USA) from three photographs per
specimen.
Scoring was performed by two researchers
independently, and discrepancies were resolved by consensus with an
additional investigator. Intensity of staining was categorized as
0–3 representing negative (−), weak (+), moderate (++), and strong
(+++) staining, respectively. Extent of immunostaining was
categorized into 0 (<10%), 1 (10–25%), 2 (25–50%), 3 (>50%).
The final score of each section was determined by multiplying score
of stained cell numbers with score of staining intensity, ranging
from 0–9, and scores of 0–3 represent low expression level of Foxp3
and 4–9 represent high level. IOD of each photograph was evaluated
by using Image Pro-Plus software according to published literature
(17,18).
Cell culture
Human lung squamous cell carcinoma cell line H520
was purchased from American Type Culture Collection (ATCC;
Manassas, VA, USA) and lung adenocarcinoma cell line A549 was
obtained from the Institute of Biochemistry and Cell Biology,
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). All cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum
(FBS) at 37°C and 5% CO2.
Transient transfection and cell
proliferation assay
For transient transfection assay, cells were seeded
in 6-well culture plates at an appropriate density. Then cells were
transfected with siRNAs or plasmids with Lipofectamine 2000
(Invitrogen, Carslbad, CA, USA) at 60–70% confluence. qRT-PCR and
western blot assay were used to examine transfection efficiency.
Cell function analysis was conducted 48 h after transfection.
Number of viable cells was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays (EZ4U; Biomedica, Vienna, Austria).
RNA isolation and qRT-PCR
Total RNAs were extracted with TRIzol reagent
according to the manufacturer's instructions. cDNA was synthesized
using PrimeScript RT Master Mix (RR036A; Takara, Dalian, China).
The expression level of target genes were detected by real-time
qRT-PCR using SYBR-Green PCR Master Mix (Invitrogen) on the Applied
Biosystems 7900HT sequence detection system. The relative mRNA
levels (fold-change) of the specific genes were analyzed by the
2−ΔΔCt (ΔCt = Cttarget – CtGAPDH;
ΔΔCt = ΔCtexpressing vector − ΔCtcontrol
vector) method. The primers were listed in Table I.
 | Table ISequences of oligonucleotides used in
this study. |
Table I
Sequences of oligonucleotides used in
this study.
| Name | Sequences
(5′–3′) |
|---|
| GAPDH | F:
5′-AGCCACATCGCTCAGACAC-3′ |
| R:
5′-GCCCAATACGACCAAATCC-3′ |
| Foxp3 | F:
5′-GTGGCCCGGATGTGAGAAG-3′ |
| R:
5′-GGAGCCCTTGTCGGATGATG-3′ |
| Vimentin | F:
5′-GCCCTAGACGAACTGGGTC-3′ |
| R:
5′-GGCTGCAACTGCCTAATGAG-3′ |
| ZO-1 | F:
5′-CGGTCCTCTGAGCCTGTAAG-3′ |
| R:
5′-GGATCTACATGCGACGACAA-3′ |
| E-cadherin | F:
5′-CGAGAGCTACACGTTCACGG-3′ |
| R:
5′-GGGTGTCGAGGGAAAAATAGG-3′ |
| Slug | F:
5′-AGCAGTTGCACTGTGATGCC-3′ |
| R:
5′-ACACAGCAGCCAGATTCCTC-3′ |
| N-cadherin | F:
5′-TTTGATGGAGGTCTCCTAACACC-3′ |
| R:
5′-ACGTTTAACACGTTGGAAATGTG-3′ |
| Laminin | F:
5′-AGGAACCCGAGTTCAGCTAC-3′ |
| R:
5′-CACGTCGAGGTCACCGAAAG-3′ |
| Snail | F:
5′-ACTGCGACAAGGAGTACACC-3′ |
| R:
5′-GAGTGCGTTTGCAGATGGG-3′ |
| ZEB-1 | F:
5′-ACCTCTTCACAGGTTGCTCCT-3′ |
| R:
5′-AGTGCAGGAGCTGAGAGTCA-3′ |
| β-catenin | F:
5′-GATTTGATGGAGTTGGACATGG-3′ |
| R:
5′-TGTTCTTGAGTGAAGGACTGAG-3′ |
| LMO2 | F:
5′-GCTCCTTGAAATCGACCAGAA-3′ |
| R:
5′-GCGAGTCTGTTCGGTGATGT-3′ |
| TAL1 | F:
5′-CCCAACGCCAACTGGAGATTT-3′ |
| R:
5′-AGTCGGATGGTCTTCTCAGTC-3′ |
| p53 | F:
5′-ACTTGTCGCTCTTGAAGCTAC-3′ |
| R:
5′-GATGCGGAGAATCTTTGGAACA-3′ |
| CD44 | F:
5′-CTGCCGCTTTGCAGGTGTA-3′ |
| R:
5′-CATTGTGGGCAAGGTGCTATT-3′ |
| RUNX1 | F:
5′-TGAGCTGAGAAATGCTACCGC-3′ |
| R:
5′-ACTTCGACCGACAAACCTGAG-3′ |
Immunoprecipitation (IP) and western blot
assay
For IP assay, 2 µg corresponding antibodies
were immunoprecipitated with cell lysates at 4°C overnight. Then
the IP lysates were incubated with protein A/G agarose (Invitrogen)
for 4 h and centrifuged for western blot assay. For western blot
analysis, RIPA buffer containing PMSF (both from Solarbio, Beijing,
China) and protease inhibitor cocktail (Roche Applied Science,
Mannheim, Germany) were used to extract total tissue and cell
proteins. Western blot analysis was done according to the standard
protocol, with primary antibodies against Foxp3 (Abcam, Cambridge,
MA, USA), ZO-1 and vimentin (both from CST, Beverly, MA, USA), LMO2
(Abcam). The signaling pathway antibodies were purchased from CST.
GAPDH (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) on the
same membrane was used as a loading control.
Cell migration and invasion assay
Transwell with or without Matrigel was employed for
migration and invasion assay, respectively. Briefly, cells were
resuspended and added into the upper chamber of Transwell at a
density of 1×105 cells/well. The upper chamber was added
with 200 µl serum-free DMEM and the lower chamber was added
with 600 µl DMEM containing 10% FBS. After incubation at
37°C for 24 (migration assay) or 48 h (invasion assay), inserts
were taken out and cells attached inside the chambers were rubbed
off softly and stained with 0.1% crystal violet. Then stained cells
of 5 random fields were counted.
Statistical analysis
SPSS 15.0 software was employed for statistical
analysis. The correlation between clinicopathological parameters
with Foxp3 expression was examined by Pearson's correlation
analysis. The effect of Foxp3 on survival was estimated by
Kaplan-Meier curve and log-rank test. Student's t-test was used to
analyze differences between two groups and one-way ANOVA was
employed for analyzing data more than two groups. p<0.05 was
considered statistically significant.
Results
Downregulated expression of Foxp3
predicts adverse outcomes for NSCLC patients
To clarify the role of Foxp3 in NSCLC, we first
evaluated the level of Foxp3 in 74 paired human NSCLC tissues and
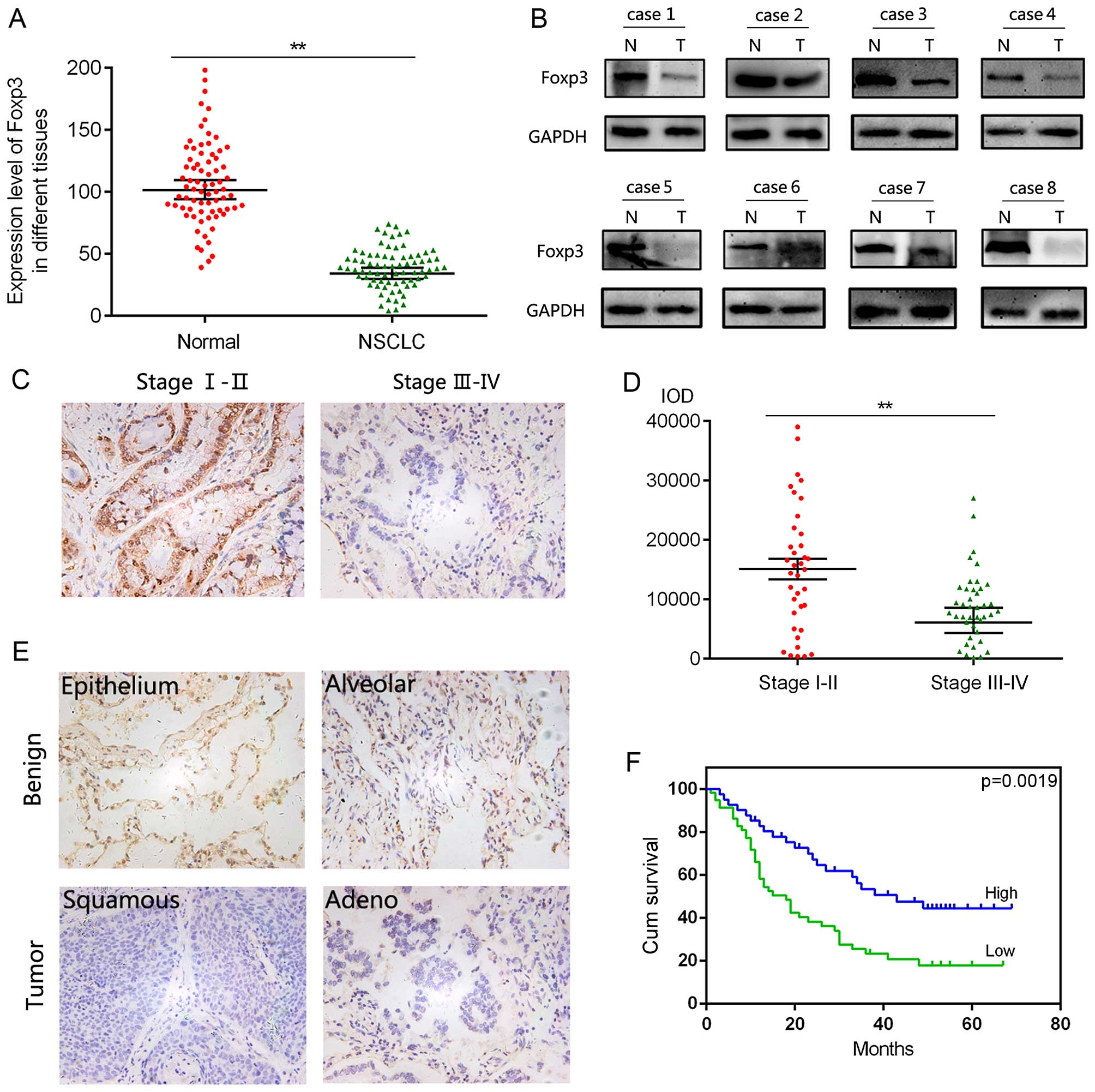
corresponding non-tumoral tissues. As shown in Fig. 1A, the mRNA level of Foxp3 was
significantly downregulated in NSCLC tissues compared with
non-tumoral tissues, which was confirmed by the result of western
blot analysis in Fig. 1B. Next, a
tissue microarray was employed to explore the relationship between
Foxp3 expression and clinicopathological parameters of NSCLC
patients. Foxp3 expression was significantly decreased in higher
stage NSCLC patients (n=41) compared with lower stage samples
(n=37) (Fig. 1C and D), suggesting
the role of Foxp3 in tumor progress. Additionally, as shown
Fig. 1E in and calculated in
Fig. 1F, patients with low
expression of Foxp3 yielded worse OS than those with high
expression of Foxp3. These data indicated that low expression of
Foxp3 predicted adverse outcomes of NSCLC patients.
Foxp3 regulates proliferation, migration
and invasion ability of NSCLC cells
We next explored the biological roles of Foxp3 in a
lung squamous cell carcinoma cell line H520 and a lung
adenocarcinoma cell line A549. Two commercialized siRNAs were
employed for silencing of FOXP3 in H520 and A549 cells. As
indicated in Fig. 2A, both siRNAs
downregulated the expression of Foxp3 effectively and siRNA#2
yielded a more effective silencing efficiency. We found that the
proliferation ability of A549 cells was enhanced after silencing of
FOXP3 (Fig. 2B) and observed that
the migrated cells through Transwell were significantly increased
after silencing of FOXP3 (Fig. 2C).
In addition, silencing of FOXP3 promoted the proliferation,
migration and invasion ability of H520 cells (Fig. 2D–F). To confirm the biological
results of Foxp3 inhibition, Foxp3 overexpression plasmid
pcDNA3.1-Foxp3 and the control plasmid were transiently transfected
into A549 cells. The transfection efficiency was examined by
western blot analysis and pcDNA3.1-Foxp3 group yield a restoration
effect of Foxp3 (Fig. 3A). The MTT
assay indicated that Foxp3 restoration undermined the proliferation
ability of A549 cells and the Transwell analysis suggested that
overexpression of Foxp3 inhibited the migration and invasion
ability of A549 cells (Fig. 3B and
C). These results indicated that Foxp3 could regulate
proliferation, migration and invasion ability of NSCLC cells.
Foxp3 regulates epithelial-mesenchymal
transition (EMT) of NSCLC cells
To clarify the preliminary mechanism of Foxp3
mediated biological behavior, we next detected the expression level
of EMT makers involved in the self-renewal ability, especially in
migration and invasion process. EMT markers including vimentin,
ZO-1, E-cadherin, Slug, N-cadherin, laminin, Snail, ZEB-1 and
β-catenin were chosen for analysis according to published
literature. Furthermore, by screening Foxp3-related literature, we
selected 5 most relevant genes involved in Foxp3-mediated diseases,
especially cancer-related diseases. By examining the mRNA level of
these genes, We observed a downregulation of epithelial marker ZO-1
and an upregulation of mesenchymal marker vimentin after Foxp3
downregulation (Fig. 3D), which
were confirmed by the results of western blot analysis (Fig. 3E). Additionally, the mRNA level of
LMO2 was decreased while the level of TAL1 was increased after
silencing of FOXP3 (Fig. 3D), which
was accompanied with the expression change of the two genes induced
by Foxp3 downregulation in T-ALL.
Foxp3 regulates NF-κB signaling in NSCLC
cells
We next explored the mechanisms of Foxp3 regulation
of EMT in NSCLC cells. In a recently published study (15), Foxp3 functioned as a tumor
suppressor of T-ALL modulating TAL1 transcriptional activity
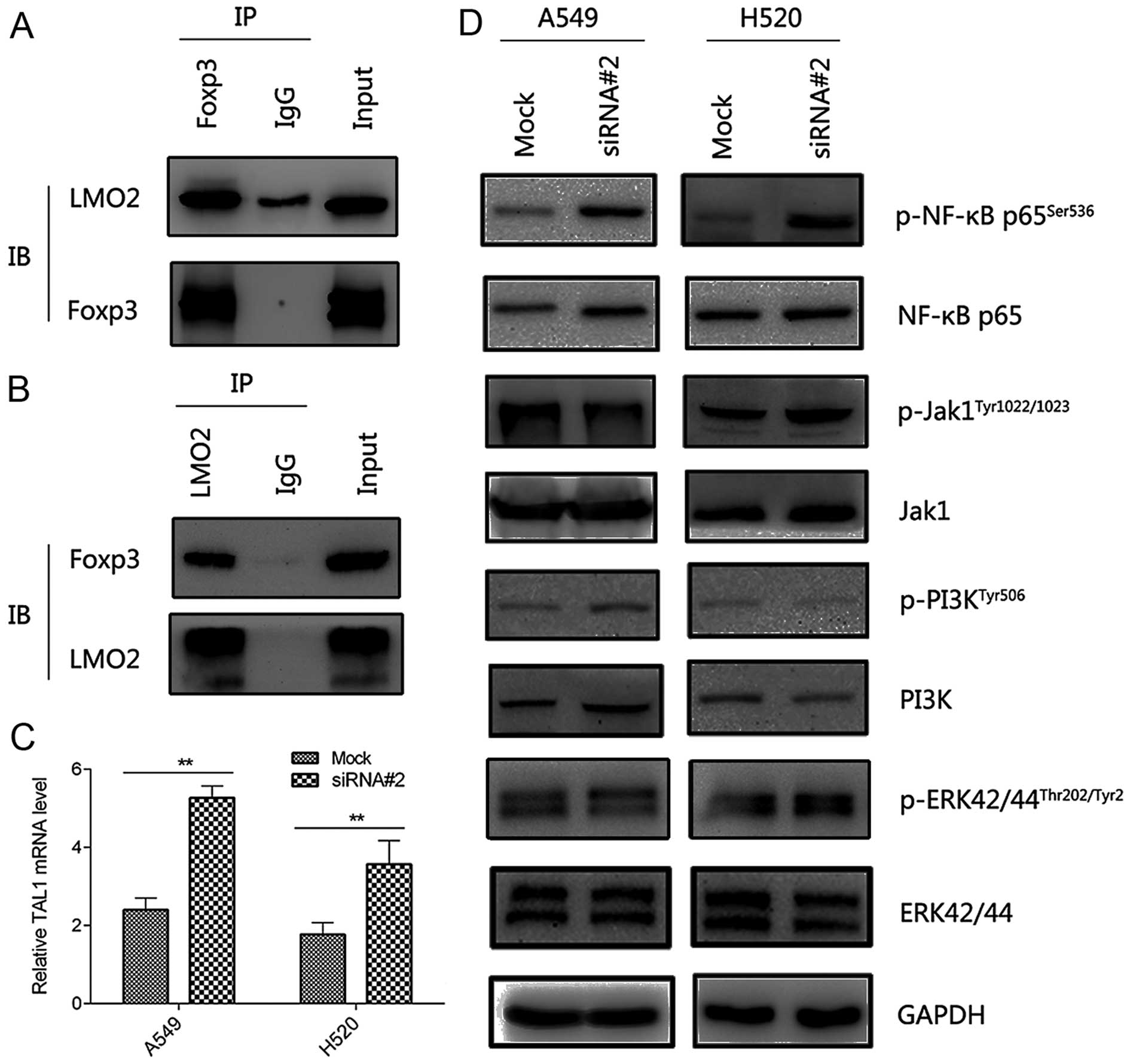
through interaction with LMO2. We verified the endogenous
interaction between LMO2 and Foxp3 by co-IP (Fig. 4A and B). Additionally, the mRNA
level of TAL1 was significantly upregulated after silencing of
FOXP3 (Fig. 4C), suggesting a
similar mechanism through which Foxp3 regulated biological behavior
of NSCLC cells. The in-depth mechanisms of these results needed to
be confirmed by further studies.
Considering the different pathogenesis and molecular
mechanisms between T-ALL and NSCLC, we determined to explore novel
mechanisms on Foxp3-mediated EMT. Published literature has revealed
that ERK, PI3K, Jak and NF-κB signaling pathways were all potential
regulatory factors involved in EMT (19–22).
In the present study, we detected the expression level of ERK,
PI3K, Jak, NF-κB and their corresponding phosphorylated proteins.
As the result, silencing of FOXP3 increased the phospho-NF-κB level
in both cell lines while higher p-ERK42/44Thr202/Tyr2
was detected in only one cell line (Fig. 4D). These results suggested that
Foxp3 could regulate EMT, at least partially, via NF-κB
pathway.
Discussion
Foxp3 gene localized at X chromosome and
three domains of Foxp3 protein have been identified so far: an
N-terminal C2H2 Zinc finger domain, a leucine zipper motif and a
conserved C-terminal forkhead DNA-binding region (FKH) (23,24).
Although high Foxp3 levels are initially detected in Tregs, the
expression of Foxp3 is not restricted to Tregs. Recent studies
indicated that Foxp3 was also expressed in breast, lung, prostate
and gastric cancer tissue (25–28).
Furthermore, the clinical roles and biological function of Foxp3
have been thoroughly studied in T-ALL and breast cancer (15,27).
These results revealed Foxp3 could induce tumor suppressor
phenotypes in human cancer. Although a variety of Foxp3-related
signaling pathways had been identified in Treg cells, the
mechanisms of Foxp3-assiociated cancers were not entirely
clear.
In the present study, we first evaluated the
clinical significance of Foxp3 expression in NSCLC patients. The
results of the qRT-PCR and western blot analysis showed that Foxp3
was decreased in NSCLC tissues compared with non-tumoral tissues.
In addition, a tissue microarray block was constructed for
evaluating the relationship between Foxp3 expression and the
clinicopathological parameters of NSCLC patients. As the result,
lower stage NSCLC samples tended to have higher Foxp3 expression
compared with higher stage patients. Higher Foxp3 expression
predicted shorter OS in NSCLC patients. These results indicated
that high expression level of Foxp3 predicted adverse outcomes of
NSCLC patients.
The role of Foxp3 in NSCLC cell proliferation has
not been thoroughly discussed before. In our study, we found that
transfection of targeted siRNA into A549 and H520 cells promoted
cell proliferation while Foxp3 re-expression in A549 cells
inhibited cell proliferation, suggesting potential
tumor-suppressive role of Foxp3. EMT refers to a complex biological
process during which polarized epithelial cells connected to the
basement membrane obtained the phenotypes of mesenchymal cells
(29,30). These phenotypes include enhanced
migration, invasion, anti-apoptotic ability, as well as a
significant increase of the extracellular matrix (30). In the microscopic view, the essence
of EMT process is the decrease of epithelial markers including
E-cadherin, laminin and the increase of representative mesenchymal
markers including Snail, Slug, N-cadherin and vimentin (30,31).
Recent evidences indicated that the EMT process was involved in the
development of pulmonary adenocarcinoma and squamous cell carcinoma
(32,33). In the present study, EMT-related
aggressive behavior including migration and invasion were promoted
after Foxp3 downregulation while these phenotypes were inhibited by
Foxp3 restoration. Additionally, the mRNA and protein levels of
epithelial marker ZO-1 was downregulated while the levels of
mesenchymal marker vimentin was upregulated in A549/siRNA#2 cells
compared with A549/vector cells. These results indicated that Foxp3
was an upstream regulator of EMT process in NSCLC cells. However,
the definite role of Foxp3 in EMT regulation requires further
study.
We next explored the mechanisms of Foxp3 regulation
of the EMT process. Recent literature revealed that Foxp3 could
modulate TAL1 transcriptional activity through interaction with
LMO2 in T-ALL (15). In the present
study, we found Foxp3 could interacted with LMO2 by co-IP assay,
furthermore, we observed that the mRNA level of TAL1 was
upregulated in both cell lines after Foxp3 downregulation. These
results indicated that Foxp3 could interact with LMO2 and affect
the expression level of TAL1, suggesting a potential mechanism of
Foxp3-mediated EMT. However, these results still needed to be
confirmed and further studied due to the different pathogenesis and
molecular mechanisms between NSCLC and T-ALL. By screening ERK,
PI3K, Jak and NF-κB signaling pathways, we found that protein level
of p-ERK42/44Thr202/Tyr2 was increased in squamous cell
carcinoma cell line H520 while no significance of
p-ERK42/44Thr202/Tyr2 level was observed in
adenocarcinoma cell line A549. These results indicated the
different molecular mechanisms between lung squamous cell carcinoma
and adenocarcinoma, which still needed further investigation.
Interestingly, we observed that silencing of FOXP3 increased the
phospho-NF-κB level in both cell lines, which was accompanied by
the relationship between NF-κB pathway and EMT process in breast
cancer, pancreatic cancer, gastric cancer and other cancer types
(22,34,35).
In conclusion, we identified the tumor suppressive
role of Foxp3 in NSCLC. Low expression level Foxp3 predicted
adverse outcomes of NSCLC patients. Foxp3 could regulate the EMT
process of NSCLC, at least partially, via NF-κB pathway. This study
provided novel insights into the role of FOXP3 in NSCLC and
expanded the growing understanding of NSCLC biology.
Acknowledgments
This study was supported by the Youth Innovation
Fund of the First Affiliated Hospital of Zhengzhou University.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
4
|
Howington JA, Blum MG, Chang AC, Balekian
AA and Murthy SC: Treatment of stage I and II non-small cell lung
cancer: Diagnosis and management of lung cancer, 3rd ed: American
College of Chest Physicians evidence-based clinical practice
guidelines. Chest. 143(Suppl 5): e278S–e313S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue A, Kobayashi K, Maemondo M, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al North-East Japan Study Group: Updated overall survival
results from a randomized phase III trial comparing gefitinib with
carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer
with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 24:54–59.
2013. View Article : Google Scholar
|
|
6
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawyers C: Targeted cancer therapy.
Nature. 432:294–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, et al: Efficacy of gefitinib, an inhibitor of the
epidermal growth factor receptor tyrosine kinase, in symptomatic
patients with non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Janku F, Stewart DJ and Kurzrock R:
Targeted therapy in non-small-cell lung cancer - is it becoming a
reality? Nat Rev Clin Oncol. 7:401–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antonicelli A, Cafarotti S, Indini A,
Galli A, Russo A, Cesario A, Lococo FM, Russo P, Mainini AF,
Bonifati LG, et al: EGFR-targeted therapy for non-small cell lung
cancer: Focus on EGFR oncogenic mutation. Int J Med Sci.
10:320–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arvey A, van der Veeken J, Samstein RM,
Feng Y, Stamatoyannopoulos JA and Rudensky AY: Inflammation-induced
repression of chromatin bound by the transcription factor Foxp3 in
regulatory T cells. Nat Immunol. 15:580–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Samstein RM, Arvey A, Josefowicz SZ, Peng
X, Reynolds A, Sandstrom R, Neph S, Sabo P, Kim JM, Liao W, et al:
Foxp3 exploits a pre-existent enhancer landscape for regulatory T
cell lineage specification. Cell. 151:153–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katoh H, Zheng P and Liu Y: FOXP3: Genetic
and epigenetic implications for autoimmunity. J Autoimmun.
41:72–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fleskens V, Mokry M, van der Leun AM,
Huppelschoten S, Pals CE, Peeters J, Coenen S, Cardoso BA, Barata
JT, van Loosdregt J, et al: FOXP3 can modulate TAL1 transcriptional
activity through interaction with LMO2. Oncogene. Dec 21–2015.Epub
ahead of print. PubMed/NCBI
|
|
16
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y, et al: Foxp3 controls an
miR-146/NF-κB negative feedback loop that inhibits apoptosis in
breast cancer cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao D, Long XD, Lu TF, Wang T, Zhang WW,
Liu YX, Cui XL, Dai HJ, Xue F and Xia Q: Metformin decreases IL-22
secretion to suppress tumor growth in an orthotopic mouse model of
hepatocellular carcinoma. Int J Cancer. 136:2556–2565. 2015.
View Article : Google Scholar
|
|
18
|
Luo Q, Zhang Y, Wang N, Jin G, Jin H, Gu
D, Tao X, Huo X, Ge T, Cong W, et al: Leukemia inhibitory factor
receptor is a novel immunomarker in distinction of
well-differentiated HCC from dysplastic nodules. Oncotarget.
6:6989–6999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suh Y, Yoon CH, Kim RK, Lim EJ, Oh YS,
Hwang SG, An S, Yoon G, Gye MC, Yi JM, et al: Claudin-1 induces
epithelial-mesenchymal transition through activation of the
c-Abl-ERK signaling pathway in human liver cells. Oncogene.
32:4873–4882. 2013. View Article : Google Scholar
|
|
20
|
Schlegel NC, von Planta A, Widmer DS,
Dummer R and Christofori G: PI3K signalling is required for a
TGFβ-induced epithelial-mesenchymal-like transition (EMT-like) in
human melanoma cells. Exp Dermatol. 24:22–28. 2015. View Article : Google Scholar
|
|
21
|
Liu R-Y, Zeng Y, Lei Z, Wang L, Yang H,
Liu Z, Zhao J and Zhang HT: JAK/STAT3 signaling is required for
TGF-β-induced epithelial-mesenchymal transition in lung cancer
cells. Int J Oncol. 44:1643–1651. 2014.PubMed/NCBI
|
|
22
|
Li C-W, Xia W, Huo L, Lim SO, Wu Y, Hsu
JL, Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bennett CL, Christie J, Ramsdell F,
Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT,
Chance PF and Ochs HD: The immune dysregulation,
polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused
by mutations of FOXP3. Nat Genet. 27:20–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perrone G, Ruffini PA, Catalano V, Spino
C, Santini D, Muretto P, Spoto C, Zingaretti C, Sisti V,
Alessandroni P, et al: Intratumoural FOXP3-positive regulatory T
cells are associated with adverse prognosis in radically resected
gastric cancer. Eur J Cancer. 44:1875–1882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Merlo A, Casalini P, Carcangiu ML,
Malventano C, Triulzi T, Mènard S, Tagliabue E and Balsari A: FOXP3
expression and overall survival in breast cancer. J Clin Oncol.
27:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Wang L, Katoh H, Liu R, Zheng P and
Liu Y: Identification of a tumor suppressor relay between the FOXP3
and the Hippo pathways in breast and prostate cancers. Cancer Res.
71:2162–2171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Liu R, Li W, Chen C, Katoh H, Chen
GY, McNally B, Lin L, Zhou P, Zuo T, et al: Somatic single hits
inactivate the X-linked tumor suppressor FOXP3 in the prostate.
Cancer Cell. 16:336–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zavadil J, Haley J, Kalluri R, Muthuswamy
SK and Thompson E: Epithelial-mesenchymal transition. Cancer Res.
68:9574–9577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cañadas I, Rojo F, Taus Á, Arpí O,
Arumí-Uría M, Pijuan L, Menéndez S, Zazo S, Dómine M, Salido M, et
al: Targeting epithelial-to-mesenchymal transition with Met
inhibitors reverts chemoresistance in small cell lung cancer. Clin
Cancer Res. 20:938–950. 2014. View Article : Google Scholar
|
|
33
|
Serresi M, Gargiulo G, Proost N, Siteur B,
Cesaroni M, Koppens M, Xie H, Sutherland KD, Hulsman D, Citterio E,
et al: Polycomb repressive complex 2 is a barrier to KRAS-driven
inflammation and epithelial-mesenchymal transition in non-small
cell lung cancer. Cancer Cell. 29:17–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng Z-X, Wang D-W, Liu T, Liu WX, Xia
WB, Xu J, Zhang YH, Qu YK, Guo LQ, Ding L, et al: Effects of the
HIF-1α and NF-κB loop on epithelial-mesenchymal transition and
chemoresistance induced by hypoxia in pancreatic cancer cells.
Oncol Rep. 31:1891–1898. 2014.PubMed/NCBI
|
|
35
|
Li J, Deng Z, Wang Z, Wang D, Zhang L, Su
Q, Lai Y, Li B, Luo Z, Chen X, et al: Zipper-interacting protein
kinase promotes epithelial-mesenchymal transition, invasion and
metastasis through AKT and NF-κB signaling and is associated with
metastasis and poor prognosis in gastric cancer patients.
Oncotarget. 6:8323–8338. 2015. View Article : Google Scholar : PubMed/NCBI
|