Introduction
As the fourth leading cause of cancer death
worldwide, pancreatic cancer is an aggressive malignant and lethal
disease with a 5-year survival rate of less than 5% (1). Although the incidence of most other
cancers have been declining, the rate of incidence for pancreatic
cancer continues to increase by 1.5% per year (1). Surgery is the selected treatment
option; however, it is only applicable to less than 20% of
pancreatic cancer patients because of the late presentation and
rapid progression of pancreatic tumors (2). Current treatments for inoperable
patients are still limited to chemotherapy, radiation or both
(3). Thus, more constructive and
effective therapeutic strategies are urgently needed. In recent
years, a variety of studies have shown that many natural agents are
able to inhibit the progression of pancreatic cancer, but the
underlying mechanisms of these agents are still not fully
understood (4,5).
Curcumin (diferuloylmethane), a natural polyphenol
present in turmeric, has many biological effects, including
anti-infectious, anti-inflammatory, and antioxidant, as well as
chemopreventive activities (6).
More recently, curcumin has also been found to possess anticancer
properties that affect a variety of biological pathways involved in
tumorigenesis, cell cycle regulation, apoptosis, angiogenesis,
immune activity regulation and metastasis (7). Extensive studies have verified that
curcumin is involved in the regulation of multiple cellular
signaling pathways, including the mitogen-activated protein kinase
(MAPK), nuclear factor-κB (NF-κB), Akt, Wnt/β-catenin and other
pathways (6).
Reactive oxygen species (ROS) generated by the
mitochondrial respiratory chain are a number of chemically reactive
molecules derived from oxygen, such as superoxide anion, hydrogen
peroxide (H2O2) and others. Accumulating
evidence indicates that the intracellular redox state plays an
important role in cellular signaling transduction and regulates
multiple events (8). On one hand,
an excessive amount of ROS production can kill cancer cells,
whereas sublethal concentrations of ROS can stimulate tumor
progression by promoting cell proliferation, survival, invasion and
metastasis (9). Additionally, our
previous study showed that H2O2 (0–200
µM) could promote pancreatic cancer progression in a
dose-dependent manner, while H2O2 was
cytotoxic at concentrations above 200 µM (10). A recent study showed that curcumin
possesses a protective effect against the epithelial-mesenchymal
transition (EMT) process in the prostate tumor-stromal interaction,
which is dependent on its ability to ameliorate cancer-associated
fibroblast-induced ROS production through the MAOA/mTOR/HIF-1α
signaling pathway (11).
The MAPK signaling pathways are important signaling
cascades downstream of ROS that are involved in tumor progression
(12). Members of the MAPK family
include extracellular signal-regulated kinase (ERK), c-jun NH-2
terminal kinase (JNK) and p38 MAPK. Our previous study demonstrated
that a moderate amount of H2O2 is able to
promote pancreatic cancer invasion via the activation of the ERK
and p38 MAPK signaling pathways (10). However, the ability of curcumin to
inhibit the H2O2-induced progression of
pancreatic cancer and the mechanisms related to this process have
not been elucidated.
In the present study, we tested the hypothesis that
curcumin is able to inhibit pancreatic cancer cell proliferation
and suppress the H2O2-induced invasion and
migration of pancreatic cancer cells. We also investigated the
effect of curcumin on H2O2-induced activation
of the ERK/NF-κB signaling pathway. The results from this study
suggest that curcumin may be a novel therapy option for pancreatic
cancer via the inhibition of the ERK/NF-κB signaling pathway.
Materials and methods
Cell culture and reagents
The human pancreatic cancer cell lines BxPC-3 and
Panc-1 were obtained from the American Type Culture Collection
(ATTC; Manassas, VA, USA). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% dialyzed
heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and
100 µg/ml streptomycin in a humidified atmosphere of 5%
CO2 at 37°C. DMEM and FBS were purchased from Gibco
(Grand Island, NY, USA). Curcumin, N-acetylcysteine (NAC) and MTT
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Millicell
Transwells used in the invasion assays were obtained from Millipore
Corp. (Billerica, MA, USA). Matrigel was purchased from BD
Biosciences (Bedford, MA, USA). The primary antibodies against
(MMP)-2 and MMP-9 were from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The anti-ERK, anti-phospho-ERK (Thr202/Tyr204),
anti-NF-κB and anti-phospho-NF-κB (Ser468) antibodies were obtained
from Cell Signaling Technology, Inc. (Beverly, MA, USA). The
nitrocellulose membranes used were from Millipore Corp. The BCA
assay kit and the chemiluminescence kit were bought from Pierce
(Rockford, IL, USA). Other reagents were purchased from common
commercial sources. All drug solutions were freshly prepared on the
day of testing.
MTT assay
BxPC-3 and Panc-1 cells were seeded in 96-well
plates at a density of 1×104 cells/well and incubated
overnight in 10% FBS medium. The cells were then treated with
curcumin (0, 5, 10, 20 and 40 µM). After incubation for 24,
48 and 72 h at 37°C, 15 µl of the MTT solution [5 mg/ml in
phosphate-buffered saline (PBS)] was added to each well, and the
cells were incubated for 4 h at 37°C. Then, 100 µl of DMSO
were added to each well. The optical density (OD) value at 490 nm
was determined using a spectrophotometer (Bio-Rad, Berkeley, CA,
USA). The results are presented as the percentage relative to the
control. The proliferation inhibition rate was calculated as (1 −
ODsample/ODcontrol) × 100%.
Measurement of intracellular ROS
The level of intracellular ROS was measured using a
ROS assay kit. In brief, the cells were incubated with
2,7-dichlorodihydrofluorecein diacetate (DCFDA) for 30 min and
washed with PBS three times, and their fluorescence intensity was
measured using a fluorometer (Becton-Dickinson, San Diego, CA, USA)
with an excitation of 488 nm and an emission of 525 nm.
Wound healing assay
The migratory ability of the cells was detected by a
wound healing assay. Pancreatic cancer cells were seeded in 24-well
plates (1.0×105 cells/500 µl). After the cells
grew to 90–100% confluency, a sterile pipette tip was used to
produce a wound line between the cells. The cellular debris was
removed by washing with PBS, and then, the cells were allowed to
migrate for 24 h. Images were taken at 0 and 24 h post-wounding
using a Nikon Diaphot TMD inverted microscope (magnification, ×10).
The relative distance traveled by the leading edge from 0 to 24 h
was assessed using the Photoshop software (n=5).
Transwell Matrigel invasion assay
The invasion of pancreatic cancer cells was tested
using Transwell chambers. The 8.0-µm pore inserts were
coated with 25 µl of Matrigel. The cell suspensions
(5×104) were added to the upper chambers containing DMEM
with 1% FBS. Simultaneously, 500 ml of DMEM containing 20% FBS was
placed in the lower chambers. The cells were allowed to migrate for
48 h at 37°C. The non-invading cells were removed from the upper
surface by a wet cotton swab. After rinsing with PBS, the filter
was fixed and stained with crystal violet. The invasion ability was
determined by counting the stained cells on the bottom surface.
Three random fields were captured at ×20 magnification (n=3).
Real-time quantitative PCR (qT-PCR)
The total RNA was extracted from the pancreatic
cancer cells using the Fastgen 200 RNA isolation system (Fastgen,
Shanghai, China) according to the manufacturer's instructions. The
total RNA was reverse-transcribed into cDNA using a PrimeScript RT
reagent kit (Takara, Dalian, China). The primer sequences were as
follows: MMP-2 forward, 5′-GAT GAT GCC TTT GCT CGT GC-3′ and
reverse, 5′-CAA AGG GGT ATC CAT CGC CA-3′; MMP-9 forward, 5′-TGG
TCC TGG TGC TCC TGG TG-3′ and reverse, 5′-GCT GCC TGT CGG TGAG ATT
GG-3′; β-actin forward, 5′-GAC TTA GTT GCG TTA CAC CCT TTC T-3′ and
reverse, 5′-GAA CGG TGA AGG TGA CAG CAG T-3′. The PCR reactions
were subjected to a thermocycler program consisting of 30 sec at
95°C followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec and
72°C for 30 sec. For each qT-PCR data set, dissociation curve
analysis was conducted. The relative gene expression was calculated
using the 2−ΔΔCt method as previously reported (13).
Western blotting
The proteins in the samples were electrophoretically
resolved on a denaturing SDS-polyacrylamide gel and
electrotransferred onto nitrocellulose membranes. The membranes
were initially blocked with 5% nonfat dry milk in Tris-buffered
saline (TBS) for 2 h and then probed with each primary antibody.
After co-incubation with the primary antibodies at 4°C overnight,
the membranes were blotted with the secondary antibody for 2 h at
37°C. The results were visualized using an ECL western blotting
substrate and photographed using GeneBox (Syngene, Frederick, MD,
USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS Inc., Chicago, IL, USA). The data are
presented as the means ± SEM of three replicate assays. Differences
between the groups were assessed by an analysis of variance
(ANOVA). Statistical significance was set at P<0.05. All
experiments were repeated independently at least three times.
Results
Effect of curcumin on the proliferation
of pancreatic cancer cells
In a previous study, curcumin (Fig. 1A) was shown to possess
anti-proliferative activity against many tumor types (6). To investigate the cytotoxicity of
curcumin on pancreatic cancer cells, BxPC-3 and Panc-1 cells were
treated with curcumin at various concentrations (5, 10, 20 or 40
µM) for 24, 48 and 72 h. The results demonstrated that the
proliferative abilities of both BxPC-3 and Panc-1 cells decreased
in response to the treatment of curcumin in a time- and
dose-dependent manner. Curcumin showed a 50% inhibitory
concentration (IC50) of approximately 20 µM, and
this concentration exhibited no cytotoxic effects in BxPC-3 or
Panc-1 cells (Fig. 1B). Therefore,
the treatment concentrations of 5, 10 and 20 µM of curcumin
were used for the subsequent experiments.
Curcumin attenuates
H2O2-induced oxidative stress in pancreatic
cancer cells
Accumulating evidence indicates that ROS may play
dual roles in cancer in a dose-dependent manner (9). Additionally, our previous study
demonstrated that H2O2 could induce
pancreatic cancer cell proliferation in a dose-dependent manner
from 0 to 200 µM, while H2O2 was
cytotoxic at concentrations above 200 µM (10). Therefore, 200 µM of
H2O2 was used to treat the cells in the
current experiments.
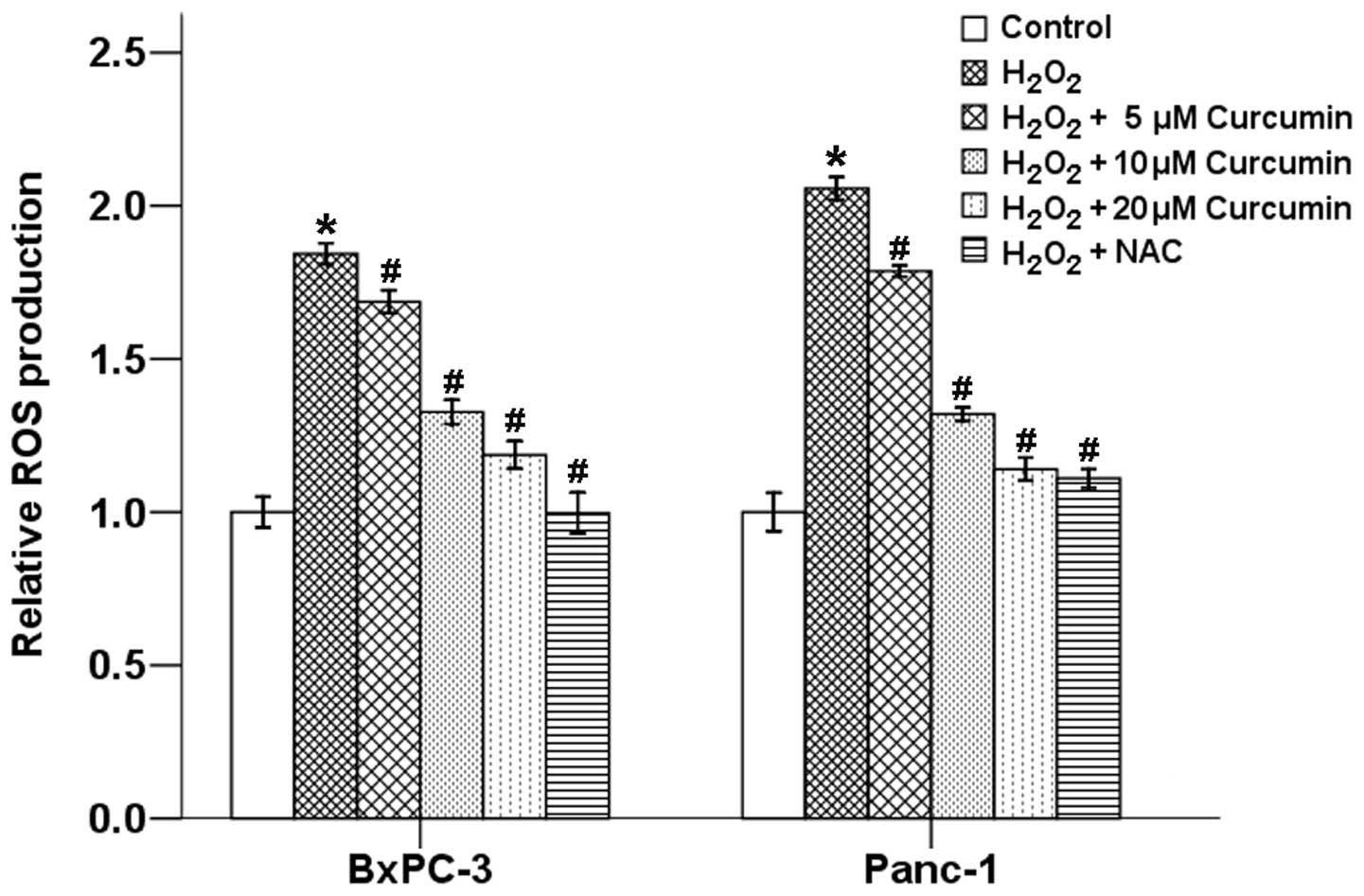
To explore the possible relationship between
curcumin and oxidative stress, we first examined the effects of
curcumin on H2O2-induced ROS production in
BxPC-3 and Panc-1 cells using the cell-permeable and
redox-sensitive compound DCFDA by flow cytometry. Our results
showed that H2O2 significantly increased
intracellular levels of ROS, while curcumin suppressed these
effects in a dose-dependent manner. NAC, a scavenger of free
radicals, also efficiently reduced the
H2O2-induced ROS level in both BxPC-3 and
Panc-1 cells (Fig. 2).
Curcumin inhibits the
H2O2-induced invasion and migration of
pancreatic cancer cells
A vital step of cancer metastasis is the invasion of
cancer cells through the basement membrane. To examine the
potential anti-invasive effects of curcumin, the invasion ability
of BxPC-3 and Panc-1 cells treated with curcumin were analyzed. As
shown in Fig. 3,
H2O2 exposure significantly increased the
invasive ability of pancreatic cancer cells, but the average cell
number that invaded into the lower chamber decreased as the
curcumin concentration increased from 5 to 20 µM. NAC also
efficiently reduced the H2O2-induced invasive
ability of the cancer cells.
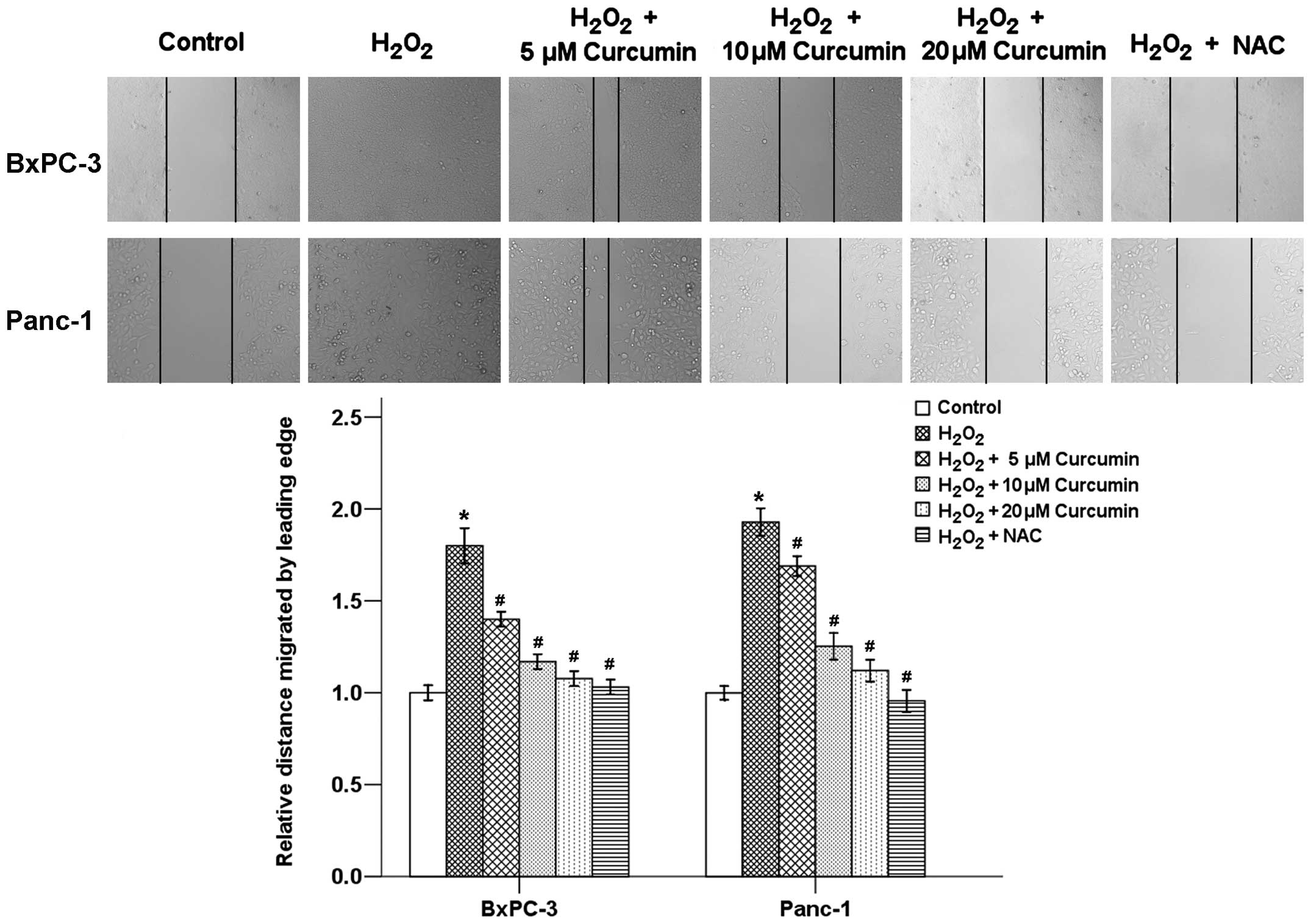
The effect of curcumin on pancreatic cancer cell
motility was determined using a wound healing assay. Our results
showed that H2O2 exposure for 24 h caused a
significant increase in the migration of both BxPC-3 and Panc-1
cells, whereas the cells treated with NAC and different
concentrations (5, 10 and 20 µM) of curcumin showed
dose-related delays in wound closure (Fig. 4). These findings indicate that
curcumin may be an effective inhibitor of
H2O2-induced migration and invasion of
pancreatic cancer cells.
Curcumin downregulates the
H2O2-induced expression of MMP-2 and MMP-9 in
pancreatic cancer cells
Matrix metalloproteinases (MMPs) are zinc-dependent
endopeptidases that degrade extracellular matrix components.
Specifically, MMP-9 and MMP-2 are thought to facilitate cancer
invasion and metastasis. As shown in Fig. 5, H2O2 exposure
significantly increased the mRNA and protein expression levels of
MMP-2 and MMP-9 in both BxPC-3 and Panc-1 cancer cells, while
curcumin counterbalanced these effects of
H2O2. The H2O2-induced
expression of MMP-2 and MMP-9 could also be downregulated by NAC
treatment.
Curcumin downregulates the activation of
the ERK/NF-κB signaling pathway
The MAPK signaling pathways are important signaling
cascades downstream of ROS that are involved in tumor migration and
invasion (12). Our previous study
showed that exposing BxPC-3 and Panc-1 cells to
H2O2 for 15 min caused a significant increase
in the phosphorylation level of ERK (10). In this study, we observed that the
H2O2-induced level of p-ERK was inhibited
after a 24-h treatment with curcumin or NAC without affecting its
total expression. In addition, the
H2O2-induced phosphorylation of NF-κB also
strongly decreased with the addition of curcumin and NAC (Fig. 6A). Moreover, the ERK inhibitor PD
98059, could inhibit the expression of p-ERK and p-NF-κB,
indicating that the NF-κB transcription factor is modulated by the
ERK pathway (Fig. 6B). Taken
together, our results demonstrate that curcumin inhibits
H2O2-induced cancer progression via the
suppression of the ERK/NF-κB signaling pathway in both BxPC-3 and
Panc-1 cells.
Discussion
The potential antioxidant and anticancer effects of
curcumin and its analogues have been extensively studied over the
last three to four decades. Curcumin has been found to suppress the
initiation, progression and metastasis of a variety of tumors
(14,15). It has been reported that curcumin
can inhibit pancreatic cancer cellular proliferation and upregulate
the extrinsic apoptotic pathway through the activation of
caspase-3, caspase-8, Bid, and Bax and the downregulation of NF-κB
and Bcl-2 genes (16). Curcumin is
also able to inhibit lipopolysaccharide-induced EMT through the
downregulation of NF-κB-Snail signaling in breast cancer cells
(17). Curcumin and its analogues
(UBS109, EF31) have been found to inhibit multiple angiogenic
pathways and suppress tumor angiogenesis (18). Toden et al (19) recently demonstrated that
curcumin-mediated sensitization to 5-FU in colorectal cancer cells
occurs through the suppression of EMT and the polycomb repressive
complex, which are regulated by miRNAs. In addition, curcumin can
also mediate several immune processes and help restore anticancer
activities, such as the restoration of the
CD4+/CD8+ T cell populations, the reduction
of the Treg cell population and the suppression of T cell apoptosis
(7,20.21). Our study demonstrated that curcumin could effectively
inhibit the H2O2-induced invasive and
migratory abilities of pancreatic cancer cells.
Accumulating evidence indicates that curcumin
inhibits tumor progression via multiple cellular signaling
pathways. Kim et al (22)
showed that curcumin inhibits TPA-induced invasion by reducing
MMP-9 activation, mainly through the PKCα, MAPK and NF-κB/AP-1
pathways, in human breast cancer cells. Curcumin can also reverse
the transforming growth factor-β1 (TGF-β1)-induced EMT of
pancreatic cancer cells by inhibiting the Hedgehog signaling
pathway (23). Shakibaei et
al (24) showed that combining
curcumin with conventional chemotherapeutic agents, such as 5-FU,
could provide more effective treatment against chemo-resistant
colon cancer cells, which may be mediated by the NF-κB/PI-3K/Src
pathways. In addition, Lu et al (25) showed that curcumin is able to
suppress the proliferation and invasion of non-small cell lung
cancer by modulating the MTA1-mediated Wnt/β-catenin pathway. Our
present study proved that curcumin could inhibit
H2O2-induced cancer invasion and migration
via the suppression of the ERK/NF-κB signaling pathway.
ROS may play dual roles in cancer progression in a
dose-dependent manner. On one hand, excess ROS production can cause
oxidative damage to DNA and genomic instability and trigger cancer
cell death; on the other hand, mild intracellular ROS can stimulate
tumor progression by promoting cell proliferation, survival,
invasion and metastasis (9). Our
previous studies have demonstrated that both hyperglycemic
conditions and superoxide dismutase (SOD)-induced mild ROS
production were able to promote the invasive and migratory activity
of pancreatic cancer (26,27). In the present study, our results
showed that the effects of H2O2-induced
intracellular ROS could be inhibited by curcumin or NAC to reduce
cell invasion and migration, which also support this perspective.
It has been reported that dietary supplementation of curcumin to
male mice was able to increase the activities of glutathione
peroxidase, glutathione reductase, glucose-6-phosphate
dehydrogenase and catalase and induce glutathione S-transferase and
quinine reductase, which can further neutralize ROS derived from
chemical carcinogens (28).
Curcumin can also induce heme oxygenase-1, an important ROS
scavenging enzyme, via nuclear factor 2-related regulation
(29). Recent studies have also
shown that curcumin could abrogate ROS production via the
MAOA/mTOR/HIF-1α signaling pathway (11). Our data showed that curcumin
inhibits H2O2-induced cancer progression via
the suppression of the ERK/NF-κB signaling pathway in pancreatic
cancer cells.
The MAPK signaling pathways are important signaling
cascades downstream of ROS that are involved in tumor migration and
invasion (12). Lee et al
(30) showed that hepatocyte growth
factor regulates H2O2 production, which
further activates the ERK pathway and regulates uPA production,
eventually increasing the invasive potential of stomach cancer
cells. Our recent study showed that SOD could promote the EMT of
pancreatic cancer cells via the activation of the
H2O2/ERK/NF-κB axis (26). We also demonstrated that
H2O2 could promote the activation of p-ERK,
p-p38, p-NF-κB and p-c-Jun, and in turn, promote pancreatic cancer
cell invasion (10). The depletion
of H2O2 by catalase inhibits the activation
of the MAPK signaling pathways and tumor invasion (10). Several studies have demonstrated
that the MAPK pathways, especially that of ERK, regulate MMP
expression (31,32). Our previous study demonstrated that
miR-106a can induce the over-expression of MMPs, which can further
promote pancreatic cancer cell invasion (33). Additionally, the expression of MMPs
is downregulated when the ROS/ERK pathway is blocked (34). The present study determined that the
inhibition of the ROS/ERK pathway by curcumin could downregulate
the expression of MMP-2 and MMP-9, which in turn attenuates cell
invasion and migration.
In conclusion, the present study demonstrates that
curcumin plays an important role in suppressing the proliferation,
migration and invasion of pancreatic cancer cells via the
ROS/ERK/NF-κB signaling pathway. These results suggest that
curcumin may be a potential anticancer agent for the treatment of
pancreatic cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant serial nos. 81502840 and
81301846).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gall TM, Tsakok M, Wasan H and Jiao LR:
Pancreatic cancer: Current management and treatment strategies.
Postgrad Med J. 91:601–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Díaz Osterman CJ, Gonda A, Stiff T,
Sigaran U, Valenzuela MM, Ferguson Bennit HR, Moyron RB, Khan S and
Wall NR: Curcumin induces pancreatic adenocarcinoma cell death via
reduction of the inhibitors of apoptosis. Pancreas. 45:101–109.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F, et al: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar
|
|
6
|
Shanmugam MK, Rane G, Kanchi MM, Arfuso F,
Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP and Sethi G:
The multifaceted role of curcumin in cancer prevention and
treatment. Molecules. 20:2728–2769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bose S, Panda AK, Mukherjee S and Sa G:
Curcumin and tumor immune-editing: Resurrecting the immune system.
Cell Div. 10:62015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee DJ and Kang SW: Reactive oxygen
species and tumor metastasis. Mol Cells. 35:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa M, Hashida M and Takakura Y:
Catalase delivery for inhibiting ROS-mediated tissue injury and
tumor metastasis. Adv Drug Deliv Rev. 61:319–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Ma Z, Ma J, Li X, Xu Q, Duan W, Chen
X, Lv Y, Zhou S, Wu E, et al: Hydrogen peroxide mediates
hyperglycemia-induced invasive activity via ERK and p38 MAPK in
human pancreatic cancer. Oncotarget. 6:31119–31133. 2015.PubMed/NCBI
|
|
11
|
Du Y, Long Q, Zhang L, Shi Y, Liu X, Li X,
Guan B, Tian Y, Wang X, Li L, et al: Curcumin inhibits
cancer-associated fibroblast-driven prostate cancer invasion
through MAOA/mTOR/HIF-1α signaling. Int J Oncol. 47:2064–2072.
2015.PubMed/NCBI
|
|
12
|
Wu WS, Wu JR and Hu CT: Signal cross talks
for sustained MAPK activation and cell migration: The potential
role of reactive oxygen species. Cancer Metastasis Rev. 27:303–314.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Duan W, Chang Y, Li R, Xu Q, Lei J, Yin C,
Li T, Wu Y, Ma Q and Li X: Curcumin inhibits hypoxia inducible
factor-1α-induced epithelial-mesenchymal transition in HepG2
hepatocellular carcinoma cells. Mol Med Rep. 10:2505–2510.
2014.PubMed/NCBI
|
|
15
|
Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, Li
X, Han L, Li W, Sun H, et al: α-Mangostin inhibits hypoxia-driven
ROS-induced PSC activation and pancreatic cancer cell invasion.
Cancer Lett. 347:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youns M and Fathy GM: Upregulation of
extrinsic apoptotic pathway in curcumin-mediated antiproliferative
effect on human pancreatic carcinogenesis. J Cell Biochem.
114:2654–2665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-κB-Snail
signaling in breast cancer cells. Oncol Rep. 29:117–124. 2013.
|
|
18
|
Nagaraju GP, Zhu S, Ko JE, Ashritha N,
Kandimalla R, Snyder JP, Shoji M and El-Rayes BF: Antiangiogenic
effects of a novel synthetic curcumin analogue in pancreatic
cancer. Cancer Lett. 357:557–565. 2015. View Article : Google Scholar
|
|
19
|
Toden S, Okugawa Y, Jascur T, Wodarz D,
Komarova NL, Buhrmann C, Shakibaei M, Boland CR and Goel A:
Curcumin mediates chemosensitization to 5-fluorouracil through
miRNA-induced suppression of epithelial-to-mesenchymal transition
in chemoresistant colorectal cancer. Carcinogenesis. 36:355–367.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanai M, Yoshimura K, Asada M, Imaizumi A,
Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y,
et al: A phase I/II study of gemcitabine-based chemotherapy plus
curcumin for patients with gemcitabine-resistant pancreatic cancer.
Cancer Chemother Pharmacol. 68:157–164. 2011. View Article : Google Scholar
|
|
22
|
Kim JM, Noh EM, Kwon KB, Kim JS, You YO,
Hwang JK, Hwang BM, Kim BS, Lee SH, Lee SJ, et al: Curcumin
suppresses the TPA-induced invasion through inhibition of
PKCα-dependent MMP-expression in MCF-7 human breast cancer cells.
Phytomedicine. 19:1085–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun XD, Liu XE and Huang DS: Curcumin
reverses the epithelial-mesenchymal transition of pancreatic cancer
cells by inhibiting the Hedgehog signaling pathway. Oncol Rep.
29:2401–2407. 2013.PubMed/NCBI
|
|
24
|
Shakibaei M, Mobasheri A, Lueders C, Busch
F, Shayan P and Goel A: Curcumin enhances the effect of
chemotherapy against colorectal cancer cells by inhibition of NF-κB
and Src protein kinase signaling pathways. PLoS One. 8:e572182013.
View Article : Google Scholar
|
|
25
|
Lu Y, Wei C and Xi Z: Curcumin suppresses
proliferation and invasion in non-small cell lung cancer by
modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitro Cell
Dev Biol Anim. 50:840–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li W, Cao L, Han L, Xu Q and Ma Q:
Superoxide dismutase promotes the epithelial-mesenchymal transition
of pancreatic cancer cells via activation of the
H2O2/ERK/NF-κB axis. Int J Oncol.
46:2613–2620. 2015.
|
|
27
|
Li W, Ma Q, Li J, Guo K, Liu H, Han L and
Ma G: Hyperglycemia enhances the invasive and migratory activity of
pancreatic cancer cells via hydrogen peroxide. Oncol Rep.
25:1279–1287. 2011.PubMed/NCBI
|
|
28
|
Iqbal M, Sharma SD, Okazaki Y, Fujisawa M
and Okada S: Dietary supplementation of curcumin enhances
antioxidant and phase II metabolizing enzymes in ddY male mice:
Possible role in protection against chemical carcinogenesis and
toxicity. Pharmacol Toxicol. 92:33–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balogun E, Hoque M, Gong P, Killeen E,
Green CJ, Foresti R, Alam J and Motterlini R: Curcumin activates
the haem oxygenase-1 gene via regulation of Nrf2 and the
antioxidant-responsive element. Biochem J. 371:887–895. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee KH, Kim SW and Kim JR: Reactive oxygen
species regulate urokinase plasminogen activator expression and
cell invasion via mitogen-activated protein kinase pathways after
treatment with hepatocyte growth factor in stomach cancer cells. J
Exp Clin Cancer Res. 28:732009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gallelli L, Falcone D, Scaramuzzino M,
Pelaia G, D'Agostino B, Mesuraca M, Terracciano R, Spaziano G,
Maselli R, Navarra M, et al: Effects of simvastatin on cell
viability and proinflammatory pathways in lung adenocarcinoma cells
exposed to hydrogen peroxide. BMC Pharmacol Toxicol. 15:672014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poudel B, Lee YM and Kim DK: DDR2
inhibition reduces migration and invasion of murine metastatic
melanoma cells by suppressing MMP2/9 expression through ERK/NF-κB
pathway. Acta Biochim Biophys Sin (Shanghai). 47:292–298. 2015.
View Article : Google Scholar
|
|
33
|
Li P, Xu Q, Zhang D, Li X, Han L, Lei J,
Duan W, Ma Q, Wu Z and Wang Z: Upregulated miR-106a plays an
oncogenic role in pancreatic cancer. FEBS Lett. 588:705–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Y, Zheng L, Liu J, Zhou Z, Cao X, Lv
X and Chen F: Shikonin inhibits prostate cancer cells metastasis by
reducing matrix metalloproteinase-2/-9 expression via AKT/mTOR and
ROS/ERK1/2 pathways. Int Immunopharmacol. 21:447–455. 2014.
View Article : Google Scholar : PubMed/NCBI
|