Introduction
Urotensin II (UII) and its receptor, G
protein-coupled receptor 14 (GPR14), are widely expressed
throughout the cardiovascular, pulmonary, central nervous, renal
and metabolic systems (1,2). UII/GPR14 signaling has been reported
to be involved in various biological functions under both
physiological and pathological conditions, such as enhancing foam
cell formation, modulating insulin and catecholamine release, and
regulating food intake (3).
Meanwhile, elevated plasma levels of UII and expression of UII and
UT have been noted in various diseased conditions, including
hypertension, atherosclerosis, heart failure, pulmonary
hypertension, diabetes, renal failure and metabolic syndrome
(3), suggesting a potential marker
of disease activity. Meanwhile, pharmacological and genetic
inhibition of the UII/GPR14 signaling pathway has been reported to
serve as a protective mechanism in various pathological conditions
(4), such as liver inflammation,
ischemia-reperfusion injury and pulmonary arterial hypertension
(5–7).
Recent studies suggest a potential immune
inflammatory function of the UII/GPR14 system (8). In lipopolysaccharide challenged mice,
inhibition of UII/GPR14 signaling by urantide treatment markedly
alleviated the production of pro-inflammatory cytokines such as
tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and
interferon-γ (IFN-γ) via inactivation of the nuclear factor-κB
(NF-κB) pathway (9), which is known
to be a major pro-inflammatory transcription factor controlling a
wide number of genes, including cytokines (10). Meanwhile, urantide, a special
antagonist of GPR14, has also been demonstrated to attenuate the
inflammatory response via inhibition of p38 mitogen-activated
protein kinase phosphorylation in lipopolysaccharide
(LPS)-stimulated Kupffer cells (9).
However, UII/GPR14 signaling in intestinal inflammation and the
potential therapeutic function in intestinal inflammatory disease
are still obscure. Thus, in the present study, we investigated
UII/GPR14 expression and the effects of inhibition of UII/GPR14 via
urantide treatment on dextran sulfate sodium (DSS)-induced
inflammation in mice and Caco-2 cells.
Materials and methods
Animal model and groups
Thirty female Kunming mice (weighing, 23.14±1.37 g)
were randomly assigned into three groups: a control group (control,
n=10), a DSS-treated group (DSS, n=10) in which mice received 5%
DSS (Kayon Biotechnology Co., Ltd., Shanghai, China) instead of tap
water for 7 days to establish an inflammatory bowel disease (IBD)
model, and a urantide group (DSS + UR, n=10) in which mice received
5% DSS and an intravenous injection of 0.6 mg/kg urantide
(Peptides, Louisville, KY, USA) dissolved in saline. The control
and untreated challenged animals received the same volume of saline
alone. The dosage of urantide used in the present study was
according to a previous study (4).
All mice were housed in polycarbonate cages in a room with
controlled temperature (25±3°C), humidity (50±5%) and a 12 h cycle
of light and dark. They were allowed free access to laboratory
strip chows throughout the experimental period.
After the experimental period, each animal was
weighted to calculate average weight gain, and then each mouse was
sacrificed and colon length and weight were measured. In addition,
colon tissues from each mice were harvested and immediately frozen
in liquid nitrogen and stored at −70°C for subsequent gene
expression and western blot analyses. The present study was
conducted according to the guidelines of the Declaration of
Helsinki and all procedures involving animal subjects were approved
by the Animal Welfare Committee of the Department of
Gastroenterology and Yantai Municipal Laiyang Central Hospital.
Clinical evaluation of DSS colitis
Rectal bleeding and diarrhea were monitored daily.
The appearance of blood in the stool was measured by hemoccult
tests (Beckman Coulter), and was given a score from 0–4, defined as
follows: 0 for no blood; 2 for positive hemoccult; and 4 for gross
bleeding. The severity of diarrhea was given a score from 0–4,
defined as follows: 0 for well-formed pellets; 2 for pasty and
semi-formed stools; and 4 for liquid stools (11). All clinical scorings were performed
in a blinded manner.
Histomorphometry determination
The morphological evaluation after DSS treatment was
carried out using hematoxylin and eosin (H&E) staining
according to a previous study (12). Briefly, one piece of each colon
sample (0.5 cm) was maintained in 4% neutral buffered 10% formalin,
processed using routine histological methods, and mounted on
paraffin blocks. Six-micrometer-thick sections were cut and stained
with H&E. All specimens were examined under a light microscope
(Nikon, Tokyo, Japan).
The histological examination was performed in a
blinded manner using a scoring system, previously validated and
described (13). Three independent
parameters were measured: severity of inflammation (0–3: none,
slight, moderate and severe), depth of injury (0–3: none, mucosal,
mucosal and submucosal and transmural), crypt damage (0–4, none,
basal 1/3 damaged, basal 2/3 damaged, only surface epithelium
intact, entire crypt and epithelium lost) and percentage of the
involved area (0–4: 0%, 1–10%, 11–25%, 26–50% and 51–100%). All
scores on the individual parameters together could result in a
total score ranging from 0 to 14.
Cytokines
Intestinal segments were fractured using an
ultrasonic disrupter (Bandelin, Berlin, Germany) and homogenized in
RIPA buffer (Takara, Tokyo, Japan). In addition, the homogenates
were centrifugated at 10,000 rpm for 20 min, and the supernatants
were used for further analysis.
IL-1β, IL-10, IL-17, TNF-α and IFN-γ were quantified
using an enzyme-linked immunosorbent assay (ELISA) kit (R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
NF-κB activity
Colonic NF-κB activity was measured according to
ELISA kits (Cell Biolabs, San Diego, CA, USA).
Cell culture and treatment
Human colorectal adenocarcinoma-derived intestinal
epithelial cells (Caco-2) were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and grown in
Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 1
mM sodium pyruvate, 20% fetal bovine serum (FBS) (HyClone, Logan,
UT, USA), and 50 U/ml penicillin-streptomycin. The cells were then
treated with 2% DSS for 4 days to induce inflammation according to
a previous study (14).
siRNA transfection
Human GPR14 siRNA was obtained from Guangzhou
RiboBio and transfected into cells using Lipofectamine RNAiMAX
reagent according to the manufacturer's instructions (Invitrogen,
Carlsbad, CA, USA). After transfection for 48 h, the medium over
the cells was changed before subsequent treatments.
Real-time PCR
Total RNA was isolated from liquid nitrogen
pulverized tissues with TRIzol reagent, and then treated with DNase
I (both from Invitrogen) according to the manufacturer's
instructions. Synthesis of the first strand (cDNA) was performed
with oligo(dT)20 and SuperScript II reverse
transcriptase (Invitrogen). Primers were designed with Primer 5.0
according to the gene sequence of mouse to produce an amplification
product. The primer sets used were as follows: β-actin, F,
GTCCACCTTCCAGCAGATGT and R, GAAAGGGTGTAAAACGCAGC; IL-1β F,
CTGTGACTCGTGGGATGATG and R, GGGATTTTGTCGTTGCTTGT; IL-6 F,
TGCAAGAGACTTCCATCCAGT and R, GTGAAGTAGGGAAGGCCG; IL-10 F,
ACAGCCGGGAAGACAATAAC and R, CAGCTGGTCCTTTGTTTGAAAG; IL-17 F,
TACCTCAACCGTTCCACGTC and R, TTTCCCTCCGCATTGACAC; IFN-γ F,
ATGAACGCTACACACTGCATCTTGGCTT and R, CCTCAAACTTGGCAATACTCATGAATGC;
TNF-α F, AGGCACTCCCCCAAAAGAT and R, TGAGGGTCTGGGCCATAGAA. Real-time
PCR was performed according to previous studies (15,16).
Relative expression was normalized and expressed as a ratio to the
expression in the control group.
Western blotting
Proteins were extracted with protein extraction
reagents in accordance with the manufacturer's instructions (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Proteins from each
sample (30 μg) were separated by SDS-polyacrylamide gel
electrophoresis and electrophoretically transferred to a
polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA,
USA). The membranes were blocked in 7% evaporated milk, diluted in
Tris-buffered saline containing 0.1% Tween-20 (TBS-226T) at room
temperature for at least 2 h, and then incubated overnight at 4°C
with the following primary antibodies: UII (ab194676), GPR14
(ab78449), IκBα (ab32518), IκBβ (ab7547), p-p65 (ab86299) and
NF-κBp65 (ab7970) (Abcam, Inc., Cambridge, MA, USA). Mouse β-actin
antibody (Sigma) was used for protein loading control. After
primary antibody incubation, the membranes were washed with TBS-T
and incubated with alkaline phosphatase-conjugated anti-mouse or
anti-rabbit IgG antibodies (Promega, Madison, WI, USA) for 2 h at
room temperature. The membranes were washed with TBS-T followed by
three washes with TBS; signals were detected by the addition of
5-bromo-4-chloro-3-238 indolylphosphate/nitroblue tetrazolium
(BCIP/NBT) solution (Sigma), then quantified and digitally analyzed
using ImageJ program (NIH, Bethesda, MD, USA). The intensity of
each band was measured and subtracted from the background. The
expression ratio of target proteins was normalized against β-actin
(17).
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software. Group comparisons were performed using one-way
analysis of variance (ANOVA) to test homogeneity of variances via
Levene's test followed by Tukey's multiple comparison test. Values
in the same row with different superscripts are significant
(P<0.05), while values with the same superscripts are not
significantly different (P>0.05).
Results
Clinical indices
As shown at Fig. 1,
altered body weight, colon length and weight, rectal bleeding and
histological scores indicated a colitis model, which is consistent
with previous studies (18,19). Compared with the IBD group, urantide
injection markedly reduced rectal bleeding and histological scores
after DSS treatment, suggesting a protective role in the colitis
model.
Inflammatory response in mice
In the present study, ileum and colon samples were
collected to test cytokine concentrations. The results exhibited
that DSS treatment increased IL-17, TNF-α and IFN-γ levels in the
ileum, and IL-1β, IL-17, TNF-α and IFN-γ levels in the colon
(P<0.05) (Table I). Compared
with the IBD group, urantide significantly reduced ileal IFN-γ and
colonic IL-17 and IFN-γ concentrations.
 | Table IEffects of urantide injection on
pro-inflammatory cytokines (pg/ml) in DSS-challenged mice. |
Table I
Effects of urantide injection on
pro-inflammatory cytokines (pg/ml) in DSS-challenged mice.
| Cytokine | Control | DSS | DSS + UR |
|---|
| Ileum |
| IL-1β | 363.16±26.14 | 392.28±13.75 | 389.38±28.17 |
| IL-10 | 103.82±8.15 | 91.73±10.65 | 112.53±9.67 |
| IL-17 |
175.39±20.33b |
253.19±17.16a |
207.27±11.56a,b |
| TNF-α |
253.38±26.47b |
297.16±24.85a |
313.47±25.14a |
| IFN-γ |
293.29±27.92b |
431.39±22.55a |
361.54±31.54b |
| Colon |
| IL-1β |
317.54±14.85b |
475.65±62.42a |
418.82±19.46a |
| IL-10 | 86.26±6.59 | 116.87±14.09 | 105.49±11.65 |
| IL-17 |
117.69±19.74b |
218.65±19.49a |
151.11±26.49b |
| TNF-α |
236.59±24.49b |
378.65±34.57a |
295.34±24.37a,b |
| IFN-γ |
251.68±15.94c |
476.15±36.11a |
387.12±25.43b |
Meanwhile, colonic expression of cytokines were
further determined via RT-PCR (Fig.
2). The results showed that colonic IL-1β, IL-10, IL-17, TNF-α
and IFN-γ were significantly upregulated in the IBD group
(P<0.05). Urantide injection markedly downregulated IL-17 and
TNF-α expression (P<0.05).
UII levels and GPR14 expression in
mice
Urantide was used to inhibit UII/GPR14 signaling in
the present study. We found that colonic UII levels were
significantly higher in the IBD group when compared to that in the
control group (P<0.05), while urantide treatment failed to
influence the colonic UII concentration (P>0.05) (Fig. 3).
Meanwhile, our data exhibited that UII and GPR14
were significantly upregulated after DSS treatment (P<0.05)
(Fig. 3), suggesting that UII/GPR14
is involved in DSS-induced inflammation. Although we failed to
observe any significant difference in colonic UII expression after
urantide injection, urantide markedly inhibited GPR14 expression
(P<0.05) (Fig. 3).
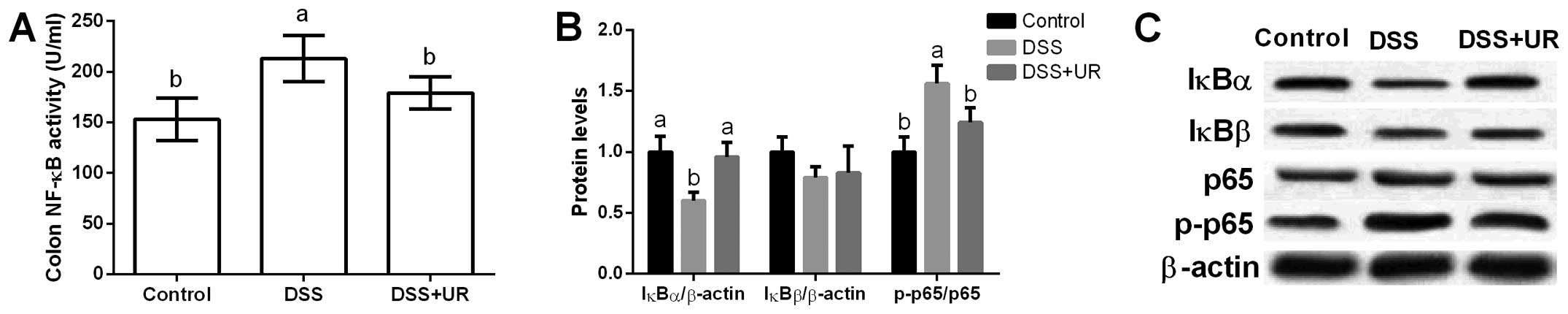
NF-κB signal in mice
Colonic NF-κB activity was significantly increased
after DSS treatment, while urantide markedly reduced NF-κB activity
in the mice (P<0.05) (Fig. 4).
Furthermore, colonic NF-κB signal-related proteins (i.e. IκBα,
IκBβ, p65 and p-p65) were assessed and the results showed that DSS
significantly increased p65 phosphorylation and urantide treatment
inhibited p65 activation (P<0.05) (Fig. 4). IκB is an inhibitory protein of
the NF-κB signaling pathway, and we found that DSS markedly
inhibited IκBα expression, while urantide alleviated IκBα
upregulation caused by DSS exposure.
UII/GPR14 mediates NF-κB signaling in
Caco-2 cells
The in vivo data indicated that UII/GPR14 is
involved in DSS-induced inflammatory response in mice. Thus, we
further validated the effect of UII/GPR14 in vitro via
GPR14-siRNA transfection. The results exhibited that GPR14-siRNA
transfection markedly reduced GPR14 expression, which further
inhibited DSS-induced IFN-γ production (P<0.05) (Fig. 5).
The western blot results showed that DSS exposure
also increased GPR14 expression in vitro and inhibition of
GPR14 via GPR14-siRNA transfection markedly reduced GPR14
expression (P<0.05) (Fig. 5).
Yet, GPR14-siRNA transfection failed to influence IκBα and IκBβ,
and GPR14 downregulation significantly inhibited p65
phosphorylation (P<0.05) (Fig.
5).
Discussion
Previous studies have suggested that the UII/GPR14
signaling pathway is involved in pro-inflammatory responses via
mediating pro-inflammatory cytokine expression (4,9,20,21).
In the present study, we used urantide, a special antagonist of
GPR14, to block GPR14 and the results indicated that GPR14
inhibition alleviated DSS-induced colitis in mice evidenced by
reduced rectal bleeding and histological injury, indicating a
protective role of urantide in the colitis model.
Pro-inflammatory cytokines and inflammation response
in the gastrointestinal tract play a vital role in the progression
of IBD (10,22–25).
However, the effect of UII/GPR14 signaling on DSS-induced
pro-inflammatory cytokine generation is not fully understood. In
the present study, we found marked increases in pro-inflammatory
cytokine (i.e. IL-1β, IL-10, IL-17, TNF-α and IFN-γ) levels and
expression in the DSS challenged mice. Inhibition of the UII/GPR14
signal using urantide significantly reduced IL-17 and TNF-α
generation, suggesting that the releases of these cytokines may be
a consequence of UII/UTR system activation induced by DSS exposure.
In lipopolysaccharide/D-galactosamine-induced liver inflammation,
urantide has been demonstrated to prevent increases in
pro-inflammatory cytokines such as IL-1β, TNF-α and IFN-γ (4). Meanwhile, the in vitro data
revealed that urantide treatment alleviated IFN-γ production caused
by DSS in Caco-2 cells. Thus, we speculated that UII/GPR14
signaling may be involved in DSS-induced inflammation and
inhibition of UII/GPR14 may exhibit an anti-inflammatory function
in in vivo and in vitro models.
UII/GPR14 signaling has been confirmed to mediate
inflammatory response (9), while
activity of the UII/GPR14 signal has never been evaluated in
DSS-induced inflammation. In the present study, we found that the
UII/GPR14 signal was significantly activated after DSS exposure
evidenced by the upregulation of UII and GPR14. Similarly, Liang
et al also reported that liver UII and GPR14 expression and
serum UII levels were markedly enhanced in
lipopolysaccharide/D-galactosamine-induced liver inflammation in
mice (4). Meanwhile, both urantide
and GPR14 siRNA transfection inhibited GPR14 expression, which
further alleviated DSS-induced inflammation in mice and Caco-2
cells.
To investigate the mechanisms underlying the
protective effect of UII/GPR14 signal inactivation on DSS-induced
inflammation, we determined the effect of urantide and GPR14 siRNA
transfection on signaling molecules of the NF-κB pathway in the
colon and Caco-2 cells. The NF-κB signaling pathway has been found
to be associated with the expression of various cytokines and
chemokines in response to cellular stimuli such as inflammation and
oxidative stress (26–29). In the present study, NF-κB signaling
was activated after DSS treatment in vivo and in
vivo, while inhibition of the UII/GPR14 signal via urantide or
GPR14 siRNA transfection significantly inactivated NF-κB via
reducing p65 phosphorylation. NF-κB activation requires IκBs, an
inhibitory protein of NF-κB (25,26,29).
The present data revealed that urantide markedly alleviated the
inhibitory effect of DSS on IκBα. Thus, we concluded that UII/GPR14
may mediate IκBα/NF-κB signaling in DSS-induced inflammation.
Inhibition of the NF-κB signaling pathway has been indicated to be
a potential therapeutic target in inflammatory diseases, including
IBD. For example, inactivation of the NF-κB pathway was found to
downregulate pro-inflammatory cytokine expression in an IBD murine
model (30). Meanwhile, pyrrolidine
dithiocarbamate, an antioxidant agent and NF-κB inhibitor, has been
confirmed to reverse the activation of the NF-κB signaling pathway
and alleviate colonic inflammation caused by DSS treatment
(31).
In conclusion, UII/GPR14 signaling is involved in
DSS-induced inflammation in mice and Caco-2 cells. Inhibition of
UII/GPR14 via urantide or GPR14 siRNA transfection alleviated
DSS-induced inflammation and NF-κB activity. Thus, the beneficial
effect of UII/GPR14 inhibition on the inflammatory response may be
associated with the NF-κB signaling pathway.
References
|
1
|
Ross B, McKendy K and Giaid A: Role of
urotensin II in health and disease. Am J Physiol Regul Integr Comp
Physiol. 298:R1156–R1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ong KL, Lam KS and Cheung BM: Urotensin
II: Its function in health and its role in disease. Cardiovasc
Drugs Ther. 19:65–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McDonald J, Batuwangala M and Lambert DG:
Role of urotensin II and its receptor in health and disease. J
Anesth. 21:378–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang DY, Liu LM, Ye CG, Zhao L, Yu FP,
Gao DY and Wang YY, Yang ZW and Wang YY: Inhibition of UII/UTR
system relieves acute inflammation of liver through preventing
activation of NF-κB pathway in ALF mice. PLoS One. 8:e648952013.
View Article : Google Scholar
|
|
5
|
Zhang JY, Chen ZW and Yao H: Protective
effect of urantide against ischemia-reperfusion injury via protein
kinase C and phosphtidylinositol 3′-kinase - Akt pathway. Can J
Physiol Pharmacol. 90:637–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Yu QX, Kong W, Gao HC, Sun B, Xie
YQ and Ren LQ: The urotensin II receptor antagonist, urantide,
protects against atherosclerosis in rats. Exp Ther Med.
5:1765–1769. 2013.PubMed/NCBI
|
|
7
|
Mei Y, Jin H, Tian W, Wang H, Wang H, Zhao
Y, Zhang Z and Meng F: Urantide alleviates monocrotaline induced
pulmonary arterial hypertension in Wistar rats. Pulm Pharmacol
Ther. 24:386–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Birker-Robaczewska M, Boukhadra C, Studer
R, Mueller C, Binkert C and Nayler O: The expression of urotensin
II receptor (U2R) is up-regulated by interferon-gamma. J Recept
Signal Transduct Res. 23:289–305. 2003. View Article : Google Scholar
|
|
9
|
Liu LM, Liang DY, Ye CG, Tu WJ and Zhu T:
The UII/UT system mediates upregulation of proinflammatory
cytokines through p38 MAPK and NF-κB pathways in LPS-stimulated
Kupffer cells. PLoS One. 10:e01213832015. View Article : Google Scholar
|
|
10
|
Hirai F and Matsui T: Status of food
intake and elemental nutrition in patients with Crohn's disease.
Integr Food Nutr Metab. 2:148–150. 2015.
|
|
11
|
Vlantis K, Polykratis A, Welz PS, van Loo
G, Pasparakis M and Wullaert A: TLR-independent anti-inflammatory
function of intestinal epithelial TRAF6 signalling prevents
DSS-induced colitis in mice. Gut. 65:935–943. 2016. View Article : Google Scholar :
|
|
12
|
Yin J, Duan J, Cui Z, Ren W, Li T and Yin
Y: Hydrogen peroxide-induced oxidative stress activates NF-κB and
Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Advances.
5:15479–15486. 2015. View Article : Google Scholar
|
|
13
|
Ranganathan P, Jayakumar C, Li DY and
Ramesh G: UNC5B receptor deletion exacerbates DSS-induced colitis
in mice by increasing epithelial cell apoptosis. J Cell Mol Med.
18:1290–1299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nighot P, Young K, Nighot M, Rawat M, Sung
EJ, Maharshak N, Plevy SE, Ma T and Blikslager A: Chloride channel
ClC-2 is a key factor in the development of DSS-induced murine
colitis. Inflamm Bowel Dis. 19:2867–2877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin J, Ren W, Liu G, Duan J, Yang G, Wu L,
Li T and Yin Y: Birth oxidative stress and the development of an
antioxidant system in newborn piglets. Free Radic Res.
47:1027–1035. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin J, Wu MM, Xiao H, Ren WK, Duan JL,
Yang G, Li TJ and Yin YL: Development of an antioxidant system
after early weaning in piglets. J Anim Sci. 92:612–619. 2014.
View Article : Google Scholar
|
|
17
|
Yin J, Liu M, Ren W, Duan J, Yang G, Zhao
Y, Fang R, Chen L, Li T and Yin Y: Effects of dietary
supplementation with glutamate and aspartate on diquat-induced
oxidative stress in piglets. PLoS One. 10:e01228932015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agren R, Otero JM and Nielsen J:
Genome-scale modeling enables metabolic engineering of
Saccharomyces cerevisiae for succinic acid production. J Ind
Microbiol Biotechnol. 40:735–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KH, Park M, Ji KY, Lee HY, Jang JH,
Yoon IJ, Oh SS, Kim SM, Jeong YH, Yun CH, et al: Bacterial β-
(1,3)-glucan prevents DSS-induced IBD by restoring the reduced
population of regulatory T cells. Immunobiology. 219:802–812. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shori AB and Baba AS: Fermented milk
derives bioactive peptides with antihypertensive effects. Integr
Food Nutr Metab. 2:178–181. 2015.
|
|
21
|
Zaki SA, Amin WSM and Nagi HM: The
functional role of tomato and carrot on histopathological lesions
of brain, small intestine and prostate in mice treated with
acrylamide. Integr Food Nutr Metab. 1:114–118. 2014.
|
|
22
|
Sánchez-Fidalgo S, Cárdeno A,
Sánchez-Hidalgo M, Aparicio-Soto M and de la Lastra CA: Dietary
extra virgin olive oil polyphenols supplementation modulates
DSS-induced chronic colitis in mice. J Nutr Biochem. 24:1401–1413.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scharl M, Vavricka SR and Rogler G:
Review: new anti-cytokines for IBD: what is in the pipeline? Curr
Drug Targets. 14:1405–1420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beloqui A, Coco R, Alhouayek M, Solinís
MÁ, Rodríguez-Gascón A, Muccioli GG and Préat V: Budesonide-loaded
nanostructured lipid carriers reduce inflammation in murine
DSS-induced colitis. Int J Pharm. 454:775–783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi A-J, Buisson N and Kim C-T: Digestion
characteristics and kinetic analysis of bio-molecules in a
simulated human intestinal system. Integr Food Nutr Metab.
2:189–192. 2015. View Article : Google Scholar
|
|
26
|
Yin J, Ren WK, Wu XS, et al: Oxidative
stress-mediated signaling pathways: A review. J Food Agric Environ.
11:132–139. 2013.
|
|
27
|
Hendy HA-RE and Gemeai AROA: Effect of
broccoli intake on antioxidant in the liver and kidney tissues of
hyperglycemic rats. Integr Food Nutr Metab. 1:83–86. 2014.
|
|
28
|
Mileva S, Galunska B, Gospodinova M, et
al: Vitamin D3 status in children with acute diarrhea. Integr Food
Nutr Metab. 1:1–6. 2014.
|
|
29
|
McCann MJ, Dalziel JE, Bibiloni R and
Barnett MPG: An integrated approach to assessing the bio-activity
of nutrients in vitro: The anti-oxidant effects of catechin and
chlorogenic acid as an example. Integr Food Nutr Metab. 2:197–204.
2015. View Article : Google Scholar
|
|
30
|
Beneficial effects of THSG on acetic
acid-induced experimental colitis: involvement of upregulation of
PPAR-γ and inhibition of the Nf-Kb inflammatory pathway. Molecules.
16:8552–8568. 2011. View Article : Google Scholar
|
|
31
|
Yin J, Wu M, Duan J, Liu G, Cui Z, Zheng
J, Chen S, Ren W, Deng J, Tan X, et al: Pyrrolidine dithiocarbamate
inhibits NF-kappaB activation and upregulates thesxpression of
Gpx1, Gpx4, occludin, and ZO-1 in DSS-induced colitis. Appl Biochem
Biotechnol. 177:1716–1728. 2015. View Article : Google Scholar : PubMed/NCBI
|