Introduction
Cysteine-rich protein 61 (CYR61) encodes a protein
with 10% cysteine residues and was first described in 1985, as an
immediate early gene, which can be induced by serum or
platelet-derived growth factor (PDGF) (1). CYR61 is a secreted, extracellular
matrix (ECM)-associated signaling molecule that belongs to the
CCN-gene family.
The CCN family includes six distinct genes:
CYR61/CCN1, connective tissue growth factor (CTGF)/CCN2,
nephroblastoma overexpressed gene (NOV)/CCN3, and Wnt-induced
secreted proteins WISP-1/CCN4, WISP-2/CCN5, and WISP-3/CCN6
(2). These are matri-cellular
regulatory factors involved in internal and external cell
signaling. They play a role in angiogenesis, chondrogenesis, and
osteogenesis, by stimulating mitosis, adhesion, apoptosis, ECM
production, and growth arrest and migration of multiple cell types
(3).
Human CYR61 maps to p22.3 on chromosome 1, encodes a
384-residue protein, and yields a 360-residue protein containing 38
conserved cysteine residues after signal peptide cleavage (4–7). To
date, a number of studies describe the involvement of CYR61 in many
cell functions, such as cell adhesion, migration, proliferation,
apoptosis and angiogenesis (8–10).
As integrin receptor, CYR61 is expressed in various
tissues and may have different physiological functions (11–14).
However, expression profiles of CYR61 in malignant melanoma have
been inconsistent. Babic et al and Kunz et al
reported CYR61 overexpression in malignant melanoma, possibly
related to cell migration (15,16).
However, in 2009, Dobroff et al demonstrated that silencing
the cAMP-response element-binding protein (CREB) in two human
metastatic melanoma cell lines, A375SM and C8161-c9, resulted in
suppressed tumor growth and metastasis and increased CYR61
expression (17). They pointed to
the possibility of inducing expression of CYR61 as potential
therapeutic method for malignant melanoma. To date, the expression
profile of CYR61 in Chinese patients with malignant melanoma has
not been studied.
Here, we determined the levels of CYR61 in samples
obtained from Chinese patients with malignant melanoma or other
skin tumors, and compared them with those of patients with no skin
pathology. Among these malignant tumors, we analyzed CYR61
expression in samples derived from different clinical stages using
anti-CYR61 monoclonal antibody (mAb). Our results showed that CYR61
was expressed at low levels in malignant melanoma, which is
consistent with previous studies (17). Furthermore, we examined the role of
CYR61 on the growth of B16 cell line. We also explored CYR61
expression in B16 cells, when exposed to either epirubicin or
interferon (IFN)-α. The results showed that epirubicin and IFN-α
inhibited B16 proliferation and subsequently significantly
decreased CYR61 expression. Moreover, the increased apoptosis in
B16 cells was consistent with the reduced expression of
proliferating cell nuclear antigen (PCNA). Taken together, we
provide evidence that epirubicin and IFN-α negatively impacted B16
cell proliferation and reduced their expression of CYR61 and PCNA,
thus indicating CYR61 as a potential target for malignant melanoma
treatment.
Materials and methods
Reagents
Monoclonal anti-CYR61 antibodies were obtained from
Sigma-Aldrich (St. Louis, MO, USA). EliVision™ plus Polyer HRP
(mouse/rabbit) immunohistochemistry (IHC) kit was provided by
Fuzhou Maxin Biotechnology Co., Ltd. (Fuzhou, China).
3H-TdR was a gift from the Shanghai Institute of Applied
Physics, Chinese Academy of Sciences (Shanghai, China).
SYBR® Green PCR Master Mix was purchased from Applied
Biosystems (Foster City, CA, USA) and TRIzol was obtained from
Invitrogen (Carlsbad, CA, USA). Cyr61 protein was supplied by
Peprotech, Inc. (Rocky Hill, NJ, USA).
Tissue microarray (TMA) and IHC
assay
The TMA containing malignant melanoma, other skin
tumors, and normal skin, was obtained from Xi'an Alena
Biotechnology Ltd., Co. (Xi'an, China). IHC studies were performed
using the standard EliVision™ method. In brief, TMA sections were
deparaffinized, rehydrated, and endogenous peroxidase activity was
blocked by treatment with 3% hydrogen peroxide for 20 min. For
antigen retrieval, TMA slides were microwave-treated in 10 mM
citrate buffer (pH 6.0) for 10 min. The slides were incubated with
mouse mAb against human CYR61 (1:100 dilution), overnight at 4°C,
followed by incubation with an HRP-conjugated goat anti-mouse
polyclonal antibody for 60 min, and subsequent reaction with
3,3′-diaminobenzidine (DAB). The nuclei were counterstained with
hematoxylin. Negative controls were performed by replacing the
primary antibody with mouse IgG. To evaluate the IHC staining of
CYR61 in malignant melanoma, other skin tumors, and normal skin, a
semi-quantitative scoring criteria for IHC of CYR61 was used, in
which both staining intensity and positive areas were recorded as −
(negative), + (weak positive), ++ (moderate positive), and +++
(strong positive).
Cell lines and culture conditions
B16, a mouse melanoma cell line derived from
spontaneous skin tumors in C57BL/6 mouse, was obtained from
Shanghai Institutes for Biological Sciences, CAS, Shanghai, China.
B16 melanoma cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; HyClone, Logan, UT, USA) supplemented with 20% fetal
calf serum (FCS), penicillin 100 U/ml, streptomycin 100
µg/ml (Gibco, North Andover, MA, USA), L-glutamine 2 mM,
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) 10 mM,
and nonessential amino acids (HyClone). Cells were grown at 37°C in
a humidified atmosphere containing 5% CO2 and were
routinely passaged every 3–4 days. For passaging cells, parental
cells and CYR61-treated cells were released from plastic culture
dishes with a trypsin (0.25%)-EDTA (1 mM) solution (Gibco) for 5
min.
B16 cells at a concentration of 1.48×106
were plated and grown in 10-cm Petri dishes with 8 ml of complete
culture medium. For the 3H-TdR method, B16 cells were
plated at a concentration of 2.0×103 cells/well in
complete culture medium, in 96-well flat bottomed culture
plates.
Cell proliferation assay
To evaluate the effects of epirubicin (0, 0.075,
0.15, 0.3, 0.6 and 1.2 µg/ml) and IFN-α (0, 102,
103, and 104 IU/ml) on cell growth, several
96-well plates were plated with 2.0×103 cells/well.
After 24 h, cells were incubated with drugs at the above mentioned
concentrations, measuring any inhibition in cell growth at 12, 24
and 48 h, by using the MTT test.
The methods of Carmichael et al (18) and Alley et al (19) were adapted to our culture
conditions. Briefly, the cultures were incubated with 20 µl
MTT (5 mg/ml in fresh medium) for 4 h (37°C and 5% CO2).
After a 10 min centrifugation to remove the medium and the
non-metabolized MTT, 150 µl of dimethyl sulfoxide (DMSO;
Fluka, Milwaukee, WI, USA) were added to each well to solubilize
the MTT formazan produced by the cells. After shaking for 10 min at
room temperature, the amount of colored formazan metabolite was
determined by absorbance at 490 nm.
3H-TdR incorporation in
vitro
B16 cells (1×104/ml) were seeded in
96-well plates (200 µl/well) and recombinant human CYR61
protein (Peprotech, Inc.) was added at concentrations of 0, 0.625,
1.25, 2.5, 5.0 and 20 ng/ml for 4 h. Six wells were set for each
treatment. Next, the cells were treated with 1 µCi
3H and cultured for another 8 h. They were then washed
three times with PBS and digested with 0.125% trypsin (HyClone). A
cell suspension was prepared and leached onto a membrane. This was
washed with 10% trichloroacetic acid, followed by addition of 0.1
mol/l NaOH. Subsequently, anhydrous ethanol was added for
decolorization and dehydration. The membrane was dried at 70°C and
placed in scintillation solution for 24 h in the dark. Counts per
minute were measured using a Trilux 1450 MicroBeta machine
(Perkin-Elmer Wallac Inc., USA).
Cell cycle analysis by FACS
B16 cells were incubated in 50-ml culture flasks in
DMEM with or without recombinant human CYR61 protein. After 72 h,
cells (1×105/ml) were collected, washed repeatedly with
PBS containing 0.1% BSA, centrifuged at 1,000 rpm/min for 10 min,
and fixed with Cy5-CD4, Cy5-CD8 antibodies at 4°C for 20 min,
respectively. Cells were then washed three times with Annexin
V+/PI− buffer. Five microliters of Annexin
V+ and 5 µl PI− (R&D Systems,
Minneapolis, MN, USA) were added to the culture for another 15 min.
The cell cycle distribution was analyzed using a FACScan flow
cytometer (BD FACSCalibur; BD Biosciences, San Jose, CA, USA).
Quantitative real-time reverse
transcriptase-polymerase chain reaction (RT-qPCR) analysis
Real-time PCR was performed as previously reported
(20). The sequence of primers were
as follows: GADPH forward, 5′-GTG AAG GTC GGA GTC AAC G-3′ and
reverse, 5′-TGA GGT CAA TGA AGG GGT C-3′; β-actin forward, 5′-TGT
CCA CCT TCC AGC AGA TGT-3′ and reverse, 5′-AGC TCA GTA ACA GTC CGC
CTA G-3′; CYR61 forward, 5′-TCC AGC CCA ACT GTA AAC ATC A-3′ and
reverse, 5′-GGA CAC AGA GGA ATG CAG CC-3′; PCNA forward, 5′-CCA ATT
GTG CCG AGA AAA GC-3′ and reverse, 5′-GAC AGA GCC AGT ATT GGG AGT
TG-3′. The above primers were designed and provided by Takara
Biotechnology, Co., Ltd. (Dalian, China). cDNA was amplified with
SYBR® Green PCR Master Mix (Takara Biotechnology, Co.,
Ltd.), by using the 7000 Real-Time PCR system (Applied Biosystems).
For target gene quantification, normalization was done based on a
Chr.21 assay, C2. Relative copy numbers (RCN) were determined on
the basis of comparative ΔΔCq (Ct) method, with a normal control
DNA as the calibration standard (21). All experiments were repeated three
times. A=0.5-fold-change in RCN was considered as benchmark for
deletion.
Western blot analysis
CYR61 protein in B16 cells was detected by western
blot analysis with specific anti-human CYR61 monoclonal antibodies.
Following electrophoresis, proteins were transferred to PVDF
membrane at 60 V for 2 h. Membranes were blocked with 5% non-fat
milk, washed with PBS, and incubated with mAb at 4°C overnight.
Subsequently, they were incubated with HRP-conjugated goat
anti-mouse IgG at room temperature for 45 min, followed by washing
with PBS. The target protein was visualized by using
autoradiography film (Fujifilm LAS-4000).
Statistical analysis
Group measures were shown as mean ± SEM. A Student's
t-test was used to analyze the differences between each two groups.
A one-way ANOVA was initially performed to assess the overall
statistical significance, followed by a two-tailed paired or
unpaired Student's t-test. A p<0.05 was considered
significant.
Results
Low CYR61 expression in malignant
melanoma
Using TMA and IHC, we examined the expression of
CYR61 in 78 skin samples from Chinese patients with different
clinical diagnoses, stages and pathological types. The expression
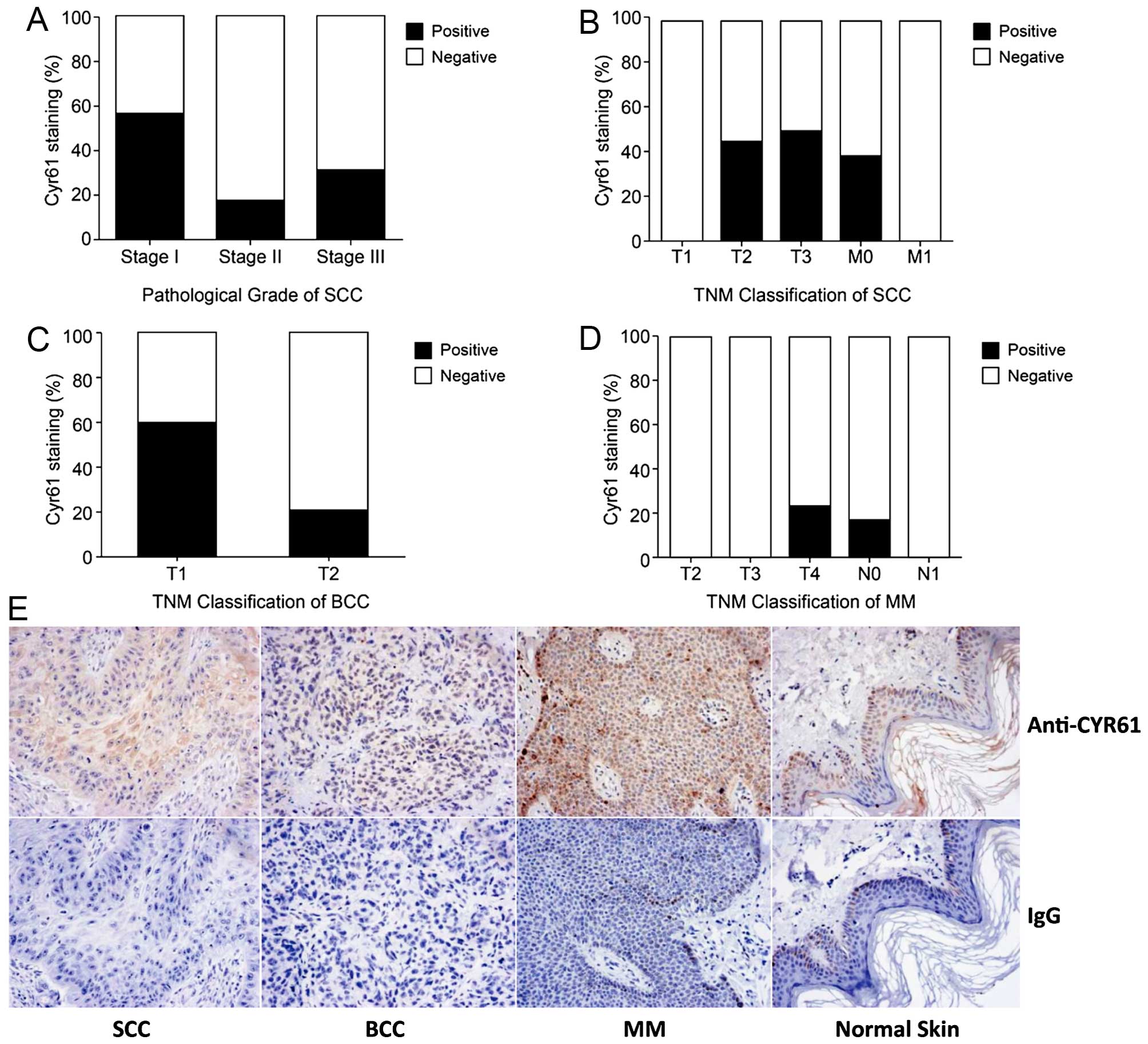
profile of CYR61 is displayed in Table
I. CYR61 levels are low in malignant melanoma (MM) and basal
cell carcinoma (BCC) tissues, and high in squamous cell carcinoma
(SCC) tissues (p<0.01), when compared with normal skin tissue.
This is different from previous studies where CYR61 was found to be
overexpressed in malignant melanoma (17). Next, we examined the correlation
between CYR61 levels with the clinical stage and pathological
diagnosis. There was no direct relationship between CYR61
expression and the pathological diagnosis or TNM stage in either
SCC (Fig. 1A and B) or BCC and MM
(Fig. 1C and D). The specificity of
the anti-CYR61 antibody was concluded from the positive CYR61
expression detected in tissues exposed to antibody as opposed to
the negative one in tissues incubated with control IgG (Fig. 1E).
 | Table IExpression of CYR61 in 78 tissues of
Chinese origin. |
Table I
Expression of CYR61 in 78 tissues of
Chinese origin.
| Sample (no.) | Age (years) | Clinical stage | Staining intensity,
n (%)
| Positive staining,
% |
|---|
| − | + | ++ | +++ |
|---|
| SCC (20) | 65.8±16.6 | I | 3 (37) | 5 (62) | 0 (0) | 0 (0) | 62.5 |
| | II | 7 (77) | 2 (22) | 0 (0) | 0 (0) | 22.2 |
| | III | 2 (66) | 1 (3) | 0 (0) | 0 (0) | 33.3 |
| BCC (15) | 65.3±10.1 | T1N0M0 | 4 (40) | 6 (60) | 0 (0) | 0 (0) | 60 |
| | T2N0M0 | 4 (80) | 1 (20) | 0 (0) | 0 (0) | 20 |
| MM (15) | 53±13.6 | T2N0M0 | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 |
| | T3N0M0 | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 0 |
| | T3N1M0 | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 |
| | T4N0M0 | 5 (71) | 2 (28) | 0 (0) | 0 (0) | 28.6 |
| | T4N1M0 | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 |
| | T4N1M1 | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 |
| Inflammation
(8) | 55.1±10.3 | – | 2 (25) | 6 (75) | 0 (0) | 0 (0) | 75 |
| Normal skin
(20) | 41.8±17.4 | – | 4 (20) | 16 (80) | 0 (0) | 0 (0) | 80 |
Recombinant human CYR61 inhibits
proliferation and promotes apoptosis in B16 cells
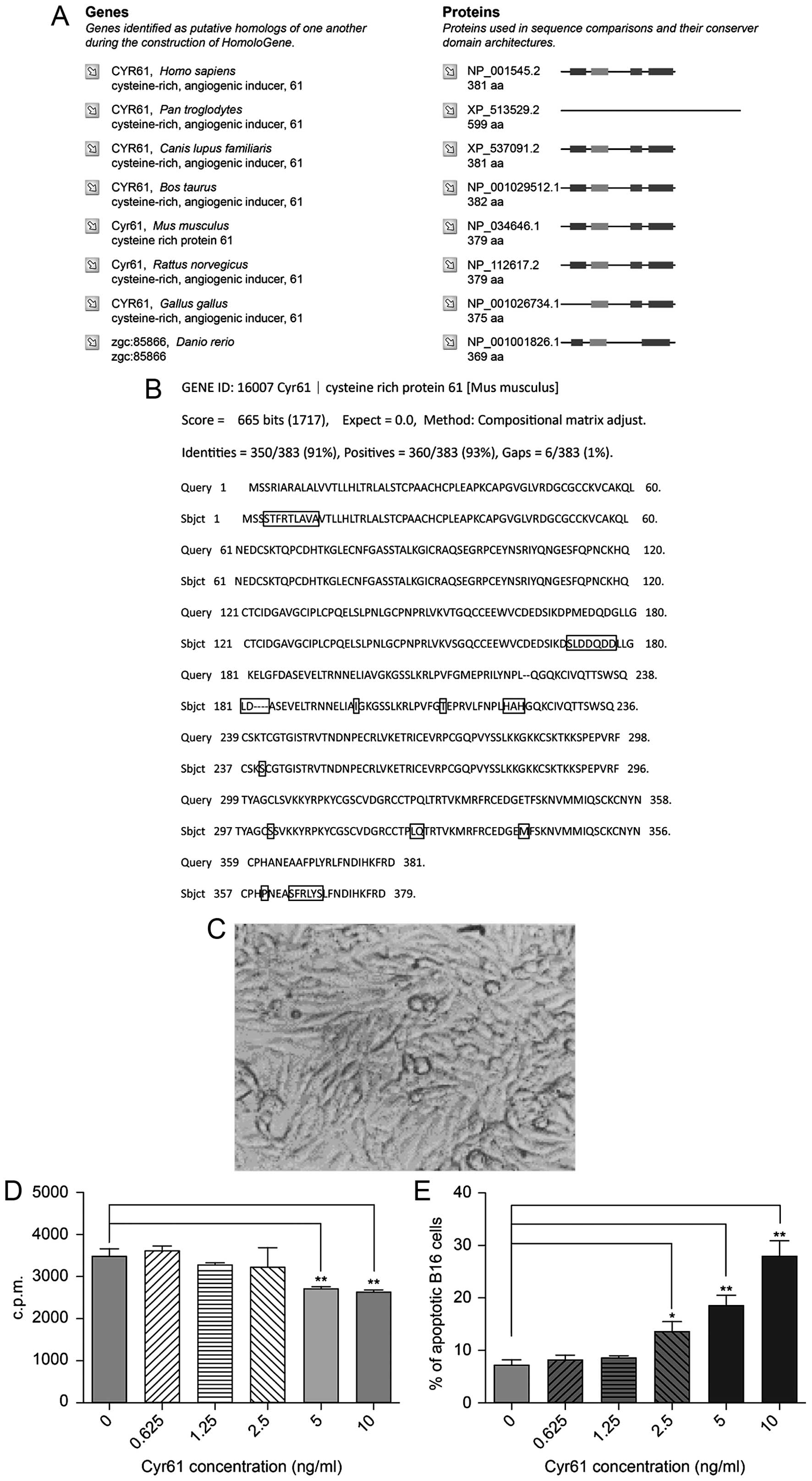
Based on the search results from NCBI HomoloGene,
human CYR61 has a high degree of homology to the protein in other
species (Fig. 2A). We also found
93% sequence similarity between CYR61 in human and mouse,
confirming reliability of using human CYR61 in B16 cells in
vitro (Fig. 2B). B16 cells
appeared to grow well under normal conditions (Fig. 2C). However, 3H-TdR
incorporation suggested that human CYR61 suppressed B16
proliferation in vitro, an effect enhanced progressively
with increasing concentration of the protein added, up to 5 ng/ml
(Fig. 2D).
Additional to growth suppression, CYR61 protein also
had apoptosis promoting effect, proportional with the concentration
of the recombinant protein. At 10 ng/ml, 30% of cells were
apoptotic, significantly more than when exposed to low CYR61
concentrations or control (without CYR61) (Fig. 2E).
Anti-tumoral effect of epirubicin and
IFNa on B16 cells
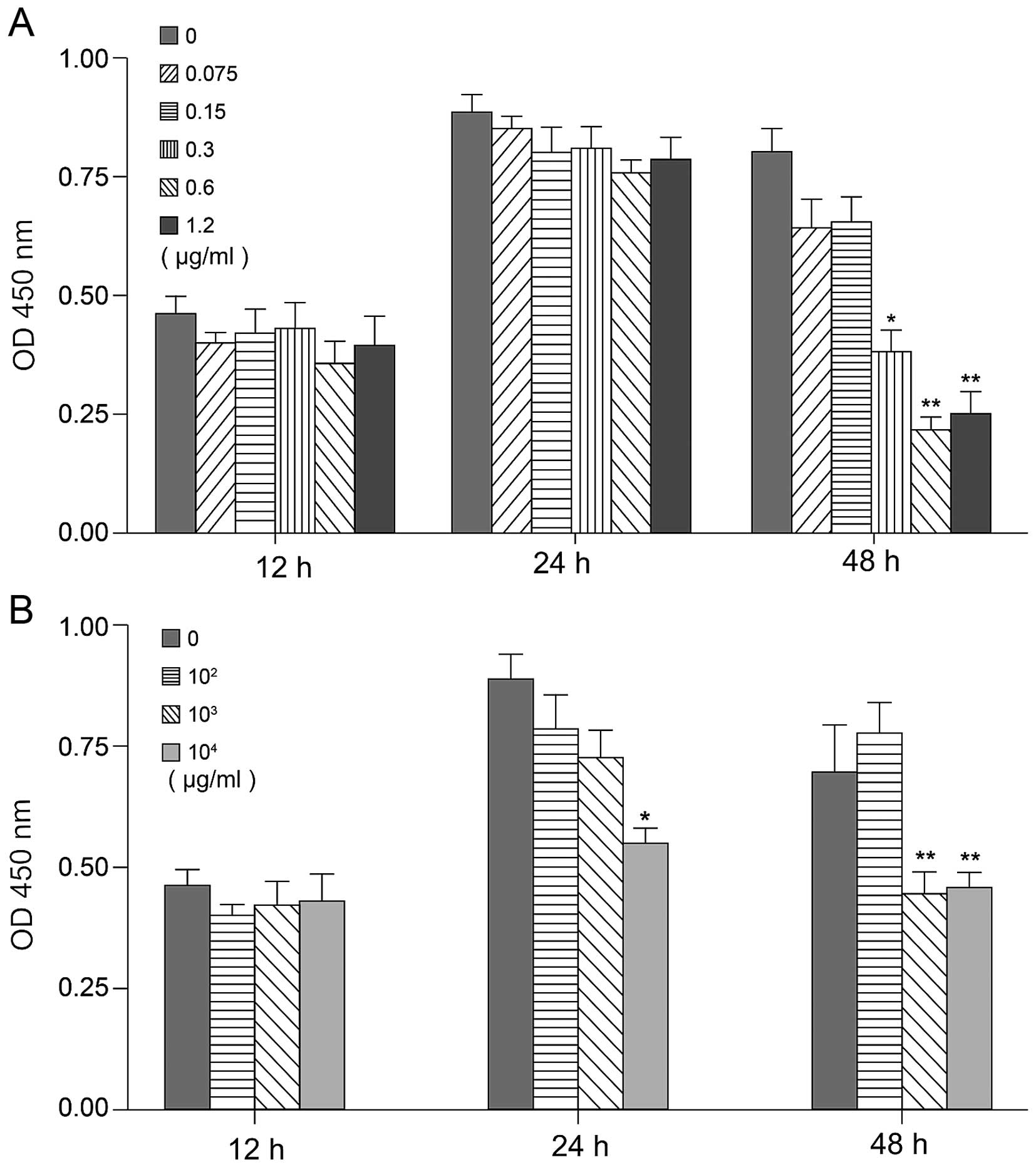
Epirubicin and IFN-α were added to B16 cells and the
proliferation rate was monitored to determine their effect on cell
growth. To evaluate the antitumor effect of different individual
concentrations of epirubicin (0, 0.075, 0.15, 0.3, 0.6 and 1.2
µg/ml) and IFN-α (0, 102, 103 and
104 IU/ml), cells (2.0×103/well) were seeded
into 96-well plates and 24 h later, they were incubated with
various doses of drugs. Cell growth was evaluated by the MTT assay
after 12, 24 and 48 h of treatment. As shown in Fig. 3, both epirubicin and IFN-α had the
ability to inhibit B16 proliferation. After 12 h of treatment with
different concentrations of drugs, there was no difference in cell
growth, suggesting that cells were probably not in the
proliferative stage yet. After 24 h, the 6 different doses of
epirubicin did not show a statistically significant cell growth,
while 104 IU/ml IFN-α clearly inhibited proliferation
(p<0.05). Forty-eight hours later, both 0.075–1.2 µg/ml
of epirubicin and 103–104 IU/ml IFN-α
strongly inhibited B16 growth, compared with other doses tested. In
addition, the number of cells in the 0.3 or 0.6 µg/ml
epirubicin treatment groups were decreased (p<0.05 and
p<0.01, respectively) (Fig.
3A).
CYR61 and PCNA expression when B16
proliferation is inhibited
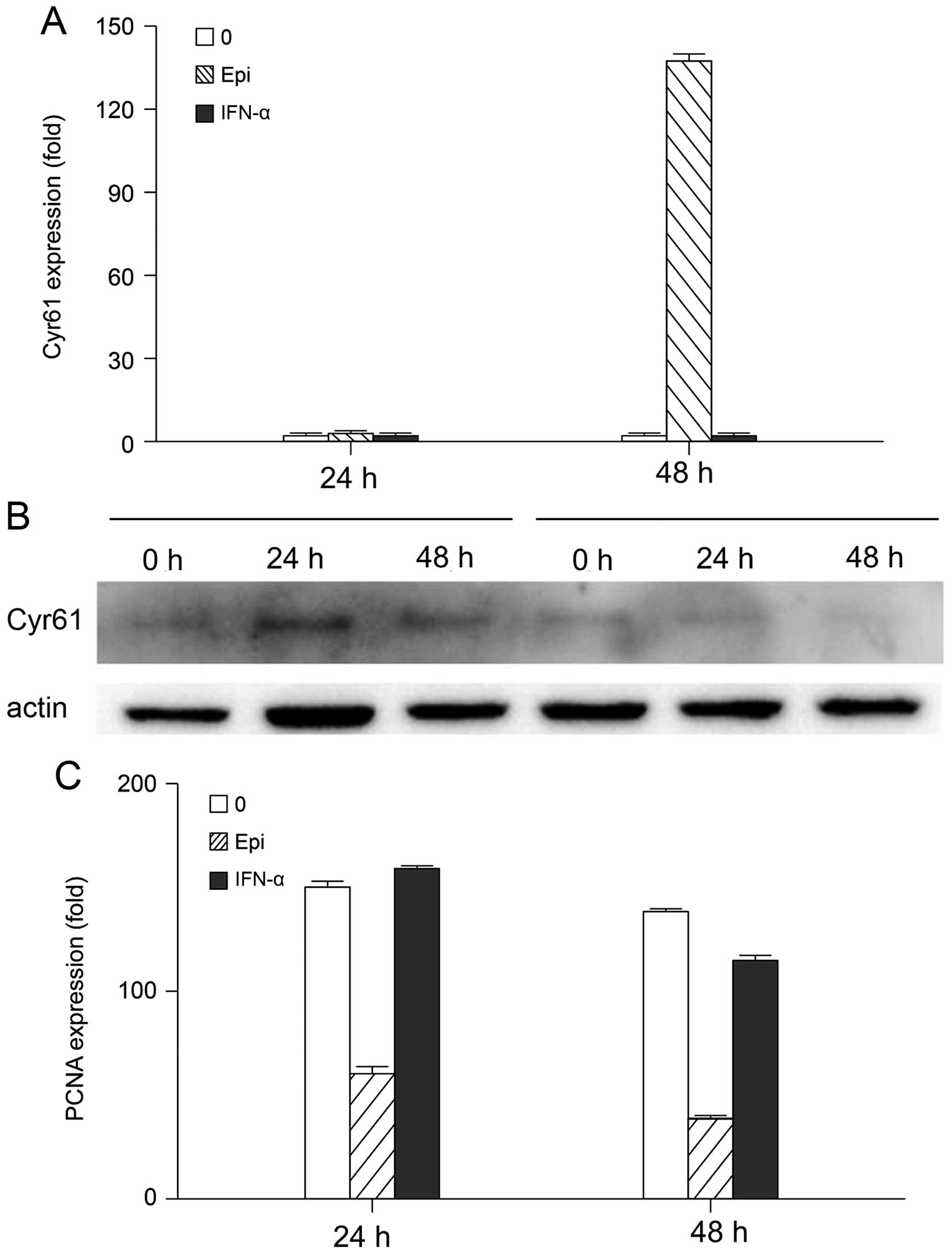
We further determined CYR61 and PCNA expression in
B16 cells treated with 0.3 µg/ml epirubicin or
103 IU/ml IFN-α. To analyze the relationship between
cell growth and CYR61 expression, we evaluated CYR61 gene
expression at different time-points using quantitative real-time
PCR, and CYR61 protein expression using western blot analysis.
In Fig. 4, we show
that after 24 h of treatment, neither 0.3 µg/ml epirubicin
nor 103 IU/ml IFN-α inhibited B16 proliferation or
promoted CYR61 expression. However, 48 h later, epirubicin
inhibited cell growth and promoted apoptosis, while IFN-α had no
effect (Fig. 4B). PCNA is an
important indicator for proliferation and differentiation of tumor
cells. As shown in Fig. 4C, at the
24 h point, 0.3 µg/ml epirubicin inhibited PCNA expression,
although the proliferative status of the cells did not change.
After 48 h, both 0.3 µg/ml epirubicin and 103
IU/ml IFN-α inhibited the proliferation of B16 cells, a process
accompanied by decreased PCNA expression. This suggests that
epirubicin had an earlier and sharper effect than IFN-α on PCNA
expression. Also, they probably have a different mechanism of
action, since solely epirubicin inhibits proliferation and promotes
apoptosis by upregulating CYR61.
Discussion
CYR61 has been reported to be overexpressed in
different clinical stages of several types of tumors, such as
breast cancer (22,23), glioma (24), and pancreatic cancer (25). However, in other cancers, such as:
non-small cell lung cancer (NSCLC) (11,26),
endometrial cancer, and papillary thyroid carcinoma (27–29),
the levels of CYR61 were shown to be reduced. Currently, the
expression profile of CYR61 in Chinese patients with malignant
melanoma is unclear.
Malignant melanoma has a complex array of
pathological features. During early stages, it is often
misdiagnosed as hyperpigmentation or nevi, due their similarities
in appearance. The incidence and exact mechanism of malignant
melanoma remain unclear, and some of the etiological factors that
have been implicated are malignant transformation of nevi,
ultraviolet radiation, racial and genetic background, trauma, viral
infection, and reduced immunity (30–33).
Several studies have reported abnormal gene and protein expression
in malignant melanoma, especially of the malignant melanoma
growth-related factors (34–37).
Tumor suppressor genes may also play an important role in melanoma
progression and aggression.
Here, we analyzed the expression profile of CYR61 in
skin samples from 78 Chinese patients with different pathologies,
including three common skin tumors (BCC, SCC, and malignant
melanoma), inflammatory lesions, and normal skins. Samples were
placed on TMAs, balanced and normalized by age and gender. The
level of CYR61 was examined by IHC and analyzed in correlation with
the pathological diagnosis.
CYR61 stained positive in normal skin and skin with
chronic inflammation around 80 and 75%, respectively. Compared with
levels found in normal skin, CRY61 was reduced in malignant
melanoma and BCC tissues, and overexpressed in SCC tissues.
Interestingly, we found no direct relationship between the CRY61
expression and the TNM stage. The percentage of CRY61-positive
cells was significantly lower in malignant tumors than in normal or
chronic inflammation skin. CYR61 expression was decreased in most
tumor tissues, mostly in malignant melanoma. This suggests that
CYR61 could become a potential therapeutic target and marker for
this cancer. Thus, we used a murine malignant melanoma cell line
(B16) to examine the effects of exogenous CYR61 on cell
proliferation and apoptosis in vitro. Our results show that
exogenous CYR61 inhibits cell growth and increases apoptosis.
In cancer therapy, proto-oncogenes are targeted to
block or reduce cancer cell activities, and tumor suppressor are
targeted to restore or increase their activity (38–43).
CYR61 could be classified as a tumor suppressor gene, since when
mutated, the inhibition of tumor growth is lost. While it seems to
act similarly to p53 a tumor growth suppressor gene, its role has
not been reported yet. This could be a significant finding for the
treatment and outcome of malignant melanoma.
Two anti-tumoral drugs, epirubicin and IFN-α, were
used to study the B16 cell growth and apoptosis. We first focused
on determining the optimal dose and time of action for IFN-α and
epirubicin. The results showed that 0.3 µg/ml epirubicin or
103 IU/ml IFN-α had negative effects on B16 cells. We
also found that no cell inhibition appeared before 24 h of
treatment, and that 48 h of treatment was best for suppressing
growth. Based on the cell growth curve, at 12 h post-treatment,
cells were yet to enter the proliferative phase, and at 24 h, when
proliferation reached a peak, the inhibitory effect of IFN-α or
epirubicin began to appear. CYR61 expression increased after 48 h
of treatment with epirubicin, but not with IFN-α. Western blot
analysis confirmed that IFN-α may affect B16 cell growth through a
non-CYR61 pathways.
PCNA is an essential protein for eukaryotic DNA
synthesis, closely related to several cell cycle regulators
(44,45). It expresses abnormally in a variety
of malignant tumors. Therefore, PCNA can be an important indicator
of cell proliferation and DNA synthesis. Previous studies confirmed
that intracellular microinjection of PCNA antisense
oligonucleotides or antibodies can inhibit DNA synthesis and cell
cycle progression (46–50). Studies on the expression of
survivin, an inhibitor of apoptosis, show that it may be associated
with the development of choroidal melanoma. Since PCNA is directly
related to survivin protein, the PCNA proliferation index increase
parallels the survivin increase in choroidal melanoma. This
suggests that PCNA may be an inhibitor of apoptosis during
choroidal melanoma development.
Our data indicated that after 24 h of treatment with
epirubicin, PCNA expression was inhibited, while B16 growth did not
change significantly. When epirubicin and IFN-α inhibited B16
proliferation, CYR61 expression decreased significantly. There was
an increase in apoptotic cells, consistent with the low levels of
PCNA. At 48 h, the PCNA expression was severely reduced, suggesting
that epirubicin suppresses B16 cell growth, by inhibiting either
survivin or another apoptosis suppressor gene. The effect of IFN-α
on PCNA expression was minimal and discernible only after 48 h.
These results showed that both drugs inhibit B16 cell proliferation
by decreasing PCNA expression. Epirubicin had an earlier and
sharper effect than IFN-α on PCNA expression, probably due to
different mechanisms of action. While epirubicin inhibited
proliferation and promoted apoptosis by upregulating CYR61, IFN-α
is likely to have a different target.
IFN-α, a soluble glycoprotein, is produced by a
variety of cells with a various anti-viral, anti-tumoral and
immunomodulatory roles (51).
Recent studies have shown that IFN also inhibits tumor angiogenesis
(52–54). Currently, IFN type I is widely used
in treatment of hematological cancers (55), follicular lymphoma (56), chronic myeloid leukemia (57), multiple myeloma (58) and solid tumors (59), AIDS related Kaposi's sarcoma
(60), renal carcinoma (61), and endocrine pancreatic tumors
(62). IFN I inhibits DNA synthesis
and slows the mitosis rate in a selective manner, since tumor cells
are 500–1,000 times more resilient than normal cells. In our study,
IFN-α inhibited cell proliferation, but did not affect the CRY61
expression. CYR61 inhibits B16 cell proliferation and promotes
apoptosis, when survivin and PCNA expression is reduced, a
mechanism confirmed when using epirubicin. IFN-α did not inhibit
cell growth by activating CYR61 and had only a slight inhibiting
effect on PCNA expression.
In conclusion, in this study, we found that CYR61
expression was lower in Chinese patients with malignant melanoma
compared with that of patients with other skin tumors or normal
skin. CYR61 expression was also reduced in proliferative B16 cells.
Using epirubicin and IFN-α to inhibit B16 proliferation, we also
found increased CYR61 and decreased PCNA expression in arrested B16
cells. In conclusion, our study provides evidence that CYR61 may be
a potential therapeutic target for malignant melanoma patients with
high CYR61.
Acknowledgments
This study is supported by grants from the
Department of Dermatology and Cutaneous Surgery of Shanghai Ninth
People's Hospital (Affiliated to Shanghai Jiaotong University
School of Medicine).
References
|
1
|
Lau LF and Nathans D: Identification of a
set of genes expressed during the G0/G1 transition of cultured
mouse cells. EMBO J. 4:3145–3151. 1985.PubMed/NCBI
|
|
2
|
Brigstock DR, Goldschmeding R, Katsube KI,
Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, et
al: Proposal for a unified CCN nomenclature. Mol Pathol.
56:127–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perbal B: CCN proteins: Multifunctional
signalling regulators. Lancet. 363:62–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Brien TP, Yang GP, Sanders L and Lau LF:
Expression of cyr61, a growth factor-inducible immediate-early
gene. Mol Cell Biol. 10:3569–3577. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brunner A, Chinn J, Neubauer M and Purchio
AF: Identification of a gene family regulated by transforming
growth factor-beta. DNA Cell Biol. 10:293–300. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jay P, Bergé-Lefranc JL, Marsollier C,
Méjean C, Taviaux S and Berta P: The human growth factor-inducible
immediate early gene, CYR61, maps to chromosome 1p. Oncogene.
14:1753–1757. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martinerie C, Viegas-Pequignot E, Nguyen
VC and Perbal B: Chromosomal mapping and expression of the human
cyr61 gene in tumour cells from the nervous system. Mol Pathol.
50:310–316. 1997. View Article : Google Scholar
|
|
8
|
Brigstock DR: The CCN family: A new
stimulus package. J Endocrinol. 178:169–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Menéndez JA, Mehmi I, Griggs DW and Lupu
R: The angiogenic factor CYR61 in breast cancer: Molecular
pathology and therapeutic perspectives. Endocr Relat Cancer.
10:141–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leask A and Abraham DJ: All in the CCN
family: Essential matricellular signaling modulators emerge from
the bunker. J Cell Sci. 119:4803–4810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong X, O'Kelly J, Xie D, Mori A, Lemp N,
McKenna R, Miller CW and Koeffler HP: Cyr61 suppresses the growth
of non-small-cell lung cancer cells via the beta-catenin-c-myc-p53
pathway. Oncogene. 23:4847–4855. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SY, Hahn HG, Nam KD, Park KC, Yun HY,
Baek KJ, Kwon NS and Kim DS: A derivative of 2-aminothiazole
inhibits melanogenesis in B16 mouse melanoma cells via glycogen
synthase kinase 3β phosphorylation. J Pharm Pharmacol.
63:1031–1036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klebanoff CA, Gattinoni L, Palmer DC,
Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD,
Finkelstein SE, et al: Determinants of successful CD8+
T-cell adoptive immunotherapy for large established tumors in mice.
Clin Cancer Res. 17:5343–5352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao H, Peng Y, Hong Y, Liu Y, Guo ZS,
Bartlett DL, Fu N and He Y: Lentivector prime and vaccinia virus
vector boost generate high-quality CD8 memory T cells and prevent
autochthonous mouse melanoma. J Immunol. 187:1788–1796. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Babic AM, Kireeva ML, Kolesnikova TV and
Lau LF: CYR61, a product of a growth factor-inducible immediate
early gene, promotes angiogenesis and tumor growth. Proc Natl Acad
Sci USA. 95:6355–6360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kunz M, Moeller S, Koczan D, Lorenz P,
Wenger RH, Glocker MO, Thiesen HJ, Gross G and Ibrahim SM:
Mechanisms of hypoxic gene regulation of angiogenesis factor Cyr61
in melanoma cells. J Biol Chem. 278:45651–45660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dobroff AS, Wang H, Melnikova VO, Villares
GJ, Zigler M, Huang L and Bar-Eli M: Silencing cAMP-response
element-binding protein (CREB) identifies CYR61 as a tumor
suppressor gene in melanoma. J Biol Chem. 284:26194–26206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: Assessment of radiosensitivity. Cancer Res.
47:943–946. 1987.PubMed/NCBI
|
|
19
|
Alley MC, Scudiero DA, Monks A, Hursey ML,
Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH and Boyd
MR: Feasibility of drug screening with panels of human tumor cell
lines using a microculture tetrazolium assay. Cancer Res.
48:589–601. 1988.PubMed/NCBI
|
|
20
|
Zhang Q, Wu J, Cao Q, Xiao L, Wang L, He
D, Ouyang G, Lin J, Shen B, Shi Y, et al: A critical role of Cyr61
in interleukin-17-dependent proliferation of fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Rheum.
60:3602–3612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun M, Ma F, Zeng X, Liu Q, Zhao XL, Wu
FX, Wu GP, Zhang ZF, Gu B, Zhao YF, et al: Triphalangeal
thumb-polysyndactyly syndrome and syndactyly type IV are caused by
genomic duplications involving the long range, limb-specific SHH
enhancer. J Med Genet. 45:589–595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin MT, Chang CC, Chen ST, Chang HL, Su
JL, Chau YP and Kuo ML: Cyr61 expression confers resistance to
apoptosis in breast cancer MCF-7 cells by a mechanism of
NF-kappaB-dependent XIAP up-regulation. J Biol Chem.
279:24015–24023. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Menendez JA, Vellon L, Mehmi I, Teng PK,
Griggs DW and Lupu R: A novel CYR61-triggered 'CYR61-alphavbeta3
integrin loop' regulates breast cancer cell survival and
chemosensitivity through activation of ERK1/ERK2 MAPK signaling
pathway. Oncogene. 24:761–779. 2005. View Article : Google Scholar
|
|
24
|
Xie D, Yin D, Wang HJ, Liu GT, Elashoff R,
Black K and Koeffler HP: Levels of expression of CYR61 and CTGF are
prognostic for tumor progression and survival of individuals with
gliomas. Clin Cancer Res. 10:2072–2081. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holloway SE, Beck AW, Girard L, Jaber MR,
Barnett CC Jr, Brekken RA and Fleming JB: Increased expression of
Cyr61 (CCN1) identified in peritoneal metastases from human
pancreatic cancer. J Am Coll Surg. 200:371–377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tong X, Xie D, O'Kelly J, Miller CW,
Muller-Tidow C and Koeffler HP: Cyr61, a member of CCN family, is a
tumor suppressor in non-small cell lung cancer. J Biol Chem.
276:47709–47714. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sampath D, Zhu Y, Winneker RC and Zhang Z:
Aberrant expression of Cyr61, a member of the CCN
(CTGF/Cyr61/Cef10/NOVH) family, and dysregulation by 17
beta-estradiol and basic fibroblast growth factor in human uterine
leiomyomas. J Clin Endocrinol Metab. 86:1707–1715. 2001.PubMed/NCBI
|
|
28
|
Wasenius VM, Hemmer S, Kettunen E,
Knuutila S, Franssila K and Joensuu H: Hepatocyte growth factor
receptor, matrix metalloproteinase-11, tissue inhibitor of
metalloproteinase-1, and fibronectin are up-regulated in papillary
thyroid carcinoma: A cDNA and tissue microarray study. Clin Cancer
Res. 9:68–75. 2003.PubMed/NCBI
|
|
29
|
Chien W, Kumagai T, Miller CW, Desmond JC,
Frank JM, Said JW and Koeffler HP: Cyr61 suppresses growth of human
endometrial cancer cells. J Biol Chem. 279:53087–53096. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dika E, Fanti PA, Vaccari S, Patrizi A and
Maibach HI: Causal relationship between exposure to chemicals and
malignant melanoma? A review and study proposal. Rev Environ
Health. 25:255–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong Y, Kumar SM and Xu X: Molecular
pathogenesis of sporadic melanoma and melanoma-initiating cells.
Arch Pathol Lab Med. 134:1740–1749. 2010.PubMed/NCBI
|
|
32
|
Mihić LL, Bulat V, Situm M, Krolo I and
Seserko A: The role of apoptosis in the pathogenesis of malignant
melanoma. Coll Antropol. 34(Suppl 2): 303–306. 2010.
|
|
33
|
Khalid U, Saleem T, Imam AM and Khan MR:
Pathogenesis, diagnosis and management of primary melanoma of the
colon. World J Surg Oncol. 9:142011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gruber F, Kastelan M, Brajac I, Saftić M,
Peharda V, Cabrijan L, Stanić Zgombić Z and Simonić E: Molecular
and genetic mechanisms in melanoma. Coll Antropol. 32(Suppl 2):
147–152. 2008.
|
|
35
|
Ibrahim N and Haluska FG: Molecular
pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol.
4:551–579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Russo AE, Torrisi E, Bevelacqua Y,
Perrotta R, Libra M, McCubrey JA, Spandidos DA, Stivala F and
Malaponte G: Melanoma: Molecular pathogenesis and emerging target
therapies (Review). Int J Oncol. 34:1481–1489. 2009.PubMed/NCBI
|
|
37
|
Ugurel S, Utikal J and Becker JC: Tumor
biomarkers in melanoma. Cancer Control. 16:219–224. 2009.PubMed/NCBI
|
|
38
|
Chandeck C and Mooi WJ: Oncogene-induced
cellular senescence. Adv Anat Pathol. 17:42–48. 2010.
|
|
39
|
Parsons BL, Myers MB, Meng F, Wang Y and
McKinzie PB: Oncomutations as biomarkers of cancer risk. Environ
Mol Mutagen. 51:836–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Adhikari AS and Iwakuma T: Mutant p53 gain
of oncogenic function: In vivo evidence, mechanism of action and
its clinical implications. Fukuoka Igaku Zasshi. 100:217–228.
2009.PubMed/NCBI
|
|
41
|
Bar J, Moskovits N and Oren M: Involvement
of stromal p53 in tumor-stroma interactions. Semin Cell Dev Biol.
21:47–54. 2010. View Article : Google Scholar :
|
|
42
|
Lane D and Levine A: p53 Research: The
past thirty years and the next thirty years. Cold Spring Harb
Perspect Biol. 2:a0008932010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Solomon H, Brosh R, Buganim Y and Rotter
V: Inactivation of the p53 tumor suppressor gene and activation of
the Ras oncogene: Cooperative events in tumorigenesis. Discov Med.
9:448–454. 2010.PubMed/NCBI
|
|
44
|
Dervan PA, Magee HM, Buckley C and Carney
DN: Proliferating cell nuclear antigen counts in formalin-fixed
paraffin-embedded tissue correlate with Ki-67 in fresh tissue. Am J
Clin Pathol. 97(Suppl 1): S21–S28. 1992.PubMed/NCBI
|
|
45
|
Bolton WE, Mikulka WR, Healy CG,
Schmittling RJ and Kenyon NS: Expression of proliferation
associated antigens in the cell cycle of synchronized mammalian
cells. Cytometry. 13:117–126. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakano H, Namatame K, Suzuki T, Takahashi
H, Sakai H, Nakamura T and Kumada K: Histopathological response to
preoperative chemotherapy including 5-fluorouracil additionally
assessed by immunocytochemical and pharmacologic parameters in
patients with advanced gastric cancer. Surg Today. 26:482–488.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hiraga Y, Tanaka S, Haruma K, Yoshihara M,
Sumii K, Kajiyama G, Shimamoto F and Kohno N: Immunoreactive MUC1
expression at the deepest invasive portion correlates with
prognosis of colorectal cancer. Oncology. 55:307–319. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kunihiro M, Tanaka S, Haruma K, Yoshihara
M, Sumii K, Kajiyama G and Shimamoto F: Combined expression of
HLA-DR antigen and proliferating cell nuclear antigen correlate
with colorectal cancer prognosis. Oncology. 55:326–333. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jia X and Han C: Biomarkers in the studies
on chemoprevention of colorectal cancer. Wei Sheng Yan Jiu.
29:109–111. 2000.In Chinese.
|
|
50
|
Reszeć J, Kańczuga-Koda L, Sulkowska M,
Koda M, Cylwik J, Barwijuk-Machała M and Sulkowski S: An evaluation
of Ki-67 and PCNA expression in conjunctival and eyelid tumours.
Folia Morphol (Warsz). 63:95–98. 2004.
|
|
51
|
Meyer O: Interferons and autoimmune
disorders. Joint Bone Spine. 76:464–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Heng DY and Bukowski RM: Anti-angiogenic
targets in the treatment of advanced renal cell carcinoma. Curr
Cancer Drug Targets. 8:676–682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Agarwala SS and Case S: Everolimus
(RAD001) in the treatment of advanced renal cell carcinoma: A
review. Oncologist. 15:236–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ather MH, Masood N and Siddiqui T: Current
management of advanced and metastatic renal cell carcinoma. Urol J.
7:1–9. 2010.PubMed/NCBI
|
|
55
|
Ramakrishna R and Manoharan A: Sustained
long-term remissions with weekly interferon maintenance therapy in
hairy cell leukemia. Asia Pac J Clin Oncol. 6:210–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baldo P, Rupolo M, Compagnoni A, Lazzarini
R, Bearz A, Cannizzaro R, Spazzapan S, Truccolo I and Moja L:
Interferon-alpha for maintenance of follicular lymphoma. Cochrane
Database Syst Rev. CD0046292010.PubMed/NCBI
|
|
57
|
Burchert A and Neubauer A: Interferon
alpha and T-cell responses in chronic myeloid leukemia. Leuk
Lymphoma. 46:167–175. 2005. View Article : Google Scholar
|
|
58
|
Khoo TL, Vangsted AJ, Joshua D and Gibson
J: Interferon-alpha in the treatment of multiple myeloma. Curr Drug
Targets. 12:437–446. 2011. View Article : Google Scholar
|
|
59
|
Garbe C, Eigentler TK, Keilholz U,
Hauschild A and Kirkwood JM: Systematic review of medical treatment
in melanoma: Current status and future prospects. Oncologist.
16:5–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Aversa SM, Cattelan AM, Salvagno L,
Crivellari G, Banna G, Trevenzoli M, Chiarion-Sileni V and
Monfardini S: Treatments of AIDS-related Kaposi's sarcoma. Crit Rev
Oncol Hematol. 53:253–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chowdhury S, Larkin JM and Gore ME: Recent
advances in the treatment of renal cell carcinoma and the role of
targeted therapies. Eur J Cancer. 44:2152–2161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Massironi S, Sciola V, Peracchi M,
Ciafardini C, Spampatti MP and Conte D: Neuroendocrine tumors of
the gastro-entero-pancreatic system. World J Gastroenterol.
14:5377–5384. 2008. View Article : Google Scholar : PubMed/NCBI
|