Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies in the world (1). Although the surgical approaches and
adjuvant chemotherapy have improved, the survival rate of HCC
patients at advanced stages remains low (2–4).
Therefore, it is imperative to investigate the molecular mechanisms
of HCC to provide novel therapeutic approaches.
Epithelial-mesenchymal transition (EMT) is a crucial
step in tumor progression. During EMT, epithelial cells lose their
characteristic marker E-cadherin and gain mesenchymal markers
including N-cadherin and vimentin (5,6).
Hypoxia is an essential component of the neoplastic
microenvironment. It contributes to the progression of various
cancers by activating adaptive transcriptional programs that
promote cell survival, motility, and tumor angiogenesis. In HCC,
regions of hypoxia are present throughout the tissue because of
areas of necrosis and irregular blood flow (7). Previous studies showed that hypoxia
induces EMT in HCC cells (8,9). Under
hypoxic conditions, cancer cells develop escape mechanisms to
survive and leave the unfavorable environment (10). Afterwards, they acquire increased
potential for local invasion and ability to evade to distant
organs. Thus, preventing hypoxia-induced EMT is a promising
approach for treatment of HCC.
The frequently rearranged in advanced T-cell
lymphomas-1 (FRAT1) gene, located on human chromosome 10q24.1,
encodes a 29-kDa protein comprising 279 amino acids (11). It is a positive regulator of
β-catenin in the Wnt pathway (12).
Previous studies have shown that FRAT1 plays a critical role in
different types of tumors (13–15).
For example, Guo et al reported that silencing of FRAT1
inhibits human glioblastoma cell growth, migration and invasion
(16). However, the expression and
role of FRAT1 in HCC has not been elucidated. In this study, we
investigated the effect of FRAT1 on EMT process in HCC cells
induced by hypoxia.
Materials and methods
Tissue specimens
A total of 12 HCC and 12 non-cancerous liver tissue
samples were obtained from the Department of Infectious Disease,
The First Affiliated Hospital of Xi'an Jiaotong University (China),
during the period from 2014 to 2015. Dissected samples were frozen
immediately after surgery and stored at −80°C until needed. A
protocol for the use of patient samples was approved by the Medical
Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong
University (China) and written informed consent was obtained from
each patient.
Cell culture and hypoxia treatment
Three human HCC lines (HepG2, 97H and HCCLM3) a
hepatocyte cell line (HL-7702) were purchased from the American
Type Culture Collection (ATCC, Manassas, VA, USA). These cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% (v/v) fetal bovine serum (FBS; Gibco, Rockville, MD, USA)
and 100 U/ml streptomycin and penicillin (Gibco). The cells were
incubated in a humidified atmosphere containing 5% CO2
incubator at 37°C. For hypoxic culture, cells were incubated in a
hypoxic chamber with 1% oxygen, or grown in culture media
containing cobalt chloride (CoCl2) for 24 h.
Quantitative real-time reverse
transcription PCR (qRT-PCR)
Total RNA was extracted with Tri-reagent (Sigma, St.
Louis, MO, USA). Aliquots (5 µg) of RNA were reverse
transcribed to cDNA using Superscribe First-Strand Synthesis system
(Invitrogen, Carlsbad, CA, USA). RT-qPCR was performed with
Brilliant SYBR Green Master Mix (Bio-Rad Laboratories, Hercules,
CA, USA). Primers pairs used were: FRAT1, (forward,
5′-GGCAGAACCTGGCTACTCTG-3′; reverse,
5′-CACGAGCTTGATTGCAAGTTCAGG-3′); and β-actin (forward,
5′-AAATCGTGCGTGACATCAAAGA-3′; and reverse,
5′-GGCCATCTCCTGCTCGAA-3′). A comparative CT method
(2−ΔΔCt) was used to analyze the relative changes in
gene expression.
Western blotting
The HCC cells were washed twice with ice-cold PBS
and then lysed with cell lysis buffer (50 mM NaF, 10 mM
Na2P2O7, 2% SDS, 1 mM PMSF).
Protein concentration was measured using a BCA protein assay kit
(Bio-Rad). Equal amount of protein was separated by SDS-PAGE and
transferred to Immobilon-P Transfer membranes (Millipore, Boston,
MA, USA). Blots were blocked with 5% fat-free milk for 1 h at room
temperature. Immunodetection of target proteins (FRAT1, E-cadherin,
N-cadherin, vimentin, β-catenin, cyclin D1 and c-myc) and β-actin
was performed using mouse monoclonal antibody (1:1,500, Santa
Cruz), and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), respectively. After washing with TBST buffer (0.05 mol/l
Tris, 0.15 mol/l NaCl and 0.05% Tween-20), the membranes were
incubated with goat anti-mouse horseradish peroxidase-conjugated
secondary antibody (Santa Cruz Biotechnology) for 1 h.
Subsequently, immunoblots were visualized by enhanced
chemiluminescence detection (ECL, Amersham, Bucks, UK).
RNA interference and cell
transfection
Small interfering RNA (siRNA) against FRAT1 was
transfected into HepG2 cells in 24-well culture plates using
Lipofectamine 2000 reagent (Invitrogen) according to the
manufacturer's instructions. The siRNA targeting FRAT1 was as
follows: FRAT1, 5′-GCAGTTACGTGCAAAGCTT-3′.
Cell migration and invasion assay
Cell migration assay was performed using Transwell
cell culture inserts with 8-µm pore size polycarbonate
membrane (Millipore). In brief, HepG2 cells were transfected with
si-Mock or si-FRAT1 for 24 h, and then were seeded in the upper
compartment of the chamber, and 500 µl DMEM medium with 10%
FBS was added into the lower compartment. Filtered CoCl2
was then added to the upper insert, and free-CoCl2 was
used as controls. The cells were allowed to migrate for 24 h, after
which the upper surface of the membrane was wiped to remove
non-migratory cells. The migratory cells were fixed in 95% ethanol
and stained with 0.1% crystal violet, photographed using a
fluorescent microscope (Nikon, Japan), and counted under a
microscope. The invasion assay was done by the same procedure,
except that the membrane was coated with Matrigel to form a matrix
barrier.
Statistical analysis
Statistical analysis was conducted using SPSS
version 16.0 (Chicago, IL, USA). Data are expressed as the mean ±
SD of at least three independent experiments. Statistical
comparisons were performed using one-way analysis of variance
(ANOVA), followed by Tukey's post hoc multiple comparison tests.
The value of P<0.05 was considered statistically
significant.
Results
FRAT1 is highly expressed in HCC tissues
and cell lines
To investigate the role of FRAT1 in the development
of HCC, we first detected the expression of FRAT1 in HCC tissues.
The results demonstrated that the mRNA expression levels of FRAT1
in HCC tissues were significantly higher than those in the
non-cancerous liver tissues (Fig.
1A). Subsequently, we analyzed FRAT1 expression in human HCC
lines (HepG2, 97H and HCCLM3) using qRT-PCR and western blotting.
The expression levels of FRAT1 mRNA and protein were increased
significantly in human HCC cell lines, as compared with the control
group (Fig. 1B and C).
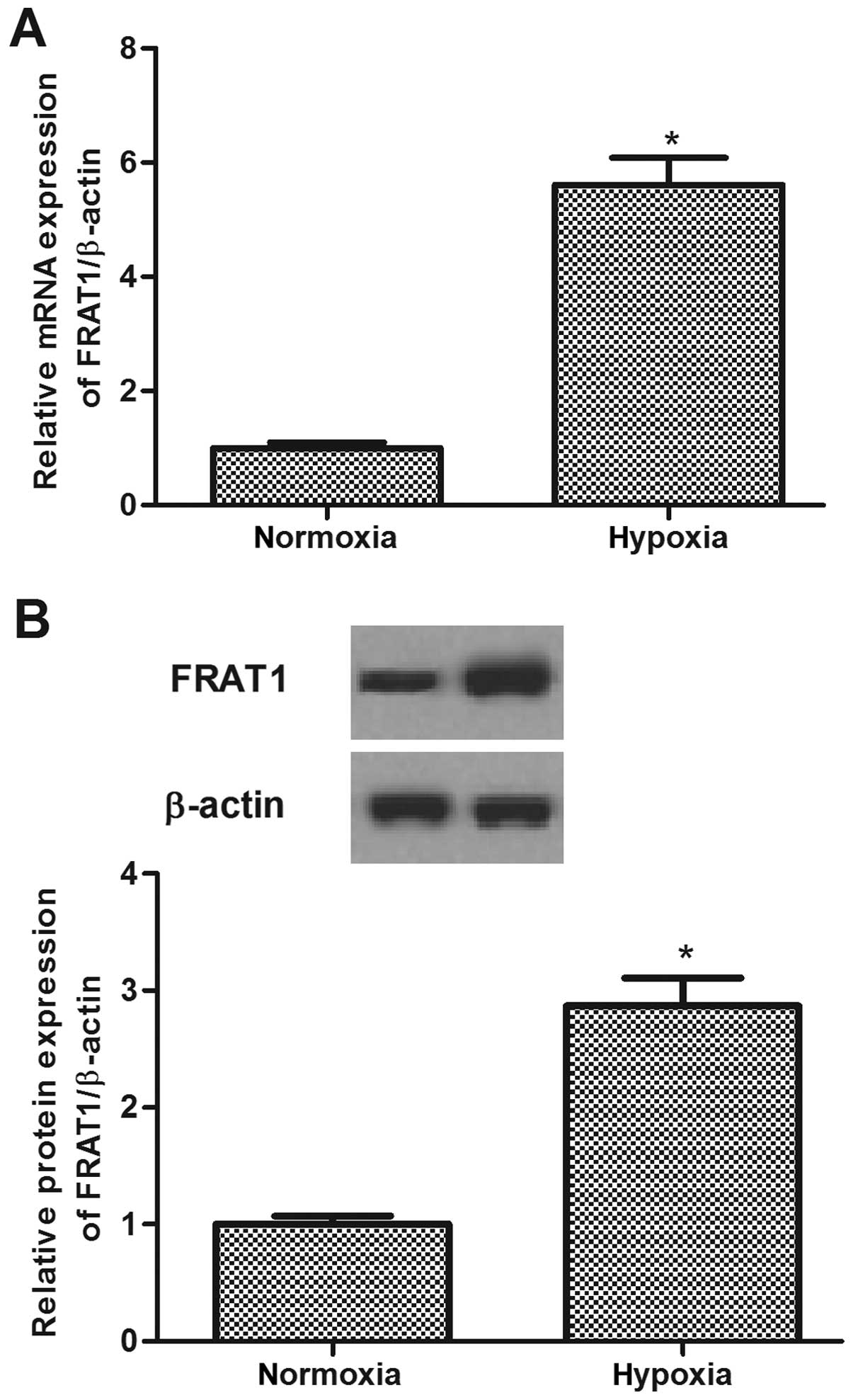
Hypoxia induces FRAT1 expression in HepG2
cells
To determine whether FRAT1 expression is regulated
by hypoxia, we detected the expression level of FRAT1 in HepG2
cells incubated during hypoxia vs. normoxia. As shown in Fig. 2, after exposure to hypoxia, the
expression of FRAT1 at both mRNA and protein levels was obviously
increased in HepG2 cells.
Silencing FRAT1 inhibits hypoxia-induced
migration and invasion in HepG2 cells
To investigate the role of FRAT1 in hypoxia-induced
migration and invasion, we examined the effects of FRAT1 siRNA on
the migration and invasion in HCC cells induced by hypoxia. The
effect of FRAT1 gene silencing was confirmed by western blotting
(Fig. 3A). Moreover, Transwell
assay indicated that hypoxia significantly increased migration of
HepG2 cells. In addition, cells migrated through the
Matrigel-coated membrane (invasion assay) also greatly increased by
hypoxia in HepG2 cells. Knockdown of FRAT1 resulted in a decreased
migration and invasion compared to si-Mock-transfected cells in the
presence of hypoxia, respectively (Fig.
3B and C).
Silencing FRAT1 suppresses
hypoxia-induced EMT in HepG2 cells
The role of FRAT1 in hypoxia-induced EMT was
investigated. As indicated in Fig.
4, hypoxia treatment decreased the expression of epithelial
maker E-cadherin, and increased the expression levels of
mesenchymal makers N-cadherin and vimentin in human HepG2 cells.
However, FRAT1 knockdown partially restored the expression of
E-cadherin and decreased the expression levels of N-cadherin and
vimentin.
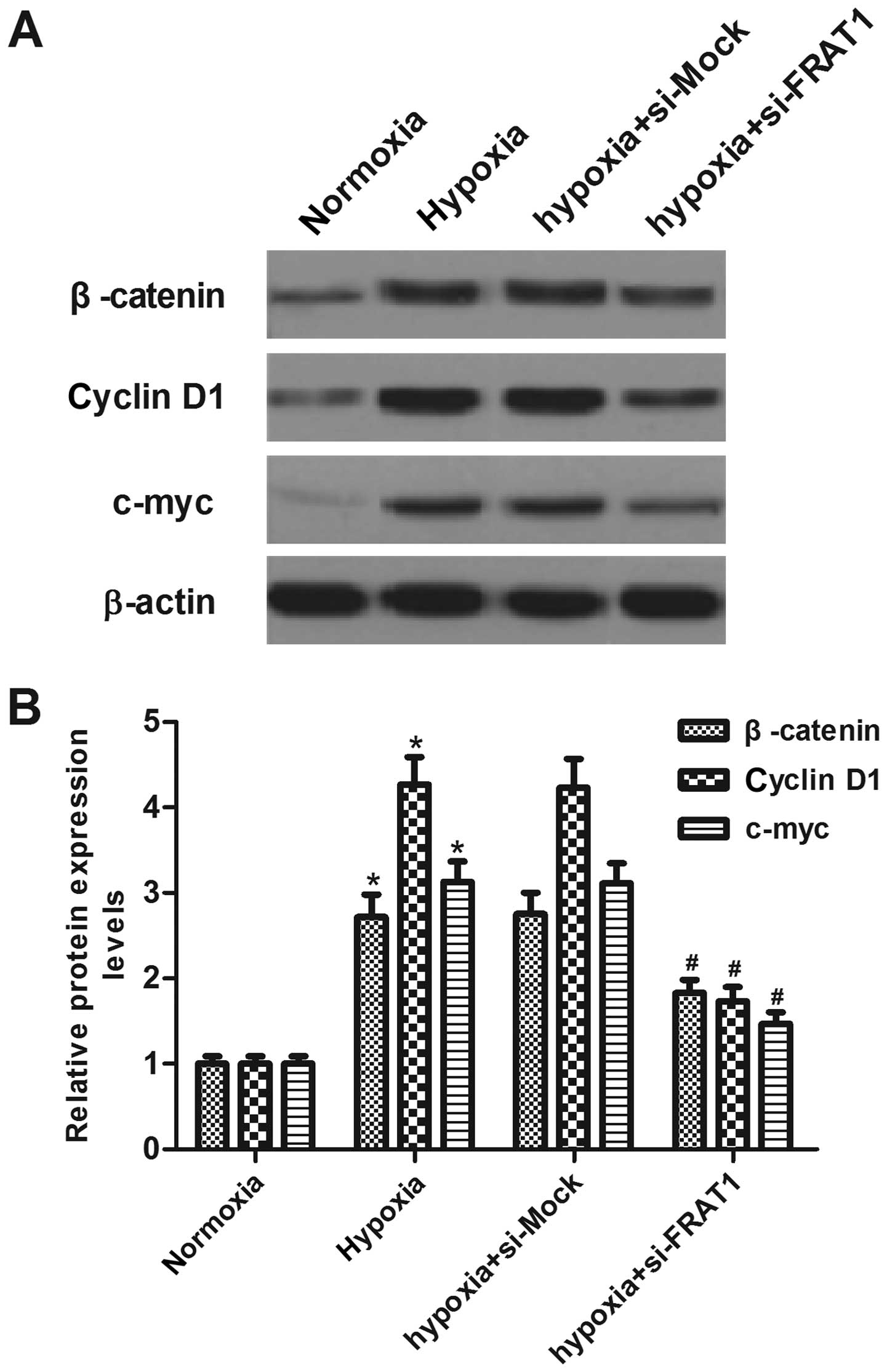
Silencing FRAT1 suppresses
hypoxia-induced Wnt/β-catenin pathway activation in HepG2
cells
To gain insight into the molecular mechanisms
involved in FRAT1-mediated EMT induced by hypoxia in HepG2 cells,
we analyzed the key components of the Wnt signaling pathway.
Western blotting showed that hypoxia significantly increased
protein levels of β-catenin, cyclin D1 and c-myc, as compared to
the normoxia group. However, FRAT1 knockdown partially suppressed
the expression levels of β-catenin, cyclin D1 and c-myc in HepG2
cells under the same hypoxic condition (Fig. 5).
Discussion
In this study, we found that FRAT1 is markedly
expressed in HCC tissues and cell lines. Hypoxia induced FRAT1
expression in HepG2 cells. FRAT1 knockdown inhibited
hypoxia-induced cell migration/invasion, downregulation of
epithelial markers and upregulation of mesenchymal markers.
Moreover, FRAT1 knockdown suppressed the expression levels of
β-catenin, cyclin D1 and c-myc in HepG2 cells under the same
hypoxic condition.
Recently, several reports have indicated that FRAT1
expression was high in various cancer tissues compared with normal
tissues. Our results are consistent with the previous reports. In
this study, we observed that FRAT1 is markedly expressed in HCC
tissues and cell lines. Hypoxia is an important micro-environmental
pressure present in the majority of solid tumors. We also found
that hypoxia obviously induced the expression of FRAT1 in HepG2
cells. These data suggest that FRAT1 may function as an oncogene
involved in the development and progression of HCC.
EMT is a process by which epithelial cells lose
epithelial characteristics and gain a mesenchymal phenotype.
Reduction or a loss of E-cadherin expression has a crucial role in
tumor progression to invasive cancer and is also one of the
well-established hallmarks of EMT (17). Previous studies demonstrated that
hypoxia might stimulate EMT of HCC cells. Hypoxia increased the
expression of Slug and Snail in HCC cells, which in turn inhibited
E-cadherin expression (10,18). Consistent with these results, in
this study, we found that hypoxia induced the migration/invasion
and EMT process in HepG2 cells, whereas, FRAT1 knockdown inhibited
these hypoxia-induced effects. These data suggest that silencing
FRAT1 inhibits hypoxia-induced EMT, consequently affects cell
migration and invasion in HCC cells.
Accumulated evidence has demonstrated a significant
role for the Wnt/β-catenin signaling pathway in the development and
progression of HCC. Aberrantly activated Wnt/β-catenin signaling
pathway due to overexpression of upstream components of this
pathway such as Frizzled (FZD) receptors and Wnt ligands is a
common early event in the pathogenesis of HCC (19–21).
β-catenin is a critical end component of the Wnt signaling pathway
that regulates HCC cell growth, migration and invasion (22,23).
Studies have demonstrated that β-catenin-mediated transcription can
induce the expression of Slug and Twist1, thereby contributing to
the EMT program (24,25). FRAT1 is a positive regulator of the
Wnt/β-catenin signaling pathway and one study demonstrated that
silencing of FRAT1 could increase the phosphorylation of β-catenin
and lead to a decreased β-catenin level (26). Moreover, hypoxia causes dysfunction
of the E-cadherin/β-catenin complex with an accumulation of
β-catenin in the nucleus and induces an invasive phenotype of tumor
cells in the development and progression of cancer (27). It was reported that HIF-2α directly
interacts with β-catenin and thereby modulate TCF-4-mediated
transcriptional activity, resulting in the EMT progress (28). In this study, we observed that RAT1
knockdown suppressed the expression levels of β-catenin, cyclin D1
and c-myc in HepG2 cells under hypoxic condition. These data
suggest that knockdown of FRAT1 inhibits hypoxia-induced EMT via
suppression of the Wnt/β-catenin pathway in HCC cells.
In conclusion, our results revealed that FRAT1 is a
hypoxia factor that is critical for the induction of EMT in HCC
cells. These data suggest a potential role for targeting FRAT1 in
the prevention of hypoxia-induced HCC cancer progression and
metastasis mediated by EMT.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernandez-Gea V, Toffanin S, Friedman SL
and Llovet JM: Role of the microenvironment in the pathogenesis and
treatment of hepatocellular carcinoma. Gastroenterology.
144:512–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villanueva A, Hernandez-Gea V and Llovet
JM: Medical therapies for hepatocellular carcinoma: A critical view
of the evidence. Nat Rev Gastroenterol Hepatol. 10:34–42. 2013.
View Article : Google Scholar
|
|
5
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan W, Fu Y, Tian D, Liao J, Liu M, Wang
B, Xia L, Zhu Q and Luo M: PI3 kinase/Akt signaling mediates
epithelial-mesenchymal transition in hypoxic hepatocellular
carcinoma cells. Biochem Biophys Res Commun. 382:631–636. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jing Y, Han Z, Zhang S, Liu Y and Wei L:
Epithelial-mesenchymal transition in tumor microenvironment. Cell
Biosci. 1:292011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Huang G, Li X, Zhang Y, Jiang Y,
Shen J, Liu J, Wang Q, Zhu J, Feng X, et al: Hypoxia induces
epithelial-mesenchymal transition via activation of SNAI1 by
hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC
Cancer. 13:1082013. View Article : Google Scholar
|
|
11
|
Saitoh T and Katoh M: FRAT1 and FRAT2,
clustered in human chromosome 10q24.1 region, are up-regulated in
gastric cancer. Int J Oncol. 19:311–315. 2001.PubMed/NCBI
|
|
12
|
Jonkers J, Korswagen HC, Acton D, Breuer M
and Berns A: Activation of a novel proto-oncogene, Frat1,
contributes to progression of mouse T-cell lymphomas. EMBO J.
16:441–450. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Yu J-H, Lin X-Y, Miao Y, Han Y,
Fan CF, Dong XJ, Dai SD and Wang EH: Overexpression of Frat1
correlates with malignant phenotype and advanced stage in human
non-small cell lung cancer. Virchows Arch. 459:255–263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Liu S, Zhu H, Zhang W, Zhang G,
Zhou X, Zhou C, Quan L, Bai J, Xue L, et al: FRAT1 overexpression
leads to aberrant activation of β-catenin/TCF pathway in esophageal
squamous cell carcinoma. Int J Cancer. 123:561–568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo G, Liu B, Zhong C, Zhang X, Mao X,
Wang P, Jiang X, Huo J, Jin J, Liu X, et al: FRAT1 expression and
its correlation with pathologic grade, proliferation, and apoptosis
in human astrocytomas. Med Oncol. 28:1–6. 2011. View Article : Google Scholar
|
|
16
|
Guo G, Kuai D, Cai S, Xue N, Liu Y, Hao J,
Fan Y, Jin J, Mao X, Liu B, et al: Knockdown of FRAT1 expression by
RNA interference inhibits human glioblastoma cell growth, migration
and invasion. PLoS One. 8:e612062013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: Are they cousins or twins? Cancers (Basel).
3:716–729. 2011. View Article : Google Scholar
|
|
18
|
Jiao M and Nan K-J: Activation of PI3
kinase/Akt/HIF-1α pathway contributes to hypoxia-induced
epithelial-mesenchymal transition and chemoresistance in
hepatocellular carcinoma. Int J Oncol. 40:461–468. 2012.
|
|
19
|
Bengochea A, de Souza MM, Lefrançois L, Le
Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, et
al: Common dysregulation of Wnt/Frizzled receptor elements in human
hepatocellular carcinoma. Br J Cancer. 99:143–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HC, Kim M and Wands JR: Wnt/Frizzled
signaling in hepatocellular carcinoma. Front Biosci. 11:1901–1915.
2006. View Article : Google Scholar
|
|
21
|
Merle P, de la Monte S, Kim M, Herrmann M,
Tanaka S, Von Dem Bussche A, Kew MC, Trepo C and Wands JR:
Functional consequences of frizzled-7 receptor overexpression in
human hepatocellular carcinoma. Gastroenterology. 127:1110–1122.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou
F, Shan X, Cai X, Chen Q, Ling N, et al: Enhancement of canonical
Wnt/β-catenin signaling activity by HCV core protein promotes cell
growth of hepatocellular carcinoma cells. PLoS One. 6:e274962011.
View Article : Google Scholar
|
|
23
|
Thompson MD and Monga SP: WNT/β-catenin
signaling in liver health and disease. Hepatology. 45:1298–1305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, Blechman J, Savagner P and Ben-Ze'ev A: Autoregulation of
E-cadherin expression by cadherin-cadherin interactions: The roles
of β-catenin signaling, Slug, and MAPK. J Cell Biol. 163:847–857.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin R, Liu W, Menezes S, Yue F, Zheng M,
Kovacevic Z and Richardson DR: The metastasis suppressor NDRG1
modulates the phosphorylation and nuclear translocation of
β-catenin through mechanisms involving FRAT1 and PAK4. J Cell Sci.
127:3116–3130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Demir R, Dimmler A, Naschberger E, Demir
I, Papadopoulos T, Melling N, Sturzl M and Hohenberger W: Malignant
progression of invasive tumour cells seen in hypoxia present an
accumulation of β-catenin in the nucleus at the tumour front. Exp
Mol Pathol. 87:109–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi H, Chun Y-S, Kim T-Y and Park J-W:
HIF-2α enhances β-catenin/TCF-driven transcription by interacting
with β-catenin. Cancer Res. 70:10101–10111. 2010. View Article : Google Scholar : PubMed/NCBI
|