Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide. Non-small cell lung cancer (NSCLC) accounts
for approximately 80% of lung cancers. Despite considerable
progress in diagnosis and treatment, the overall 5-year survival
rate of NSCLC patients still remains <15% (1,2). These
findings underscore that elucidating molecular mechanism of
pathogenesis and progression of lung cancer will offer novel
targets for effective therapies.
Cancer stem cells (CSCs) or cancer initiating cells
(CICs) are a rare subpopulation of undifferentiated cells that are
responsible for tumor initiation, maintenance and spreading
(3–5). They have been identified in various
human malignancies, including breast, brain, prostate, pancreatic,
colon and lung cancer (6–11). CSCs have been blamed for playing a
critical role in drug resistance and cancer metastasis, which may
explain why it is difficult to completely eradicate cancer and why
recurrence is a real threat in eradication of tumors completely
(12–14).
MicroRNAs (miRNAs/miRs) are small non-coding RNAs of
20–22 nucleotides that have been implicated in various types of
cancer (15–17). Abnormal miRNA expression has been
linked to diseases, including lung cancer and it has been found
implicated in a multitude of cellular processes including
proliferation, differentiation, invasion, migration and apoptosis
16,18–24.
MicroRNAs (miRNAs) regulate both normal stem cells and CSCs and
miRNA dysregulation has been implicated in tumorigenesis 16,25–28.
Recently, it has been reported that miR-520a-3p can inhibit
proliferation, apoptosis and metastasis in NSCLC by targeting
MAP3K2, and miR-520a-3p may be used as a prognostic marker for
NSCLC in clinical research (29).
However, its roles still keep emerging in lung cancer.
In the present study, we found that miR-520a-3p
expression is downregulated in NSCLC (non-small cell lung cancer)
and SCLC (small cell lung cancer). miR-520a-3p can inhibit
proliferation and cancer stem cell phenotype in NSCLC and SCLC
cells. Overexpressing miR-520a-3p can degrade HOXD8 mRNA in NSCLC
cells, but its overexpression cannot suppress HOXD8 in SCLC cells.
HOXD8 protein is upregulated in NSCLC tissues and its
overexpression can promote proliferation, formation of cancer stem
cells, migration and invasion in NSCLC cells. MET amplification
play a pivotal role in gefitinib resistance in lung cancer. We
found that miR-520a-3p can downregulate MET protein expression and
HOXD8 can upregulate MET protein expression, implying that
miR-520a-3p and HOXD8 might be potential targets for the therapy of
NSCLC. Thus, we concluded that microRNA-520a-3p inhibits
proliferation and cancer stem cell phenotype by targeting HOXD8 in
NSCLC cells and restoration of microRNA-520a-3p might be a
therapeutic strategy to reverse gefitinib resistance.
Materials and methods
NSCLC and SCLC tissues
Lung cancer tissues and adjacent normal tissues were
obtained from the First Affiliated Hospital of Zhengzhou
University. All tissues (20 NSCLC tissues and 19 SCLC tissues) were
examined histologically, and pathologists confirmed the diagnosis.
Medical ethics committee has approved the experiments undertaken.
The use of humans tissue samples follows internationally recognized
guidelines as well as local and national regulations. Informed
consent was obtained from each individual.
NSCLC and SCLC cell lines
NSCLC cell line A549 and SCLC cell line NCI-H1688
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Cells were grown in culture medium, as
recommended by the ATCC. Cells were cultured in Dulbeccos modified
Eagles medium (DMEM; Gibco-Invitrogen, Carlsbad, CA, USA)
containing 10% fetal calf serum (FCS), 2 mM L-glutamine, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified 5%
CO2 atmosphere.
Pre-miR-520a-3p/control miR, HOXD8
expressing plasmids/empty vectors and transfection
Pre-miR-520a-3p and control-miR were purchased from
Ambion (Austin, TX, USA). A final concentration of 50 nM of
Pre-miR-520a-3p and its respective negative control (control-miR)
were used for each transfection. HOXD8 expressing plasmids/empty
vectors were purchased from Tiangen Biotech, Co., Ltd. (Beijing,
China). A final concentration of 10 µg of HOXD8 expressing plasmids
and its respective negative control (empty vectors) were used for
each transfection. For the transfection experiments, the cells were
cultured in serum-free medium without antibiotics at 60% confluence
for 24 h, and then transfected with transfection reagent
(Lipofectamine 2000; Invitrogen) according to the manufacturers
instructions. After incubation for 6 h, the medium was removed and
was replaced with normal culture medium for 48 h, unless otherwise
specified.
Real-time PCR for miRNA
Total RNA from cultured cells, with efficient
recovery of small RNAs, was isolated using the mirVana miRNA
isolation kit (Ambion). Detection of the mature form of miRNAs was
performed using the mirVana qRT-PCR miRNA detection kit, according
to the manufacturers instructions (Ambion). The U6 small nuclear
RNA was used as an internal control.
MTT assay
MTT assay was performed as previously described
(31).
Sphere growth. Cells (103/ml) in
serum-free RPMI-1640/1 mM Na-pyruvate were seeded on 0.5% agar
precoated 6-well plates. After 1 week, half the medium was changed
every third day. Single spheres were picked and counted.
Western blot analysis
Western blot analysis was performed as previously
described (22). Mainly, after
incubation with primary antibody anti-ASCL1 (1:500; Abcam,
Cambridge, MA, USA), antibody anti-ALDH1A1 (1:500; Abcam),
anti-HOXD8 (1:500; Abcam), anti-p53 (1:500; Abcam), anti-PTEN
(1:500; Abcam), anti-p21 (1:500; Abcam) anti-GRP78 (1:500; Abcam),
anti-ZEB1 (1:500; Abcam), anti-MET (1:500; Abcam) and anti-β-actin
(1:500; Abcam) overnight at 4°C, IRDye™-800 conjugated anti-rabbit
secondary antibodies (Li-COR, Biosciences, Lincoln, NE, USA) were
used for 30 min at room temperature. The specific proteins were
visualized by Odyssey™ Infrared Imaging System (Neogen Corp.,
Lincoln, NE, USA).
Immunofluorescence analyses
Immunofluorescence analyses were performed as
previously described (31).
Methods of bioinformatics
The analysis of potential microRNA target sites
using the commonly used prediction algorithm-miRanda (http://www.targetscan.org//).
Migration and invasion assay
It was performed as previously described (32).
Reverse transcription-polymerase chain
reaction and real-time for mRNA
It was performed as previously described (33). Primers for HOXD8:
forward-5-TTCCCTGGATGAGAC CACAAGCAGC-3 and
reverse-5-GTCTCTCCGTGAGGG CCAGAGT-3. Primers for GAPDH:
forward-5-CGGAGTC AACGGATTTGGTCGTAT-3 and reverse-5-AGCCTTCT
CCATGGTGGTGAAGAC-3.
Statistical analysis
Data are presented as mean ± SEM. Students t-test
(two-tailed) was used to compare two groups (P<0.05 was
considered significant), unless otherwise indicated (χ2
test).
Results
Expression of miR-520a-3p is
downregulated in NSCLC and SCLC
In an attempt to identify miR-520a-3p expression
between the lung cancer tissues and the adjacent normal tissues, we
performed real-time PCR in cancer tissues vs. normal tissues. mRNA
was isolated from 39 pairs of lung cancer tissues (20 NSCLC tissues
and 19 SCLC tissues) and adjacent normal tissues (ANT). We found
that miR-520a-3p was significantly decreased in NSCLC and SCLC
tissues, compared with their adjacent normal tissues (Fig. 1). It implied that miR-520a-3p could
be a tumor suppressive gene in NSCLC and SCLC.
miR-520a-3p inhibits proliferation in
NSCLC and SCLC
To investigate whether miR-520a-3p can affect
proliferation of NSCLC and SCLC cells, using real-time PCR, we
tested whether pre-miR-520a-3p could stably express miR-520a-3p in
A549 cells (NSCLC cells) and NCI-H1688 cells (SCLC cells). The
results showed that miR-520a-3p could be significantly increased by
pre-miR-520a-3p in the two cell lines (Fig. 2A and B). Next, we performed MTT
assay to detect proliferation of A549 cells and NCI-H1688 cells
transfected with pre-miR-520a-3p. The results showed that
miR-520a-3p inhibited proliferation in the two cell lines (Fig. 2C and D).
miR-520a-3p inhibits stem cell-like
phenotypes in NSCLC and SCLC
To determine whether miR-520a-3p could affect
formation of CSCs in NSCLC and SCLC, we performed sphere forming
assay to assess the capacity of CSC or CSC-like cell self renewal
in the present study. We found that formation of spheres was
decreased by miR-520a-3p in A549 cells and NCI-H1688 cells
(Fig. 3A and C). Achaete-scute
complex homolog 1 (ASCL1) is critical for enhanced tumor-initiating
capacity in the CD133high SCLC sub-population (34). In order to detect whether
miR-520a-3p could regulate ASCL1 protein expression in A549 cells
and NCI-H1688 cells, we performed western blot analysis to assess
ASCL1 protein levels. The results showed that miR-520a-3p cannot
regulate ASCL1 protein in A549 cells, but it could significantly
suppress the protein in NCI-H1688 cells (Fig. 3B and D). Aldehyde dehydrogenase 1
(ALDH1A1) is a tumor stem cell-associated marker in lung cancer
(35). We also performed western
blot analysis to detect whether miR-520a-3p could regulate ALDH1A1.
The results demonstrated that ALDH1A1 was downregulated in A549
cells and NCI-H1688 cells (Fig. 3B and
D).
miR-520a-3p can degrade HOXD8 in NSCLC
A549 cells
To search target genes of miR-520a-3p, we commonly
used prediction algorithm, TargetScan (http://www.targetscan.org/) to predict its target
genes. The algorithm predicted that dozens of target genes could be
targeted by miR-520a-3p. We were interested in HOXD8, because we
found that contrary to miR-520a-3p, it can promote proliferation
and cancer stem cell phenotypes (data shown below). Thus, we
reasoned that miR-520a-3p might inhibit proliferation and stem
cell-like phenotypes by regulating HOXD8 in NSCLC and SCLC.
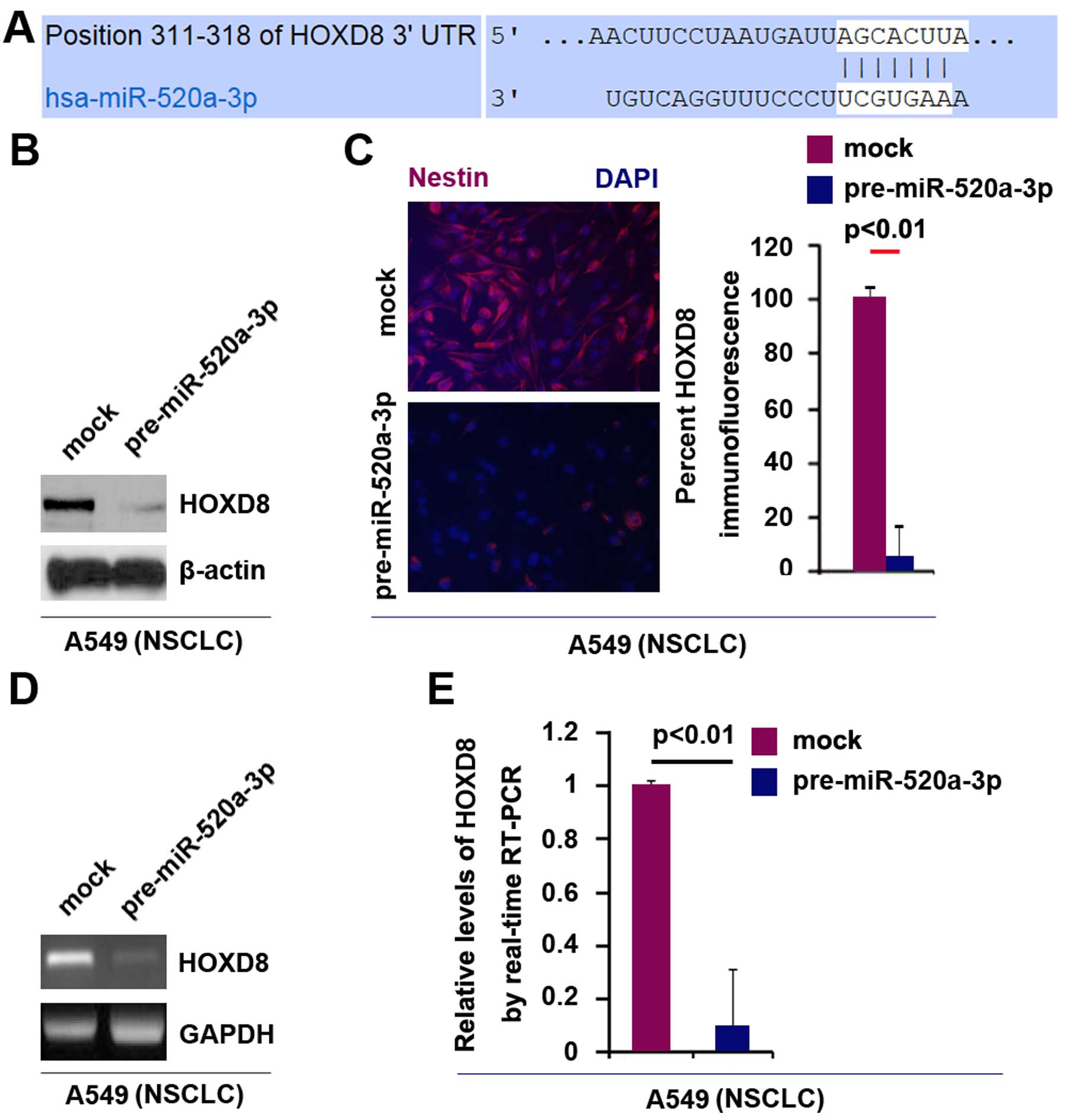
Target sites of miR-520a-3p on 3UTR of HOXD8 are
shown in Fig. 4A. In an attempt to
identify the role of miR-520a-3p in regulating HOXD8 protein
expression in A549 cells, we performed western blot analysis in
cells transfected with miR-520a-3p and control miR. The results
showed that HOXD8 protein was evidently suppressed in the cells
transfected with pre-miR-520a-3p (Fig.
4B). Moreover, we performed immunofluorescence analysis in A549
cells transfected with pre-miR-520a-3p and control miR. Consistent
with the results of western blotting, the results of
immunofluorescence showed that HOXD8 protein was evidently
suppressed in the cells transfected with pre-miR-520a-3p (Fig. 4C).
We next performed RT-PCR and real-time PCR to detect
HOXD8 mRNA expression in A549 cells transfected with
pre-miR-520a-3p or control miR. The results of RT-PCR showed that
HOXD8 mRNA was significantly downregulated in the cells transfected
with pre-miR-520a-3p (Fig. 4D).
Consistent with the results of RT-PCR, real-time PCR demonstrated
that HOXD8 mRNA was reduced in A549 cells transfected with
pre-miR-520a-3p, compared with control miR-transfected groups
(Fig. 4E). All the data
demonstrated that miR-520a-3p can degrade HOXD8 mRNA expression in
NSCLC A549 cells.
miR-520a-3p cannot suppress HOXD8
expression in SCLC NCI-H1688 cells
Having demonstrated that miR-520a-3p can degrade
HOXD8 in NSCLC A549 cells, we further studied whether HOXD8 is
regulated by miR-520a-3p in SCLC NCI-H1688 cells.
In an attempt to identify the role of miR-520a-3p in
regulating HOXD8 protein expression in NCI-H1688 cells, we
performed western blot analysis and immunofluorescence analysis in
cells transfected with pre-miR-520a-3p and control miR. The results
showed that HOXD8 protein was not changed in the cells transfected
with pre-miR-520a-3p (Fig. 5A and
B). We next performed RT-PCR and real-time PCR to detect HOXD8
mRNA expression in NCI-H1688 cells transfected with pre-miR-520a-3p
or control miR. The results of RT-PCR and real-time PCR showed that
HOXD8 mRNA was not significantly changed in the cells transfected
with pre-miR-520a-3p (Fig. 5C and
D).
HOXD8 is upregulated in cancer tissues
and its overexpression can promote proliferation, migration,
invasion and cancer stem cell phenotype in NSCLC A549 cells
To identify HOXD8 expression between NSCLC tissues
and adjacent normal tissues, we performed western blot analysis in
cancer tissues vs. normal tissues. Protein was isolated from 20
NSCLC tissues and 20 adjacent normal tissues. We found that HOXD8
expression was significantly increased in NSCLC tissues, compared
with adjacent normal tissues (Fig.
6A). It implied that HOXD8 could be an oncogene in NSCLC. To
investigate whether HOXD8 can affect proliferation of A549 cells,
using western blot, we tested whether HOXD8 expressing plasmids
could stably express HOXD8 protein in A549 cells. The results
showed that HOXD8 could be significantly increased by HOXD8
expressing plasmids in the cell line (Fig. 6B).
Next, we performed MTT assay to detect proliferation
of A549 cells transfected with HOXD8 expressing plasmids and empty
vectors. The results showed that HOXD8 promoted proliferation in
A549 cells (Fig. 6C). To further
confirm that HOXD8 could regulate proliferation, we performed
western blot analysis to detect proliferation markers, p53, PTEN
and p21 and found that HOXD8 significantly inhibited p53, PTEN and
p21 protein expression in A549 cells (Fig. 6D).
To determine whether overexpressing HOXD8 could
affect stem cell-like phenotypes in A549 cells, we performed sphere
forming assay to assess the capacity of CSC or CSC-like cell self
renewal in this study. We found that formation of spheres was
increased by HOXD8 in A549 cells (Fig.
6E). In order to detect whether HOXD8 could regulate invasion
and migration in A549 cells, we performed invasion and migration
assay to assess the role of HOXD8 on A549 cells. The results showed
that overexpressing HOXD8 promoted migration and invasion in A549
cells (Fig. 6F and H). Upregulation
of glucose-regulated protein 78 (GRP78) expression can promote
NSCLC cell invasion (36). To
indentify whether GRP78 is regulated by HOXD8, we performed western
blot analysis to assess GRP78 protein levels in A549 cells
transfected with HOXD8 expressing plasmids and empty vectors. The
results showed that GRP78 protein was significantly promoted by
HOXD8 in A549 cells (Fig. 6G).
Moreover, we found that overexpressing HOXD8 could significantly
induce ZEB1 protein expression (Fig.
6I).
Pre-miR-520a-3p inhibits MET protein
expression and HOXD8 induces MET protein expression
MET amplification play a pivotal role in gefitinib
resistance in lung cancer (30). In
order to detect the role of pre-miR-520a-3p and HOXD8 on MET
expression, we performed western blot analysis to detect MET
protein in A549 cells transfected with pre-miR-520a-3p and control
miR. The results demonstrated that miR-520a-3p inhibited MET
protein in A549 cells (Fig. 7A).
Contrary to miR-520a-3p, we found that overexpressing HOXD8 induced
MET protein expression in A549 cells (Fig. 7B). Thus, the results implied that
pre-miR-520a-3p downregulation and HOXD8 upregulation might play an
important role in gefitinib resistance in NSCLC.
Discussion
It was reported that miR-520a-3p is downregulated in
NSCLC tissues (29). But there is
no report on the expression of miR-520a-3p in SCLC. Consistent with
the report, we confirmed that miR-520a-3p was downregulated in
NSCLC tissues. Moreover, we also demonstrated that miR-520a-3p
expression is suppressed in SCLC tissues. The results indicate that
miR-520a-3p might be a tumor suppressive gene in both NSCLC and
SCLC. microRNA-520a-3p can inhibit proliferation, apoptosis and
metastasis by targeting MAP3K2 in non-small cell lung cancer
(29). Consistent with the report,
we showed that overexpressing microRNA-520a-3p suppressed
proliferation in NSCLC and SCLC cells.
CSCs assume a central role in both tumorigenesis and
metastasis (37). Better
understanding of the regulatory mechanisms of CSCs as a fundamental
component of the metastatic cascade will lead to novel therapeutic
strategies against metastatic cancer. Herein, we demonstrated that
overexpressing pre-miR-520a-3p significantly inhibited sphere
growth in NSCLC and SCLC cells. ALDH1A1 is a lung tumor stem
cell-associated marker (34). We
demonstrated that ALDH1A1 protein is inhibited by miR-520a-3p in
NSCLC and SCLC cells. Moreover, ASCL1 (achaete-scute complex
homolog 1, Mash1), a proneural basic helix-loop-helix (bHLH)
transcription factor, was initially identified as a key regulator
of early development of mitotically-active precursors for both
neurons and oligodendrocytes (38).
ASCL1 is highly expressed in classic SCLC and in NSCLC with
neuroendocrine (NE) phenotype features (39). ASCL1 regulates tumor-initiating
capacity in human small cell lung cancer (34). Our results showed that ASCL1 protein
can be suppressed by miR-520a-3p in SCLC cells, but it cannot be
suppressed in NSCLC cells highlighting that the different roles of
miR-520a-3p in NSCLC and SCLC cells.
In this study, we found that HOXD8 is another target
gene of miR-520a-3p in NSCLC, besides MAP3K2. miR-520a-3p could
degrade HOXD8 mRNA in NSCLC cells. However, we found that HOXD8 was
not suppressed in SCLC cells by miR-520a-3p, implying that
miR-520a-3p functions as a tumor suppressive gene by different
mechanism in SCLC and NSCLC. For the first time, we showed that
HOXD8 protein is upregulated in NSCLC tissues and its
overexpression promoted proliferation, cancer stem cell phenotypes,
migration and invasion in NSCLC cells.
Gefitinib-sensitive lung cancer cell line can
develop resistance to gefitinib as a result of focal amplification
of the MET proto-oncogene. Inhibition of MET signaling in lung
cells can restore their sensitivity to gefitinib (30). We showed that miR-520a-3p can
inhibit MET protein expression and HOXD8 can induce MET protein
expression, implying that miR-520a-3p downregulation and HOXD8
upregulation may play an important role in the development of
gefitinib resistance.
Glossary
Abbreviations
Abbreviations:
|
ALDH1A1
|
aldehyde dehydrogenase 1
|
|
ANT
|
adjacent normal tissues
|
|
ASCL1
|
achaete-scute complex homolog 1
|
|
CSCs
|
cancer stem cells
|
|
CICs
|
cancer initiating cells
|
|
NSCLC
|
non-small cell lung cancer
|
|
SCLC
|
small cell lung cancer
|
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang P: Epidemiology of lung cancer
prognosis: Quantity and quality of life. Methods Mol Biol.
471:469–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polyak K, Haviv I and Campbell IG:
Co-evolution of tumor cells and their microenvironment. Trends
Genet. 25:30–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collins AT and Maitland NJ: Prostate
cancer stem cells. Eur J Cancer. 42:1213–1218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaiopoulos AG, Kostakis ID, Koutsilieris M
and Papavassiliou AG: Colorectal cancer stem cells. Stem Cells.
30:363–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sullivan JP, Minna JD and Shay JW:
Evidence for self-renewing lung cancer stem cells and their
implications in tumor initiation, progression, and targeted
therapy. Cancer Metastasis Rev. 29:61–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shafee N, Smith CR, Wei S, Kim Y, Mills
GB, Hortobagyi GN, Stanbridge EJ and Lee EY: Cancer stem cells
contribute to cisplatin resistance in Brca1/p53-mediated mouse
mammary tumors. Cancer Res. 68:3243–3250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
To K, Fotovati A, Reipas KM, Law JH, Hu K,
Wang J, Astanehe A, Davies AH, Lee L, Stratford AL, et al: Y-box
binding protein-1 induces the expression of CD44 and CD49f leading
to enhanced self-renewal, mammosphere growth, and drug resistance.
Cancer Res. 70:2840–2851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McManus MT: MicroRNAs and cancer. Semin
Cancer Biol. 13:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melton C, Judson RL and Blelloch R:
Opposing microRNA families regulate self-renewal in mouse embryonic
stem cells. Nature. 463:621–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: let-7 regulates
selfrenewal and tumorigenicity of breast cancer cells. Cell.
31:1109–1123. 2007. View Article : Google Scholar
|
|
28
|
Shimono Y, Zabala M, Cho RW, Lobo N,
Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al:
Downregulation of miRNA-200c links breast cancer stem cells with
normal stem cells. Cell. 138:592–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu J, Tan Q, Deng B, Fang C, Qi D and Wang
R: The microRNA-520a-3p inhibits proliferation, apoptosis and
metastasis by targeting MAP3K2 in non-small cell lung cancer. Am J
Cancer Res. 5:802–811. 2015.PubMed/NCBI
|
|
30
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang L, Chen F, Pang EJ, Zhang ZQ, Jin BW
and Dong WF: MicroRNA-182 inhibits proliferation through targeting
oncogenic ANUBL1 in gastric cancer. Oncol Rep. 33:1707–1716.
2015.PubMed/NCBI
|
|
32
|
Dai X, Ge J, Wang X, Qian X, Zhang C and
Li X: OCT4 regulates epithelial-mesenchymal transition and its
knockdown inhibits colorectal cancer cell migration and invasion.
Oncol Rep. 29:155–160. 2013.PubMed/NCBI
|
|
33
|
Zhang HY, Li JH, Li G and Wang SR:
Activation of ARK5/miR-1181/HOXA10 axis promotes
epithelial-mesenchymal transition in ovarian cancer. Oncol Rep.
34:1193–1202. 2015.PubMed/NCBI
|
|
34
|
Jiang T, Collins BJ, Jin N, Watkins DN,
Brock MV, Matsui W, Nelkin BD and Ball DW: Achaete-scute complex
homologue 1 regulates tumor-initiating capacity in human small cell
lung cancer. Cancer Res. 69:845–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak
J, Xing L, Wang H, Liu Z, Su Y, Stass SA, et al: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu T, Guo Z, Fan H, Song J, Liu Y, Gao Z
and Wang Q: Cancer-associated fibroblasts promote non-small cell
lung cancer cell invasion by upregulation of glucose-regulated
protein 78 (GRP78) expression in an integrated bionic microfluidic
device. Oncotarget. Mar 21–2016.(Epub ahead of print). doi:
10.18632/oncotarget.8232.
|
|
37
|
Li F, Tiede B, Massagué J and Kang Y:
Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res.
17:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ball DW: Achaete-scute homolog-1 and Notch
in lung neuroendocrine development and cancer. Cancer Lett.
204:159–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sriuranpong V, Borges MW, Strock CL,
Nakakura EK, Watkins DN, Blaumueller CM, Nelkin BD and Ball DW:
Notch signaling induces rapid degradation of achaete-scute homolog
1. Mol Cell Biol. 22:3129–3139. 2002. View Article : Google Scholar : PubMed/NCBI
|