Introduction
Ovarian cancer is the major cause of deaths from
gynecologic malignancies and the 5th leading cause of
cancer-related deaths among women in the United States. According
to the National Cancer Institute (NCI) report prediction, ~21,290
new cases would be diagnosed with ovarian cancer in America in
2015, and 14,180 patients would die of this disease (1).
Most cancer-related deaths result from the formation
of metastases (2), which are
difficult to detect and can induce relapse for years after
treatment of the primary tumor (3).
Ovarian cancer patients die of their disease mainly attributed to
metastasis (4). Unfortunately, the
mechanism of metastasis is still unclear.
Collagen triple helix repeat containing-1 (CTHRC1)
is a 30-kDa secreted protein that has the ability to inhibit
collagen matrix synthesis. It is highly expressed in cartilage,
developing bones, and myofibroblasts during skin wound healing
(5). A human tumor complementary
DNA array analysis has shown that the CTHRC1 gene is expressed in
the majority of human solid cancers (6). Recent studies reported that CTHRC1
promoted tumor cell metastasis by different signaling pathways in
different cancers. In gastric cancer, CTHRC1 has been shown to be
upregulated by promoter demethylation and in response to TGF-β
signaling (7), while in human
non-small cell lung cancer, CTHRC1 mediates aggressiveness via
GSK-3β/β-catenin pathway (8).
Epidermal growth factor receptor (EGFR) has received
considerable attention in ovarian cancer research, because up to
75% of primary epithelian ovarian cancers (EOC) overexpress EGFR
(9). Overexpression of EGFR is
associated with advanced-stage disease and poor prognosis (10). Furthermore, EGFR and its family
members are the major contributors of a complex signaling cascade
that modulates growth, signaling, differentiation, adhesion,
migration and survival of cancer cells (11). Published data demonstrates that EGFR
plays an important role in tumor progression (12). Thus EGFR is considered as a critical
molecular target for therapy in advanced ovarian cancer.
EGFR is closely related to ovarian cancer metastasis
(13) and a number of cellular
signals such as protein kinase (AKT) and extracellular
signal-regulated kinase1/2 (ERK1/2) (14–16)
can be activated due to EGFR activation. Although recent study
showed CTHRC1 may promote EOC metastasis through Wnt/catinen signal
pathway (17), but little is known
about whether CTHRC1 was involved in EGFR signaling to modulate EOC
cell metastasis.
Zhou et al reported that microRNA-7 inhibited
tumor metastasis and reversed EMT through AKT and ERK1/2 pathway
inactivation by reducing EGFR expression in EOC cell lines
(18), this led us to investigate
whether the EGFR and its downstream signaling pathways were
involved in the effect on ovarian cancer cell migration and
invasion induced by CTHCR1.
In this study, we examined the CTHRC1 expression in
EOC and found that CTHRC1 played an important role in ovarian
cancer cell metastasis. EGFR inhibitors can block recombinant
CTHRC1 induced promotion of ovarian cancer cell migration and
invasion. Silencing of CTHRC1 with siRNA blocked phosphorylation of
EGFR, ERK1/2 and Akt in the ES-2 cell line. We demonstrated that
CTHRC1 may promote migration and invasion of ovarian cancer cells
through activated signaling of EGFR/ERK1/2/AKT.
Materials and methods
Cell culture
Human ovarian cancer cell line SKOV3, ES2, CAOV3,
HEY, COV318 were obtained as gifts from Shanghai Cancer Institute.
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen, USA) supplemented with 10% (v/v) fetal bovine serum
(Invitrogen), 100 U/ml penicillin and 100 µg/ml streptomycin, at
37°C in an incubator with 5% CO2 condition.
Clinical samples
The studied human ovary tissue microarray contained
83 cases of ovarian cancer. These tissues were obtained from
Department of Gynecology, Obstetrics and Gynecology Hospital, Fudan
University from 2006 to 2009. None of them had received
chemotherapy, radiotherapy and other related antitumor therapies
before surgery. Eighteen cases of normal ovarian tissues were
selected as the control group. All human ovary tissues were
obtained with informed consent and all protocols were approved by
the ethics review committee of the World Health Organization
Collaborating Center for Research in Human Production.
Tissue microarray construction
Human ovary tissue microarray was constructed by
Suzhou Xinxin Biotechnology Co. Ltd. (Xinxin Biotechnology Co.,
Suzhou, China). Two 1.6-mm cores per donor block were transferred
into a recipient block tissue microarray. Three-micron thick
sections were cut from the recipient block and transferred to glass
slides with an adhesive tape transfer system for ultraviolet cross
linkage.
Immunohistochemical staining
The tissue microarray glass slides were baked at
55°C for 1 h, and then de-paraffinized gradually through xylene,
50% xylene, gradient concentrations of ethanol until immersed in
tap water. Tissue sections were blocked for peroxidase activity
with 0.3% hydrogen peroxide at 37°C for 30 min. Antigen retrieval
was carried out by boiling in 10 mmol/l citrate buffer (pH 6.0) for
15 min. Then the tissues were incubated with CTHRC1 antibody
(rabbit, 1:200 dilution, Abcam Biotechnology) overnight at 4°C. The
next day, the tissues were washed with phosphate buffer solution
(PBS) three times and incubated with HRP-labeled anti-rabbit
secondary antibody (1:200 dilution, Dako, Carpinteria, CA, USA) for
1 h at room temperature. Immunostaining was carried out using
diaminobenzidine substrate chromogen method. After immunostaining
tissues were immersed into hematoxylin for nuclear staining. The
TMA slides were then dehydrated through gradient concentrations of
ethanol, cleared with xylene, and coverslipped with neutral balsam.
Five high-power fields were randomly chosen, and ≥300 cells were
counted per field. Expression score was determined by staining
intensity and immunoreactive cell percentage. Scoring was conducted
according to the ratio and intensity of positive-staining cells:
0–5% scored 0; 6–35% scored 1; 36–70% scored 2; >70% scored 3.
The final score of CTHCR1 expression was designated as low or high
expression group as follows: low expression, score 0–1; high
expression, score 2–3. All the scores of CTHRC1 expression were
performed in a blinded manner and determined independently by two
senior pathologists.
RNA interference-based gene silencing
experiment
Stably transfected clones were selected using ES2
cells, and transfection was performed with SuperFectin according to
the manufacturer's instructions. SiRNA interference plasmid was
purchased from GenePharma and designed to target the following cDNA
sequences: CTHRC1-shRNA1, sense, 5′-CAG CGU UGG UAU UUC ACA UUT-3′;
antisense, 5′-AUG UGA AAU ACC AAC GCU GTT-3′. CTHRC1-shRNA2, sense,
5′-GCU UCU ACU GGA UGG AAU UTT-3′; antisense, 5′-AAU UCC AUC CAG
UAG AAG CTT-3′. CTHRC1-negative control: sense, 5′-UUC UCC GAA CGU
GUC ACG UTT-3′; antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′.
Stable shRNA-expressing clones were selected with 2 µg/ml puromycin
(Sangon, Shanghai, China) and the silencing effects were verified
by qRT-PCR and western blot analysis.
Western blot analysis
Cells were lysed in lysis buffer (50 mM Tris-HCl,
150 mM NaCl, 1% Triton-X 100, 1 mM each MgCl2,
MnCl2 and CaCl2, 1 mM PMSF and 10 mM sodium
fluoride) and protease inhibitor cocktail. Proteins were separated
by SDS-PAGE under reducing condition, followed by immunoblotting
with specific primary antibodies and species-specific secondary
antibodies. Bound secondary antibodies were revealed by Odyssey
imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Quantification was analyzed using ImageJ software. The antibodies
used in this study included: anti-CTHRC1 (rabbit, 1:1,000, Abcam
Biotechnology), anti-EGFR and anti-pEGFR (Tyr1148) (rabbit,
1:1,000, Cell Signaling Technology), anti-AKT and anti-pAKT (S473)
(rabbit, 1:1,000, Cell Signaling Technology), anti-ERK1/2 and
anti-pERK1/2 (Thr202/Tyr204) (rabbit, 1:1,000, Cell Signaling
Technology), anti-β-actin (rabbit, 1:1,000, Sigma
Biotechnology).
Quantitative real-time PCR
Total cellular RNA was extracted using TRIzol
reagent (Takara) and reversely transcribed through PrimeScript
RT-PCR kit (Takara) according to the protocol. The CTHRC1 mRNA
expression was determined by real-time PCR using SYBR Premix Ex Taq
(Takara) on a 7500 real-time PCR system (Applied Biosystems) at the
following cycling settings: one initial cycle at 95°C for 10 sec
followed by 40 cycles of 5 sec at 95°C and 31 sec at 60°C. Primers
were CTHRC1: (sense) 5′-TGG ACA CCC AAC TAC AAG CA-3′ and
(antisense) 5′-GAA CAA GTG CCA ACC CAG AT-3′. 18S (primers: sense
5′-TGC GAG TAC TCA ACA CCA ACA-3′, antisense 5′-GCA TAT CTT CGG CCC
ACA-3′) was used as an internal reference.
Cell viability assay (CCK8 assay)
Cell viability was detected using a standard Cell
Counting Kit-8 assay. Ovarian cancer cells were plated in 96-well
plates at a density of 3×104 cells per well with 100 µl
of complete culture medium. We added 10 µl of reagent from Cell
Counting Kit-8 (Dojindo, Kumamoto, Japan) to each well for
detection at days 1, 2, 3, 4 and 5. After 1 h of incubation at
37°C, the optical density was measured using microplate reader at a
wavelength of 450 nm. The experiment was repeated three times.
In vitro migration and invasion
assays
For the in vitro Transwell migration assay,
4×104 ovarian cancer cells were suspended in DMEM medium
and added to the upper chamber of each insert with the non-coated
membrane. In the lower chamber 700 µl DMEM medium with 5% FBS was
added. After incubation for 24 h at 37°C, 5% CO2, cells
that migrated through the membrane filter were fixed and stained
with crystal violet. The number of migrating cells was counted and
imaged through an IX71 inverted microscope (Olympus Corp., Tokyo,
Japan). Transwell invasion assay was carried out by adding 100 µl
Matrigel (BD Bioscience, Franklin Lakes, NJ, USA) into the upper
chamber of the Transwell and placing 6×104 cells onto
the Matrigel; 48 h later, cells that invaded through the membrane
filter were fixed, stained with crystal violet, counted and imaged.
At least five grids per field were counted. All assays were
independently repeated three times.
Agent
AG1478; erlotinib (EGFR inhibitor, effective dose:
100 nmol/l, MedChem Express, USA). CTHRC1 recombinant protein
(rCTHRC1) was obtained as a gift from Shanghai Cancer
Institute.
Statistical analysis
The data were analyzed as the mean ± standard
deviation (SD). Statistical analyses were conducted using SPSS 14.0
software (SPSS Inc). We performed Pearson's test in cross tables to
assess the relationships between expression levels of CTHRC1 and
clinicopathological factors. Overall survival (OS) was calculated
using Kaplan-Meier method. Student's t-test has been performed for
calculating statistical significance between groups. Difference in
P-value <0.05 is referred to as statistically significant. All
statistical tests were two-sided.
Results
CTHRC1 protein expression level is
excessively elevated in epithelial ovarian cancer tissues
We performed an immunohistochemical (IHC) analysis
of a tissue microarray that contained 83 epithelial ovarian cancer
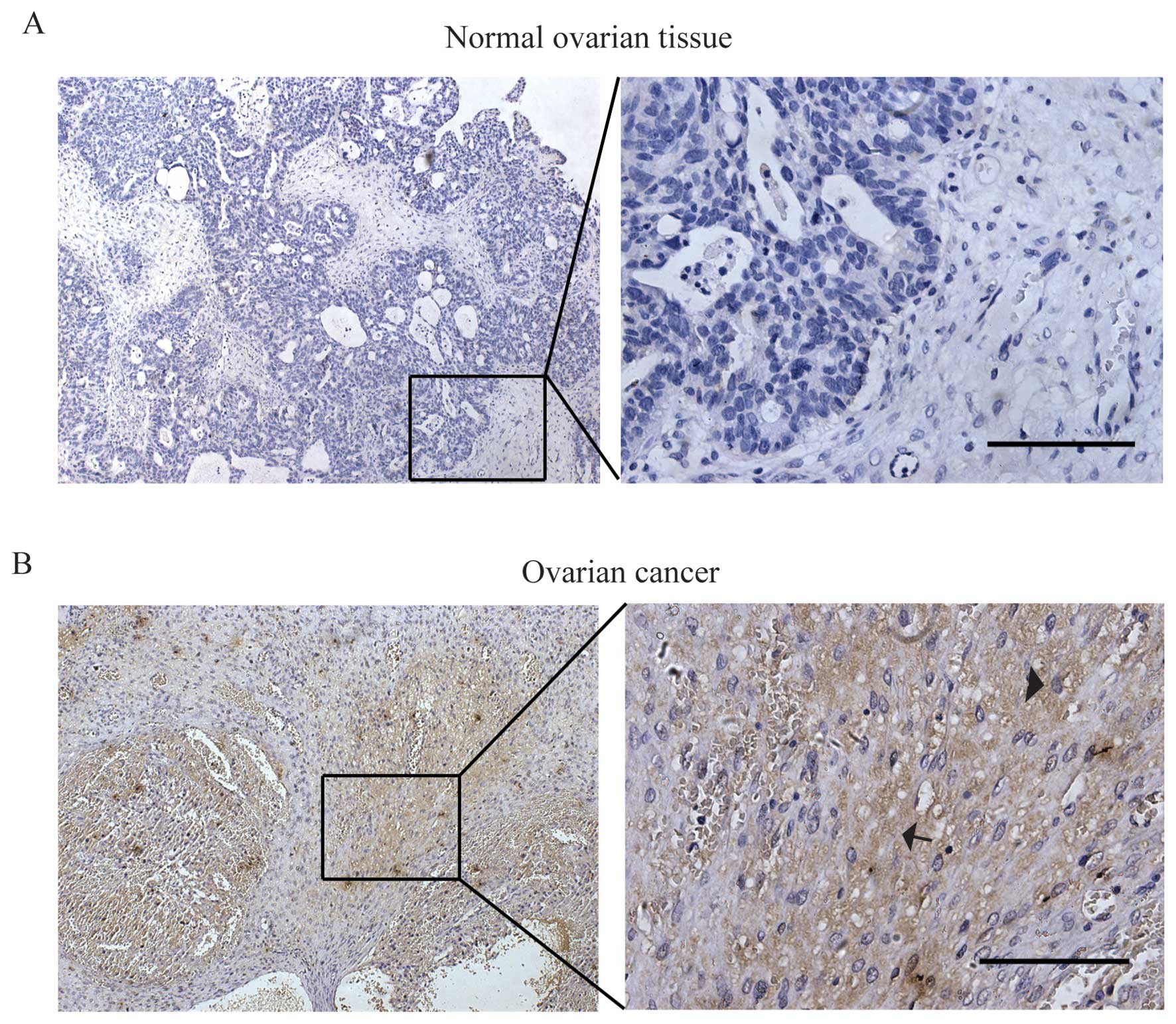
(EOC) tissue samples and 18 normal ovarian tissues. As shown in
Fig. 1, the result showed strong
CTHRC1 staining was detected in the majority of EOC samples (55.4%,
46/83) while rarely detected in the normal ovarian tissues (11.1%,
2/18). CTHRC1 was detected both as intracellular and extracellular
expression of the ovarian cancer cell as marked by arrowhead and
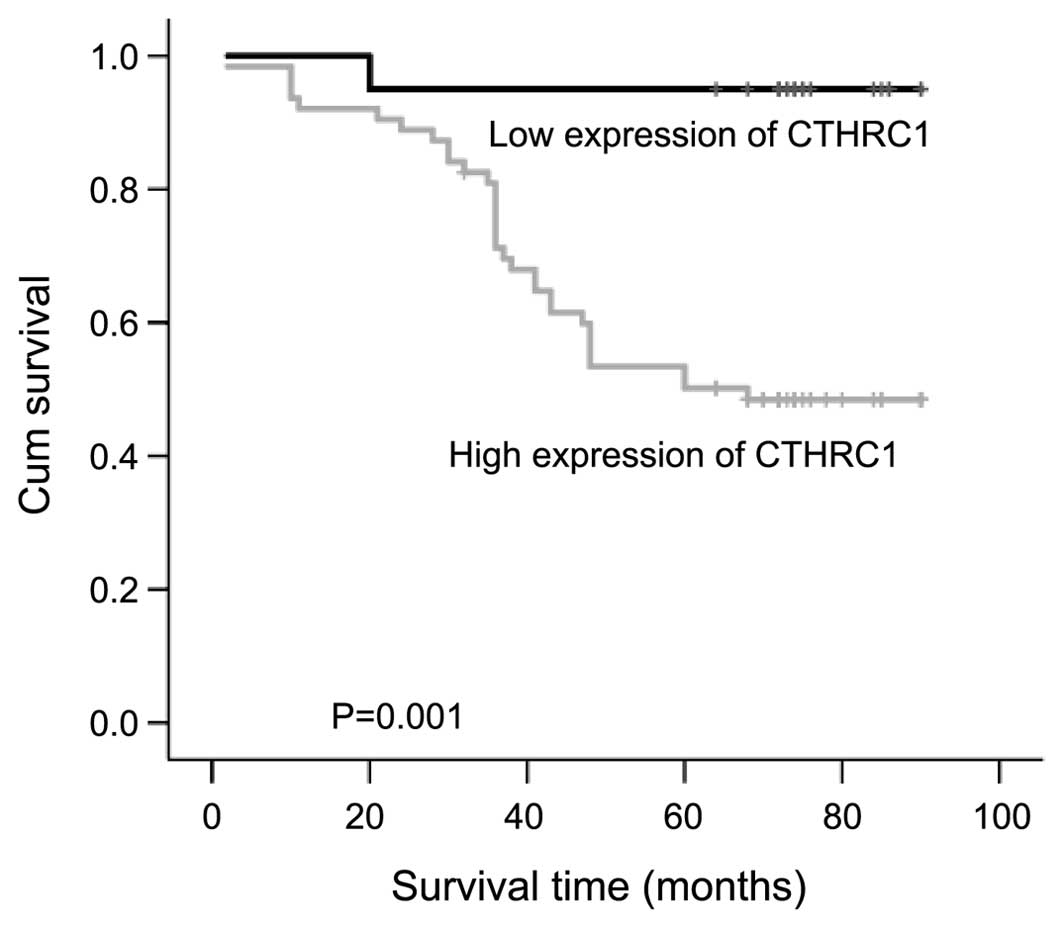
arrow in the images. Using the Kaplan-Meier analysis, we found that
patients with higher expression of CTHRC1 were significantly
associated with shorter overall survival (Fig. 2).
We further analyzed the relevance of CTHRC1
expression with patient clinicopathological parameters and found
that high expression of CTHRC1 was closely related with adverse
clinicopathological parameters of EOC, including FIGO stage, lymph
node status and tumor size. No correlation with histologic
subgroups of epithelian ovarian cancer was found (Table I).
 | Table I.Correlation between CTHRC1 expression
and clinicopathologic parameters in 83 ovarian cancer patients. |
Table I.
Correlation between CTHRC1 expression
and clinicopathologic parameters in 83 ovarian cancer patients.
|
|
| CTHRC1 |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total | Score <2, n
(%) | Score ≥2, n (%) | P-value |
|---|
| Ovarian cancer
group | 83 | 38 (45.6) | 46 (55.4) | 0.000a |
| Control group | 18 | 16 (88.9) | 2
(11.1) |
|
| Histologic
subgroups |
|
|
|
|
|
Serous | 60 | 12 (20.0) | 48 (80.0) | 0.104 |
|
Endometrioid | 11 | 5
(45.4) | 6
(54.6) |
|
| Clear
cell | 12 | 3
(25.0) | 9
(75.0) |
|
| FIGO stage |
|
|
|
|
|
I–II | 39 | 17 (43.6) | 22 (56.4) |
0.000a |
|
III–IV | 44 | 3
(6.8) | 41 (93.2) |
|
| Lymph node
status |
|
|
|
|
|
Negative | 23 | 10 (43.4) | 13 (56.6) |
0.000a |
|
|
Positive | 60 | 7
(11.6) | 51 (89.4) |
|
| Tumor size
(cm) |
|
|
<2 | 32 | 15 (46.8) | 16 (53.2) |
0.000a |
|
| ≥2 | 51 | 11 (21.5) | 40 (78.6) |
|
The expression of CTHRC1 in ES2 cells
transfected with Lenti-shCTHRC1
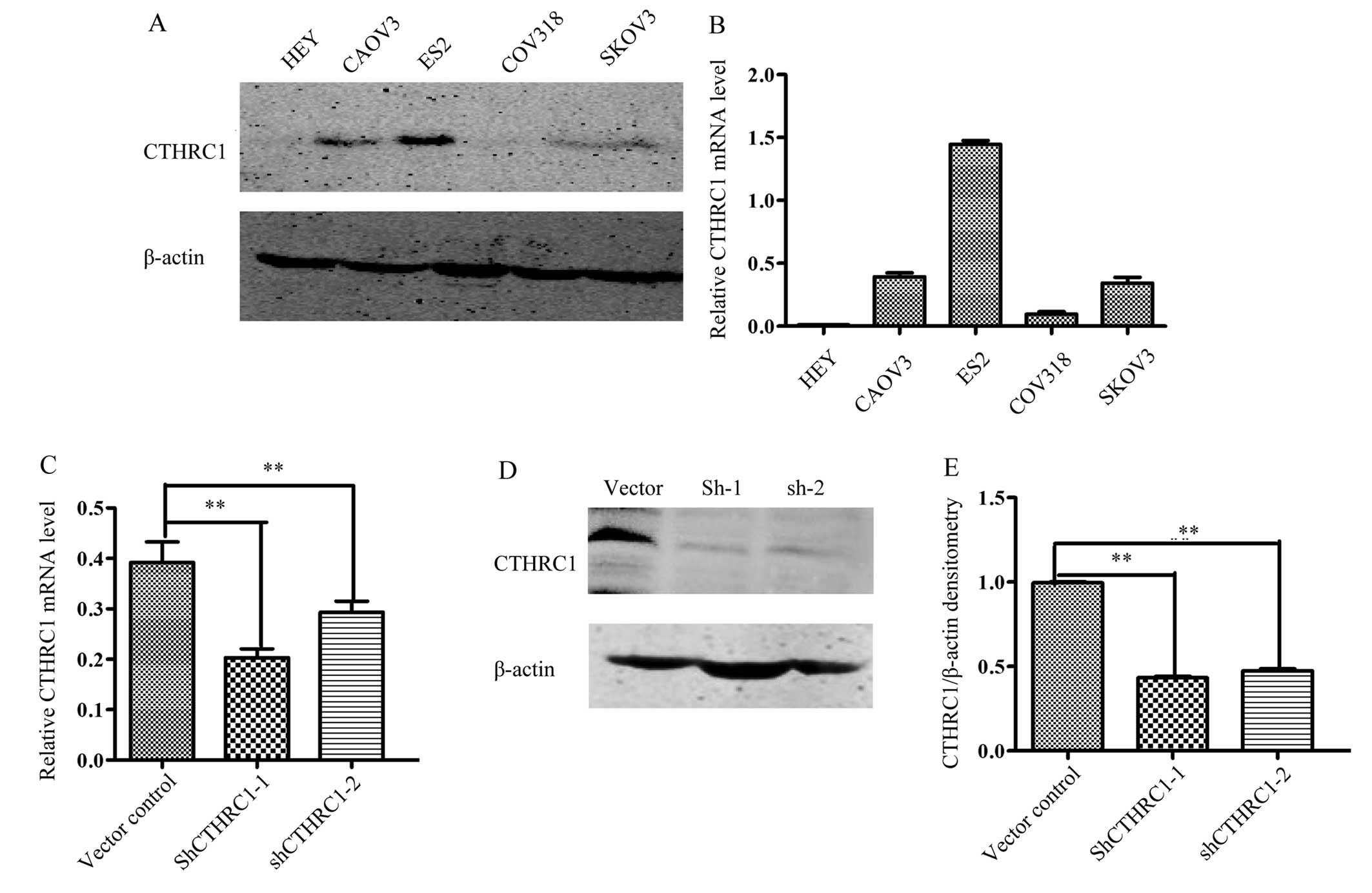
To determine the optimal cell lines for further
study, we measured the expression of CTHRC1 in five ovarian cell
lines (HEY, CAOV3, ES2, COV318 and SKOV3). Our data showed that the
expression of CTHRC1 was the highest in the ES2 cell line (Fig. 3A and B), an EOC cell line with
highly metastatic potential (17).
Therefore, ES2 cells were transfected with Lenti-shCTHRC1-(1, 2),
designated as sh1 and sh2, or a mock vector, which was labeled as
control. The silencing effects of the Lenti-shRNAs in the cell line
were validated by RT-PCR and western blotting (Fig. 3C-E). The results showed that CTHRC1
expression levels were significantly decreased by
Lenti-shCTHRC1-(1, 2).
Silencing of CTHRC1 or rCTHRC1 has no
effects on ovarian cancer cell proliferation in vitro
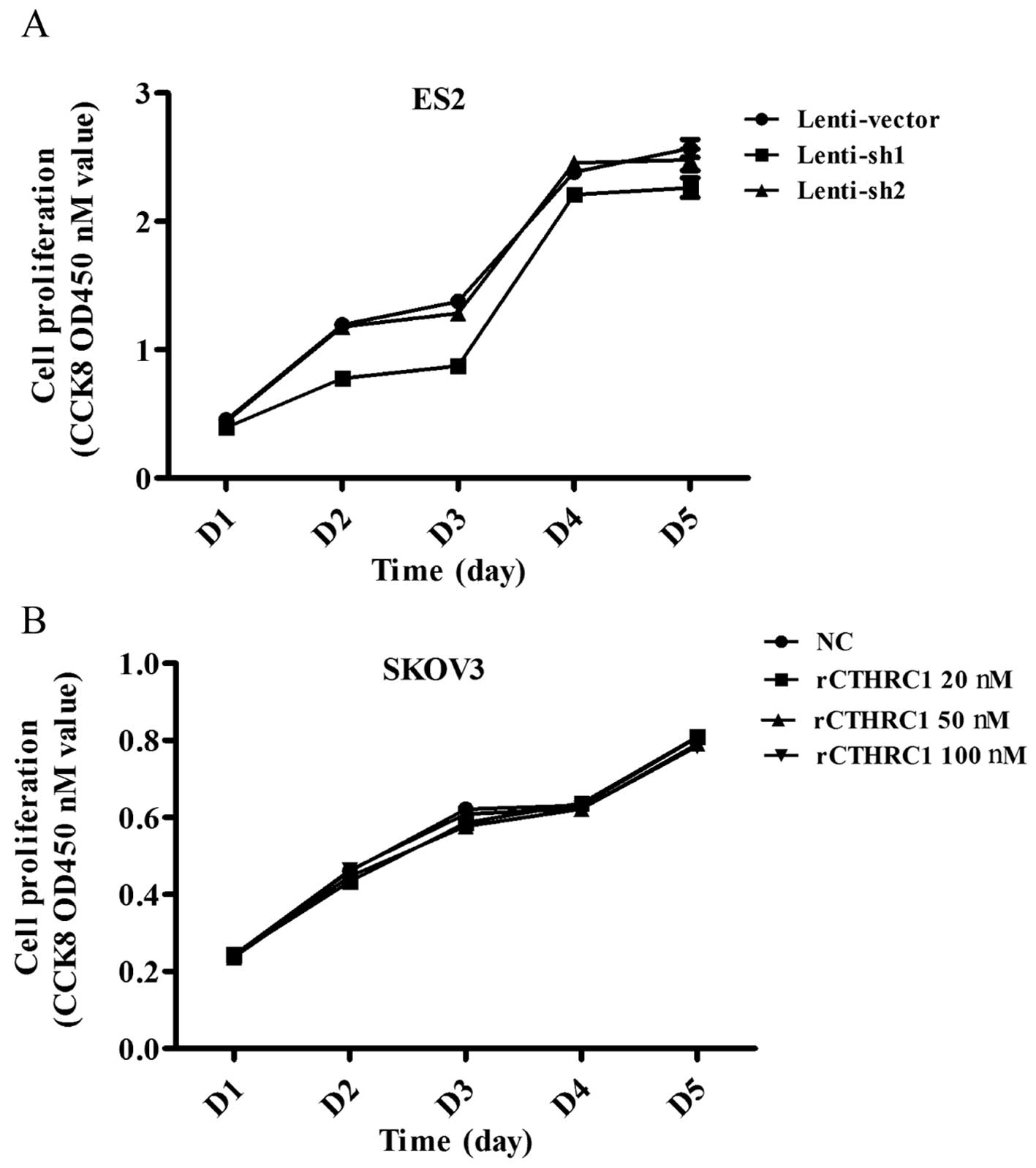
To explore the effect of CTHRC1 on ovarian cancer
cell growth, ES2 transfected with sh1 and sh2 were used to detect
cell proliferation by Cell Counting Kit-8 (CCK8) assay. The results
showed that silencing of CTHRC1 could not suppress the
proliferation of the ES2 cells (Fig.
4A).
Next we set out to investigate the effect of
purified recombinant CTHRC1 (rCTHRC1) on EOC cell viability. SKOV3
cells were seeded into 96-well plates at the same density and
treated with gradient doses of CTHRC1. Compared to the control
group, SKOV3 cell proliferation was not obviously enhanced by
rCTHRC1 protein (Fig. 4B), which
was consistent with the effect of shRNA interference.
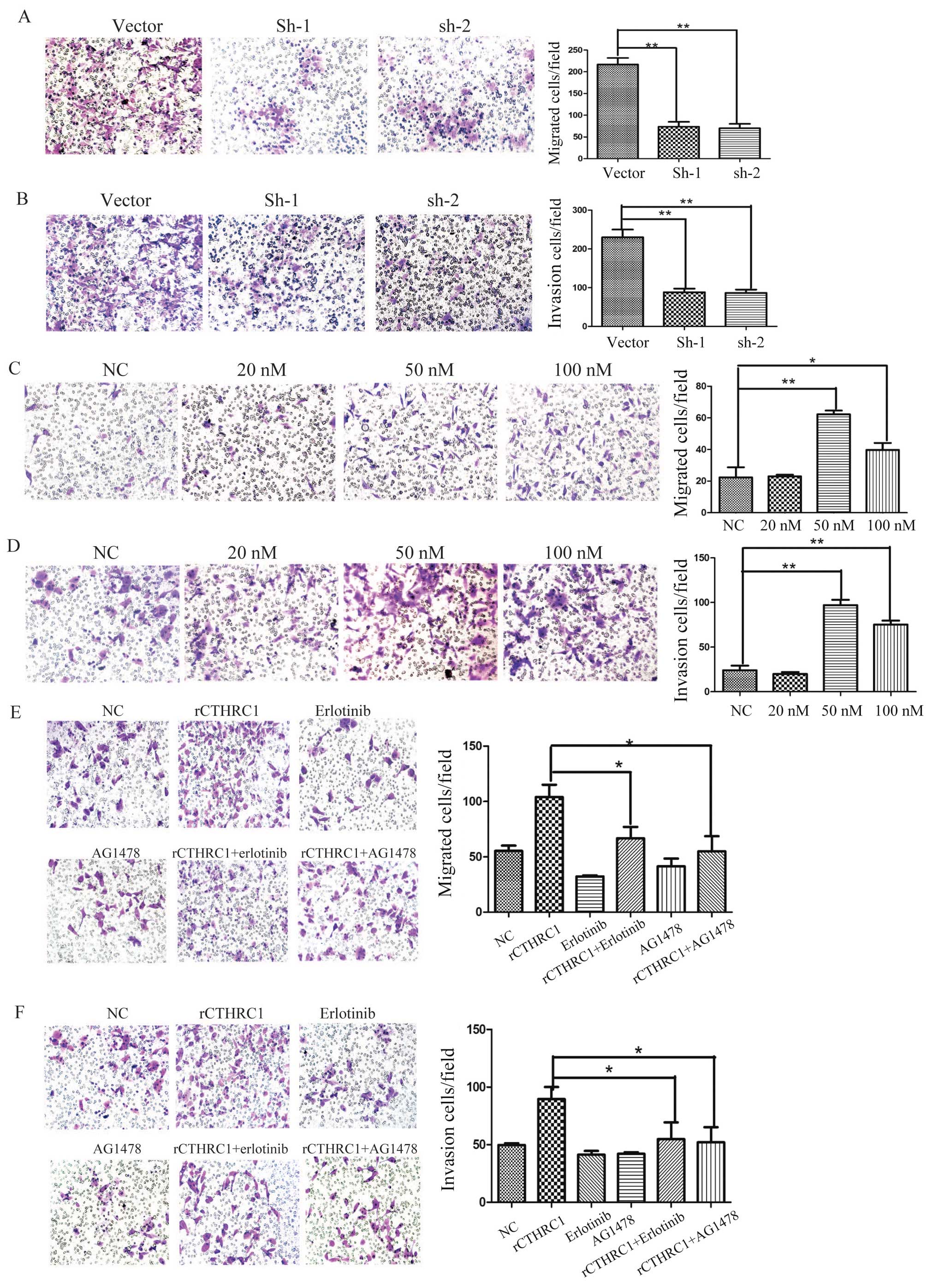
Silencing of CTHRC1 suppresses ovarian
cancer cell invasion and migration in vitro
To further investigate the functional role of CTHRC1
in ovarian cancer, we examined the effects of CTHRC1 on ovarian
cancer cell migration and invasion in vitro. Compared to the
control group, silencing of CTHRC1 significantly inhibited ES2 cell
migration and invasion in vitro (Fig. 5A and B). These data indicate that
CTHRC1 has a potent role in EOC cancer metastasis.
rCTHRC1 promotes ovarian cancer cell
invasion and migration in vitro
As a secreted protein, we explored the biological
functions of CTHRC1, so the rCTHRC1 protein was applied to SKOV3
cells in a migration and Matrigel invasion assay. Gradient doses of
0, 20, 50 and 100 nM rCTHRC1, respectively, were added into the
lower chamber and cells were added in the upper chamber. Compared
to the control group, SKOV3 cell migration and invasion were
significantly enhanced by rCTHRC1 protein at doses of 50 and 100 nM
(Fig. 5C and D). At a dose of 50
nM, the promotion effect of cell motility by the rCTHRC1 protein
was the strongest. Our data indicate that CTHRC1 contributes
greatly to the development of ovarian cancer metastasis.
EGFR inhibitors suppress ovarian
cancer cell invasion and migration induced by rCTHRC1 in vitro
Previous studies have demonstrated clear effects of
EGFR signaling on ovarian cancer cell invasion (13,19),
but whether CTHRC1 regulate EGFR signaling in ovarian cancer cells
has not been reported. Thus, we further determined EGFR signaling
in CTHRC1 induced ovarian cancer cell migration and invasion.
AG1478 and erlotinib, two inhibitors of EGFR signaling, were
applied in the Transwell assay. The results indicated that AG1478
or erlotinib partially reversed rCTHRC1 induced cell migration and
invasion (Fig. 5E and F). This
result demonstrates that CTHRC1 promotes EOC cell migration and
invasion through EGFR signaling.
CTHRC1 activates EGFR/EKR1/2/AKT
signaling in ovarian cancer cells
We further investigated whether EGFR signaling was
affected by CTHRC1. We found that the level of phosphorylated EGFR
was clearly downregulated after CTHRC1 knockdown in ES2 cells
(Fig. 6A). Existing data indicated
that the effects of EGFR signaling on cell migration and invasion
are mediated by PI3K/AKT and ERK1/2 signaling pathways in EOC
(14,20), therefore, we next investigated
whether ERK1/2 and AKT were also involved in EGFR signal induced by
CTHRC1 in EOC. We explored the pERK1/2 and pAKT expression after
CTHRC1 knockdown in ES2 cells. The results showed that the
expression of pEKR1/2 and pAKT was markedly decreased by CTHRC1
knockdown (Fig. 6B and C). We also
detected ERK/MMP9, Src and MEK/ERK signaling pathways and found
that MMP9 expression and phosphorylated Src or MEK were not altered
by CTHRC1 silencing (Fig.
6D-F).
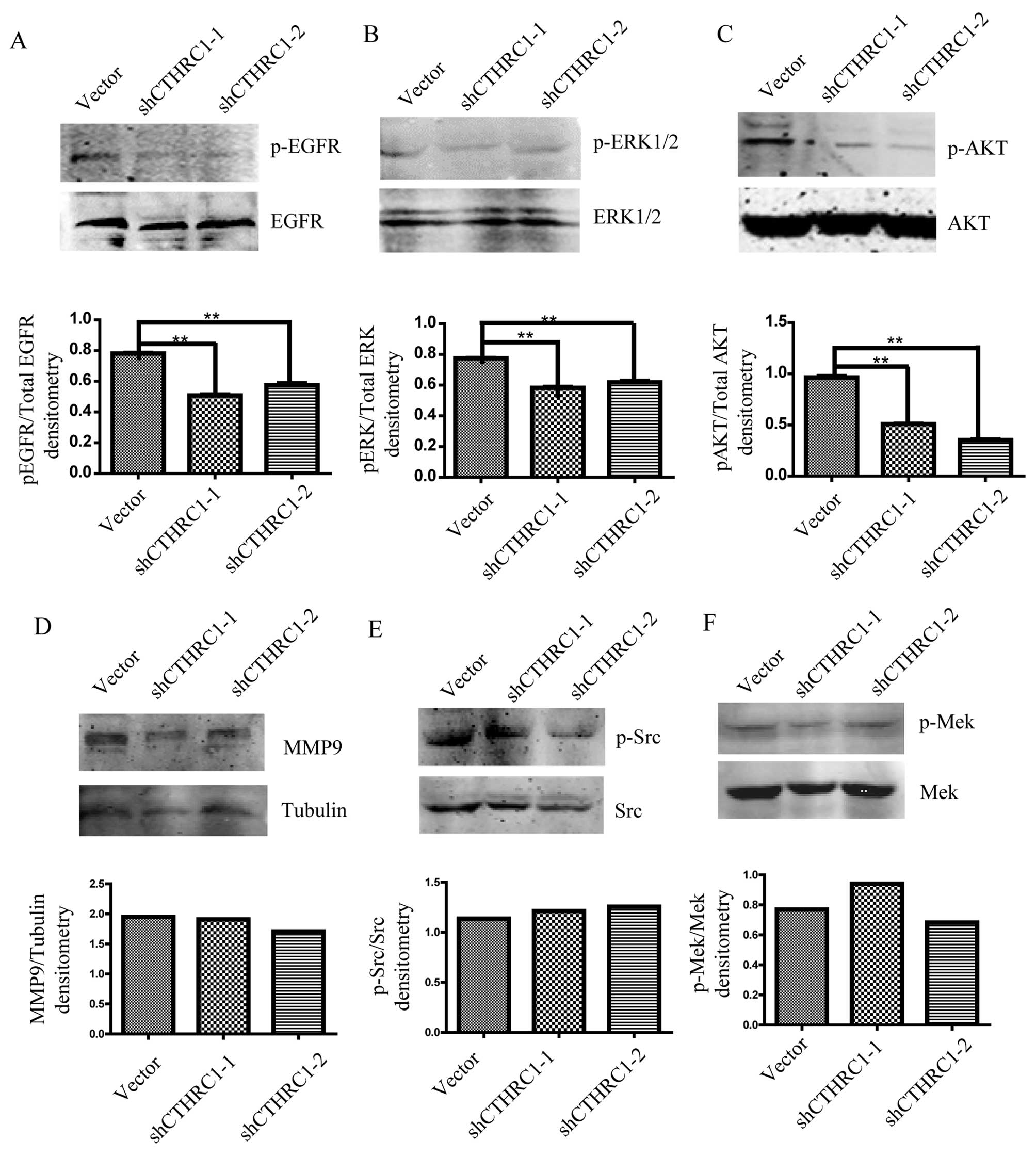 | Figure 6.The expression of EGFR and
phosphorylated EGFR (A), ERK1/2 and phosphorylated ERK1/2 (B), and
AKT and phosphorylated AKT (C) in ES2 cells transfected with
Lenti-shCTHRC1-(1, 2) or control vector detected by western
blotting. The expression of MMP9 (D), Src and phosphorylated Src
(E), and MEK and phosphorylated MEK (F) in ES2 cells transfected
with Lenti-shCTHRC1-(1, 2) or control vector detected by western
blotting. Representative images are shown in the upper lane, and
quantitative analysis of densitometry for p-ERK1/2/total EGFR,
p-ERK1/2/total EGFR, p-AKT/total AKT, MMP9/tubulin, p-Src/total Src
and p-MEK/total MEK by ImageJ software are shown below. β-actin was
used as an internal control. *P<0.05, **P<0.01. |
Collectively, our data demonstrate that CTHRC1 is
upregulated in human ovarian cancers and predicts poor prognosis.
CTHRC1 functions as a pro-migration and pro-invasion factor in
ovarian cancer, which is dependent on the EGFR/ERK1/2/AKT signaling
pathway.
Discussion
Metastasis remains the leading cause of relapse and
death from ovarian cancer. Most women are diagnosed at the advanced
stages of disease (FIGO stages III/IV) with disseminated
intraperitoneal carcinomatosis (20). Although studies have identified
components of signaling pathways whose aberrant expression and/or
activity has been linked to ovarian cancer metastasis, the
mechanisms are poorly understood. There is a critical need for
better understanding of the molecular events that drive metastasis
development and progression in ovarian cancer. CTHRC1 was found to
be aberrantly upregulated in several malignant tumors, including
melanoma, and cancers of the gastrointestinal tract, breast,
thyroid, liver and the pancreas (6,21).
Our study showed that the CTHRC1 was upregulated in
malignant epithelial ovarian tumors and associated with FIGO stage,
lymph node status and tumor size. Importantly, high CTHRC1
expression predicts poor survival of EOC patients, indicating that
CTHRC1 is of value in EOC prognosis. It is noteworthy that the
CTHRC1-positive rate is especially higher in advanced FIGO stage
ovarian cancer patients, indicating that CTHRC1 expression may be
involved in the metastasis of ovarian cancer. Further in
vivo studies are needed to clarify whether CTHRC1 is a critical
factor in EOC cancer metastasis.
Upregulation of CTHRC1 is a feature of many
aggressive human cancers, being linked to tumor cell migration and
invasion. In this study, we found that CTHRC1 silencing
significantly inhibited migration and invasion of the ovarian
cancer cell line ES2. Furthermore, as a secreted protein,
recombinant CTHRC1 protein promoted ovarian cancer cell invasion in
SKOV3. One possible reason why 50 nM CTHRC1 was more efficient than
100 nM CTHRC1 is that 50 nM may be the nearest concentration to
CTHRC1 content in the ovarian cancer microenvironment and very high
concentration of CTHRC1 may trigger different intracellular
signaling pathways that counteracts the cancer cell invasion.
Although the functional roles of CTHRC1 in tumor
cell invasion and metastasis have been well established, the
underlying mechanisms of how CTHRC1 promotes cancer cell invasion
is not fully understood. Several studies demonstrated that CTHRC1
can promote tumor cells migration and invasion by activating some
signal pathways, such as Wnt/PCP-Rho, ERK/MMP9, Src/focal adhesion
kinase and MEK/ERK signaling (22–24).
In ovarian cancer cells, Hou et al demonstrated that CTHRC1
increased the invasive capabilities by activating the Wnt/β-catenin
signaling pathway (17). However,
no report revealed the association of CTHRC1 with EGFR
signaling.
Approximately 70% of epithelial ovarian cancer
express activated EGFR (25). EGFR
overexpression and activation result in increased proliferation and
migration of solid tumors including ovarian cancer (12,26,27).
In this study, we showed that CTHRC1 promotes ovarian cancer cell
invasion by activating EGFR signaling. The following evidence
supported our finding. Western blotting showed that the
phosphorylation of EGFR expression was significantly decreased
after CTHRC1 knockdown, and the pro-invasion activity of the
rCTHRC1 protein was blocked by inhibitors of EGFR. Existing data
indicated that the effects of EGFR signaling on cell migration and
invasion are mediated by PI3K/AKT and ERK1/2 signaling pathways in
epithelial ovarian cancer (14,28,29).
In this study both expression of Akt and ERK1/2 phosphorylation
were decreased after CTHRC1 knockdown in the same ovarian cancer
cell line. Our results provide a novel mechanism mediated by CTHRC1
to induce ovarian cancer cell migration. Further deep mechanism
investigations are needed to find a binding receptor of CTHRC1 in
EOCs, a linker between secreted CTHRC1 expression and intracellular
EGFR signaling activation.
Taken together, our results indicate CTHRC1
contribute to the progression of ovarian cancer through activating
EGFR signaling and identified CTHRC1 as a potentially important
molecule for human ovarian cancer prognosis. CTHRC1 together with
EGFR can be considered as targets for treatment of epithelian
ovarian cancer.
Acknowledgements
This study was supported by The Fifth People's
Hospital of Shanghai Foundation (2015WYQJ03), Shanghai Key
Laboratory of Female Reproductive Endocrine Related Diseases
(14DZ2271700) and the National Natural Science Foundation of China
(81101600).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alonso DF, Ripoll GV, Garona J, Iannucci
NB and Gomez DE: Metastasis: Recent discoveries and novel
perioperative treatment strategies with particular interest in the
hemostatic compound desmopressin. Curr Pharm Biotechnol.
12:1974–1980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Z, Wu Z, Li J, Yang X, Wang Y, Yu Y, Ye
J, Xu C, Qin W and Zhang Z: MCAM is a novel metastasis marker and
regulates spreading, apoptosis and invasion of ovarian cancer
cells. Tumour Biol. 33:1619–1628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Durmus T, LeClair RJ, Park KS, Terzic A,
Yoon JK and Lindner V: Expression analysis of the novel gene
collagen triple helix repeat containing-1 (Cthrc1). Gene Expr
Patterns. 6:935–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang L, Dai DL, Su M, Martinka M, Li G and
Zhou Y: Aberrant expression of collagen triple helix repeat
containing 1 in human solid cancers. Clin Cancer Res. 12:3716–3722.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang P, Wang YC, Chen XY, Shen ZY, Cao H,
Zhang YJ, Yu J, Zhu JD, Lu YY and Fang JY: CTHRC1 is upregulated by
promoter demethylation and transforming growth factor-β1 and may be
associated with metastasis in human gastric cancer. Cancer Sci.
103:1327–1333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ke Z, He W, Lai Y, Guo X, Chen S, Li S,
Wang Y and Wang L: Overexpression of collagen triple helix repeat
containing 1 (CTHRC1) is associated with tumour aggressiveness and
poor prognosis in human non-small cell lung cancer. Oncotarget.
5:9410–9424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan JK, Pham H, You XJ, Cloven NG, Burger
RA, Rose GS, Van Nostrand K, Korc M, Disaia PJ and Fan H:
Suppression of ovarian cancer cell tumorigenicity and evasion of
cisplatin resistance using a truncated epidermal growth factor
receptor in a rat model. Cancer Res. 65:3243–3248. 2005.PubMed/NCBI
|
|
10
|
Phelps SL Bull, Schorge JO, Peyton MJ,
Shigematsu H, Xiang LL, Miller DS and Lea JS: Implications of EGFR
inhibition in ovarian cancer cell proliferation. Gynecol Oncol.
109:411–417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maihle NJ, Baron AT, Barrette BA, Boardman
CH, Christensen TA, Cora EM, Faupel-Badger JM, Greenwood T, Juneja
SC, Lafky JM, et al: EGF/ErbB receptor family in ovarian cancer.
Cancer Treat Res. 107:247–258. 2002.PubMed/NCBI
|
|
13
|
Zhou C, Qiu L, Sun Y, Healey S, Wanebo H,
Kouttab N, Di W, Yan B and Wan Y: Inhibition of EGFR/PI3K/AKT cell
survival pathway promotes TSA's effect on cell death and migration
in human ovarian cancer cells. Int J Oncol. 29:269–278.
2006.PubMed/NCBI
|
|
14
|
Loganathan S, Kandala PK, Gupta P and
Srivastava SK: Inhibition of EGFR-AKT axis results in the
suppression of ovarian tumors in vitro and in preclinical mouse
model. PLoS One. 7:e435772012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gan Y, Shi C, Inge L, Hibner M, Balducci J
and Huang Y: Differential roles of ERK and Akt pathways in
regulation of EGFR-mediated signaling and motility in prostate
cancer cells. Oncogene. 29:4947–4958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG,
Song GY, Kwon KI, Jeong TC and Jeong HG: Suppression of EGF-induced
tumor cell migration and matrix metalloproteinase-9 expression by
capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK,
p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 55:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou M, Cheng Z, Shen H, He S, Li Y, Pan Y,
Feng C, Chen X, Zhang Y, Lin M, et al: High expression of CTHRC1
promotes EMT of epithelial ovarian cancer (EOC) and is associated
with poor prognosis. Oncotarget. 6:35813–35829. 2015.PubMed/NCBI
|
|
18
|
Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang
W, Di W and Qiu L: MicroRNA-7 inhibits tumor metastasis and
reverses epithelial-mesenchymal transition through AKT/ERK1/2
inactivation by targeting EGFR in epithelial ovarian cancer. PLoS
One. 9:e967182014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao C, Lu S, Sowa A, Kivlin R, Amaral A,
Chu W, Yang H, Di W and Wan Y: Priming with EGFR tyrosine kinase
inhibitor and EGF sensitizes ovarian cancer cells to respond to
chemotherapeutical drugs. Cancer Lett. 266:249–262. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khalil I, Brewer MA, Neyarapally T and
Runowicz CD: The potential of biologic network models in
understanding the etiopathogenesis of ovarian cancer. Gynecol
Oncol. 116:282–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kharaishvili G, Cizkova M, Bouchalova K,
Mgebrishvili G, Kolar Z and Bouchal J: Collagen triple helix repeat
containing 1 protein, periostin and versican in primary and
metastatic breast cancer: An immunohistochemical study. J Clin
Pathol. 64:977–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H,
Zhang WM, You H, Qin W, Gu J, Yang S, et al: CTHRC1 acts as a
prognostic factor and promotes invasiveness of gastrointestinal
stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia.
16:265–278, 278.e1-278.e13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim
JT, Kim BY, Lee SJ, Choe YK, Kim DH, et al: Collagen triple helix
repeat containing 1 (CTHRC1) acts via ERK-dependent induction of
MMP9 to promote invasion of colorectal cancer cells. Oncotarget.
5:519–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park EH, Kim S, Jo JY, Kim SJ, Hwang Y,
Kim JM, Song SY, Lee DK and Koh SS: Collagen triple helix repeat
containing-1 promotes pancreatic cancer progression by regulating
migration and adhesion of tumor cells. Carcinogenesis. 34:694–702.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37:(Suppl 4). S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y,
Zhao L, Qu H, Fan Y and Wu C: Antagonism of miR-21 reverses
epithelial-mesenchymal transition and cancer stem cell phenotype
through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One.
7:e395202012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang ZG, Wei JM, Qin CF, Hao K, Tian XD,
Xie K, Xie XH and Yang YM: Suppression of the epidermal growth
factor receptor inhibits epithelial-mesenchymal transition in human
pancreatic cancer PANC-1 cells. Dig Dis Sci. 57:1181–1189. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konishi H, Takagi A, Kurita A, Kaneda N
and Matsuzaki T: PEGylated liposome IHL-305 markedly improved the
survival of ovarian cancer peritoneal metastasis in mouse. BMC
Cancer. 12:4622012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wittinger M, Vanhara P, El-Gazzar A,
Savarese-Brenner B, Pils D, Anees M, Grunt TW, Sibilia M, Holcmann
M, Horvat R, et al: hVps37A status affects prognosis and cetuximab
sensitivity in ovarian cancer. Clin Cancer Res. 17:7816–7827. 2011.
View Article : Google Scholar : PubMed/NCBI
|