Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors worldwide, and is a serious threat to human
life. HCC has the second highest cancer mortality rate, next to
lung cancer. Approximately 782,500 new liver cancer cases and
745,500 deaths occured worldwide in 2012, and most primary liver
cancers occurring worldwide are HCC. China is a country with a high
incidence of HCC, accounting for about 50% of the total number of
cancer cases and deaths (1).
Surgical and liver transplantation are still the best treatment
option for suitable patients (2–4).
However, most HCC cases are diagnosed at the advanced or
unresectable stage. Given the poor prognosis of HCC, new
therapeutic strategies and novel therapeutic targets are needed,
respectively. But current research on the main pathogenic genes and
molecular mechanisms of HCC remain unclear. In addition, coupled
with the existence of an ageing population and pollution of the
environment, we are faced with the increasingly serious situation
of HCC. Therefore, the research for pathogenic genes and molecular
mechanisms of HCC play a key role in the prevention and treatment
of HCC.
HECT domain and ankyrin repeat containing E3
ubiquitin protein ligase 1 (HACE1) is located on chromosome 6q21
which is one site of frequent mutation of malignant tumors
(5–8). HACE1 is an important tumor-suppressor
gene, which plays an important role in tumor inhibition by
mediating cell autophagy, Rac1 ubiquitination and other mechanisms.
It is also involved in a variety of biological functions including
heart protection, anti-oxidative stress and cellular dynamics
(9–13). HACE1 gene was firstly discovered as
closely related to the occurrence of Wilms' tumor (5). Futhermore, more evidence demonstrated
that lower expression or mutations of HACE1 are associated with a
variety of human malignant tumors, including breast cancer,
colorectal cancer and lymphomas (14–16).
However, there have been few available data concerning the precise
function of the HACE1 gene in HCC.
In the present study, we investigated the expression
of HACE1 in human HCC tissues and cell lines compared with matched
adjacted non-tumor HCC tissues and the normal liver cell line L02.
Immunohistochemistry (IHC) was performed to analyze the correlation
of HACE1 expression with the clinicopathological features of
patients. In addition, Cell Counting Kit-8 (CCK-8), Transwell and
wound healing assays were applied to ascertain the role of HACE1 in
the proliferation and migration of the HCC cell lines SMCC7721 and
Huh7.
Materials and methods
Patient tissue samples
A total of 40 paired tumor and their adjacted
non-tumor tissues were collected from HCC patients who underwent
routine surgical resection at The First Hospital of Lanzhou
University from 2007 to 2010. The fresh tissues were instantly
frozen in liquid nitrogen and subsequently stored at −80°C until
RNA and protein extraction for reverse-transcription quantitative
polymerase chain reaction. In order to determine the relationships
between HACE1 and the clinicopathological characteristics of HCC,
an additional 102 paraffin-embedded archived specimens from
patients who had undergone surgical resection were incorporated
into the study for IHC. These patients included 82 males and 20
females, aged between 30 and 78 years, and the median patient age
was 49 years. Histology of the tumor tissues was evaluated by two
independent pathologists who were blinded to our examination. The
clinicopathological information of all patients were collected for
analysis, including age, gender, serum AFP, ALT level, tumor size,
tumor number, vascular invasion, HBsAg, tumor differentiation,
Child-Pugh and liver cirrhosis. The present study was approved by
the Ethics Committee of The First Hospital of Lanzhou University
prior to the initiation of the study.
Cell lines and culture
The human HCC cell lines Huh7, MHCC97H, HepG2,
MHCC97L, HCCLM3, SMCC7721 and liver cell line L02 were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). All cell lines were cultured in Dulbecco's modified Eagle's
medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and
100 U/ml penicillin and 100 mg/ml streptomycin (all from Gibco,
Shanghai, China). The cells were grown at 37°C in a humidified
atmosphere of 5% CO2. The medium was changed every 2–3
days. Before further experiments, the protein and mRNA expression
levels of HACE1 in the human HCC cell lines were examined by
western blot analysis and quantitative real-time polymerase chain
reaction (qRT-PCR), respectively. According to the experimental
results, we chose the SMCC7721 and Huh7 cell lines for subsequent
experiments.
Cell infection
The Huh7 cells were cultured to 50–60% confluency in
6-well plates and were transiently transfected with siRNA of HACE1
or negative control (NC) siRNA (GeneChem, Shanghai, China) using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in accordance
with the manufacturer's instructions for HACE1 knockdown. HACE1
siRNAs were: GGGCUACAAUGGGAAUAAAdTdT (si-HACE1-1),
GCCCGAGGAUAAUGAAACUdTdT (si-HACE1-2), GCCA GUACCUAAAGAUUCUdTdT
(si-HACE1-3). The SMCC7721 cells were transfected with
pcDNA3.1-HACE1 or mock plasmid (GeneChem) using Lipofectamine 2000
transfection reagent according to the manufacturer's instructions
for HACE1 overexpression. The transfection efficiency of both Huh7
and SMCC7721 cells was evaluated using qRT-PCR and western blot
analysis. After 36 h of incubation, the cells were harvested for
further analysis. All transfections were performed in
triplicates.
qRT-PCR
Total RNA from tissues and all 7 cell lines were
extracted using TRIzol reagent (Takara, Dalian, China), according
to the manufacturer's instructions. The RNA concentration was
detected by NanoDrop 1000 (Thermo Fisher Scientific, Wilmington,
DE, USA) and complementary DNA was synthesized utilizing a
PrimeScript RT reagent kit (Takara) using 2 µg RNA. SYBR-Green dye
(Takara) and Corbet Rotor-Gene 3000 thermocycler were used to
perform the qRT-PCR reaction, according to the supplied protocol.
The primers used were as follows: HACE1 forward,
5′-TCCTTGAATGTCCTGAGTTGA-3′ and reverse,
5′-AATCTGGCTGTCCTGAATGC-3′; and GAPDH forward,
5′-GGCATCCTGGGCTACACTGA-3′ and reverse, 5′-GTGGTCGTTGAGGGCAATG-3′.
The amplification conditions of qRT-PCR were set as follows: 95°C
for 30 sec, 95°C for 5 sec, 60°C for 34 sec, 95°C for 15 sec and a
total of 40 cycles. The experiments were repeated in triplicate.
Relative expression levels of HACE1 were normalized to GAPDH
expression in each sample, and the data were analyzed according to
the comparative threshold cycle (2−ΔΔCt) method.
Protein extraction and western blot
analysis
Total protein of human HCC tissues and cells were
extracted using ice-cold lysis buffer (50 mM Tris, pH 7.4, 150 mM
NaCl, 1% SDS, 1 mM EDTA, 1% NP-40) containing 1 mM protein
inhibitor and 1 mM PMSF, for 30 min on ice. The lysates were
centrifuged at 12,000 rpm at 4°C for 15 min and the supernatants
were collected. Protein concentration was determinated using the
BCA protein assay (Beyotime, Shanghai, China). Protein samples (50
µg/lane) were separated by electrophoresis on 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto polyvinylidene difluoride (PVDF) membrane filters
(Millipore Corp., Billerica, MA, USA) in a wet transfer system
(Bio-Rad, Berkeley, CA, USA). PVDF membranes were blocked with 2%
BSA for 1 h at room temperature. Membranes were incubated with
anti-human HACE1 antibody (1:2,000 dilution; Abcam, Cambridge, UK)
overnight at 4°C. The membranes were then incubated with the
corresponding secondary antibody (1:5,000) for 1 h at room
temperature. The immunoreactive bands were visualized using
enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific,
Israel), and the relative protein expression of the membranes was
then normalized to the β-actin levels.
Cell proliferation assay
The CCK-8 assay (Dojindo Laboratories, Kunamoto,
Japan) was used to reflect the proliferation of the cells. For the
CCK-8 assay, ~5,000 viable cells were placed into 96-well plates at
a final volume of 100 µl for each well. Every 24 h, 10 µl of CCK-8
solution was added to each well, and the plate was further
incubated for 2 h at 37°C. The absorbance at 450 nm was quantitated
with a microplate reader. The experiment was performed in 6
replicates.
Cell migration assay
Transwell and wound healing assays were used to
evaluate the cell migration capability. The Transwell and migration
assays were performed using a 24-well plate, and a Transwell
chamber with a polycarbonate filter membrane (Corning Inc.,
Corning, NY, USA) was placed in the 24-well plate, and the pore
size of the filter membrane size was 8 µm. Firstly, digested cells
(5×103/chamber) with 200 µl serum-free media were added
to the upper compartment, and 400 µl DMEM containing 10% FBS was
added to the lower compartment, and further incubated for 24 h at
37°C. After incubation, the cells in the upper chamber migrated to
the lower surface of the membrane. The cells on the upper membrane
were removed carefully with a cotton tip and the penetrated
polycarbonic membrane was fixed with 95% methanol and stained with
0.1% crystal violet. The number of migrated cells was counted in 6
randomly selected fields under an inverted microscope. Independent
experiments were performed in triplicate. For the wound healing
assay, tumor cells were seeded in a 6-well plate at a density and
incubated to 70–80% confluency as a monolayer. A cell-free straight
line was scratched in the center of the well with a sterile 200-µl
pipette tip. Similarly, a second straight line was created
perpendicular to the first line to produce a cross-shaped cellular
gap in each well. The cells were subsequently washed twice with
phosphate-buffered saline (PBS) and refreshed with medium
containing 5% FBS. The cells were grown for an additional 72 h.
Digital images of the cell gap were captured at different time
points using a microscope. The gap distance was quantitatively
assessed using software.
Immunohistochemistry
The relationships between HACE1 expression and
clinicopathological characteristics of the HCC patients was
analyzed using 4-µm paraffin-embedded specimens by IHC. After
incubation at 60°C for 30 min, all tissue sections were
deparaffinized in xylene, and then rehydrated by graded ethanol
solutions before sodium citrate buffer (pH 6.0) was used as an
antigen retrieval solution. Endogenous peroxidase activity was
blocked with 0.3% hydrogen peroxide in a humidified chamber for 15
min. After washing for three times, the specimens were incubated
with rabbit monoclonal antibody against human HACE1 (1:200,
ab18056; Abcam) at 4°C overnight. Following washing with PBS for
three times, the tissue sections were incubated with a horseradish
peroxidase-labeled secondary antibody for 1 h at room temperature.
The specimens were incubated with 3,3′-diaminobenzidine (DAB)
solution for 3 min. Finally, the sections were counterstained with
hematoxylin. The primary antibody was replaced by PBS as a NC.
Tissue specimens were assessed separately by two pathologists who
were blinded to any information about the patient background or
clinical status. The percentage of positive staining was scored as
follows: 1 point (0–25%, weakly stained); 2 points, (>25–50%,
moderately stained); and 3 points (>50%, strongly stained). The
HACE1 immunostaining score was calculated from the product of the
percent positivity score × the staining intensity score which
ranged from 0 to 9. Low HACE1 expression level was defined as a
total score of <5, and high HACE1 expression was defined as a
total score of ≥5.
Statistical analysis
All statistical analysis was performed using SPSS
21.0 software. The relationships between HACE1 expression and
clinicopathological characteristics were analyzed using the
χ2 test. Student's t-test was used to compare the
statistical significance in the various groups. Survival curves
were calculated using the Kaplan-Meier method and the result was
compared using the log-rank test. Cox's proportional hazard
analysis was used to explore univariate and multivariate survival.
A difference was considered statistically significant at
P<0.05.
Results
Expression of HACE1 protein and mRNA
in HCC and paired adjacent non-tumor tissues
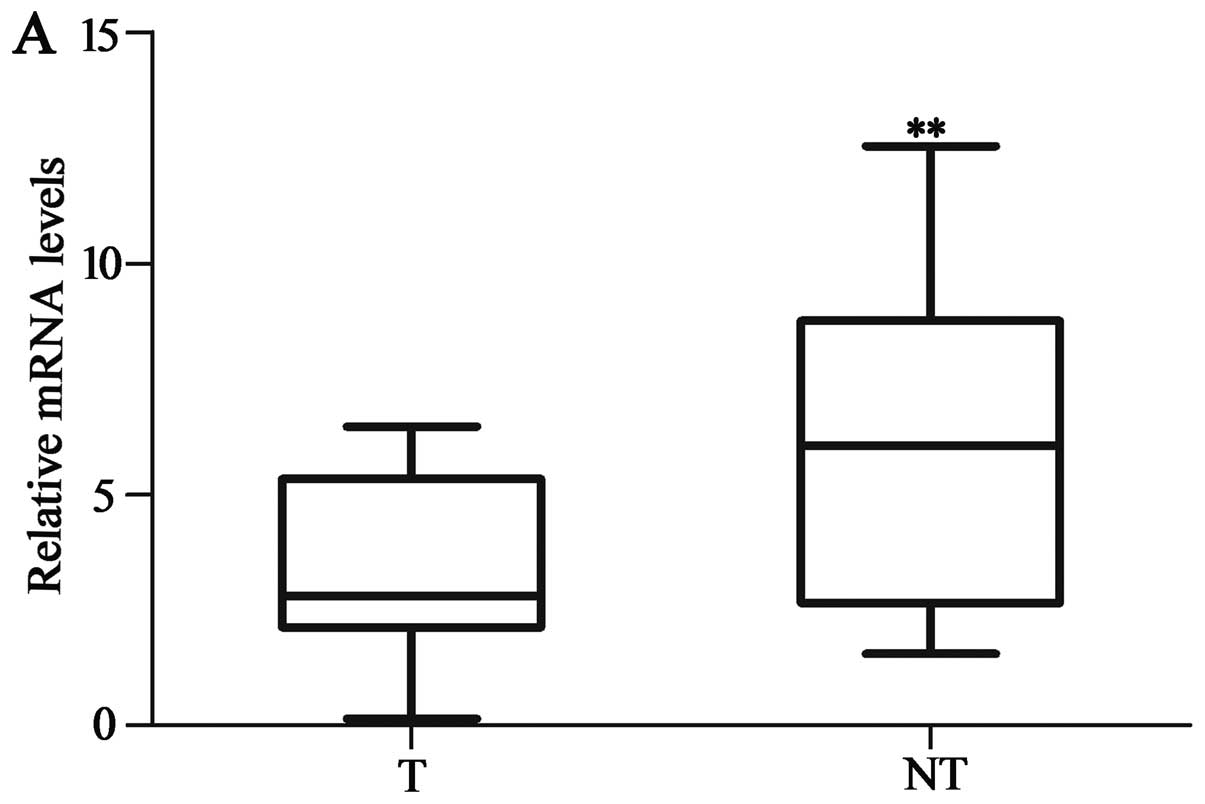
The expression of HACE1 was markedly lower in the
HCC tissues compared to that noted in the matched adjacent
non-tumor tissues at both the protein and mRNA levels. The HACE1
protein and mRNA expression in 40 HCC specimens and their adjacent
non-tumor tissues were evaluated by western blot analysis and
qRT-PCR. HACE1 mRNA expression level of HACE1 was lower in 32 of
the 40 HCC samples (80%) than in the adjacent non-tumor tissues
(P<0.01; Fig. 1A). The protein
expression of HACE1 was decreased in 30 of the 40 HCC samples
(75%), compared with the matched adjacent non-tumor tissues
(P<0.001; Fig. 1B and C).
Expression of HACE1 protein and mRNA
in HCC cell lines
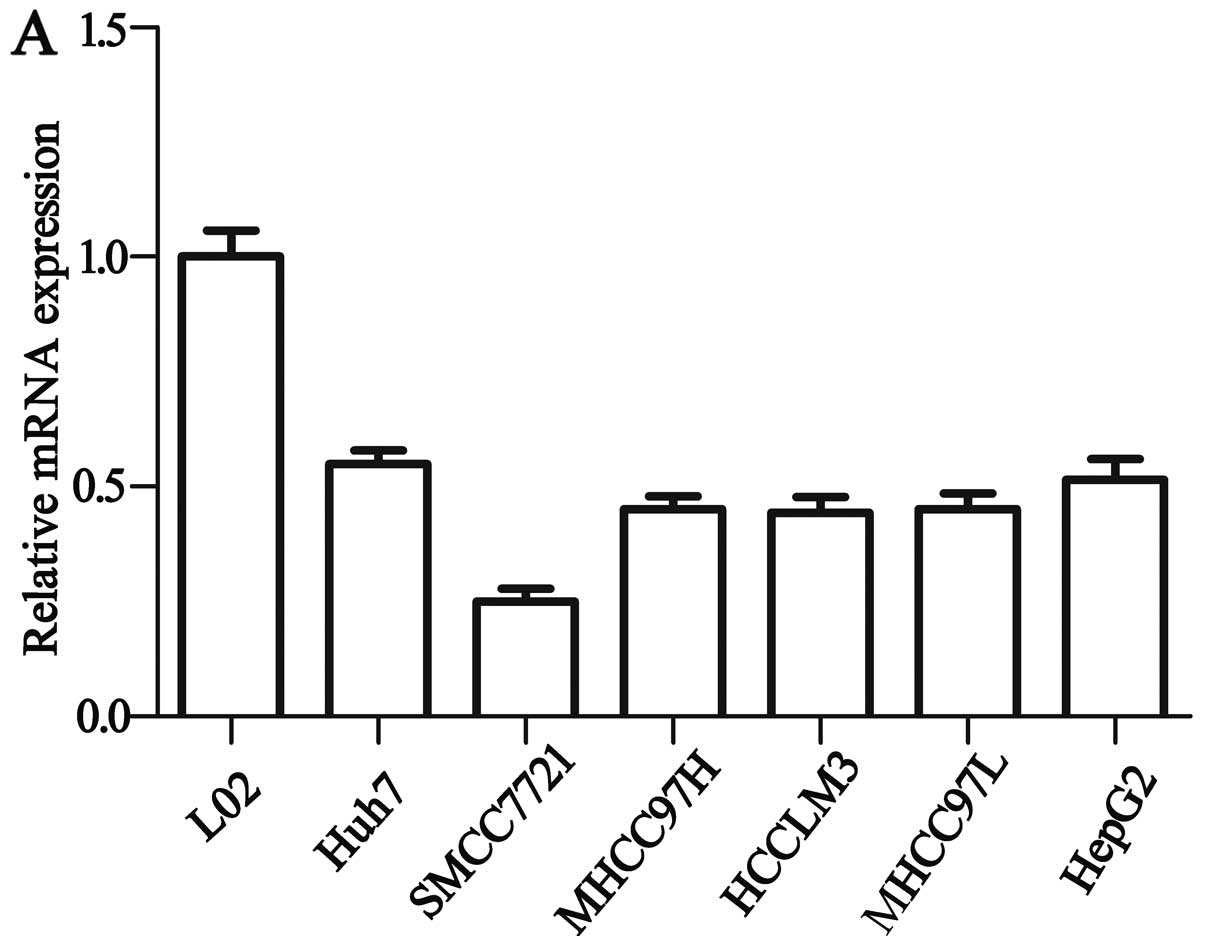
We assessed the HACE1 gene expression in HCC cell
lines by western blot analysis and qRT-PCR. Western blot analysis
and qRT-PCR determined that HACE1 mRNA (Fig. 2A) and protein expression (Fig. 2B) were significantly decreased in
the 6 HCC cell lines compared with the expression levels in the
normal liver cell line L02. As shown in Fig. 2, the highest expression of the HACE1
gene was detected in Huh7 cells, and the lowest was detected in the
SMCC7721 cells among all HCC cell lines. Therefore, to verify the
role of HACE1 in the HCC cell lines, we upregulated HACE1 by
transfection of pcDNA3.1-HACE1 into the SMCC7721 cells and Huh7
cells were transfected with siRNA targeting HACE1 for
downregulation.
Knockdown and overexpression
efficiency as determined by western blot analysis and qRT-PCR
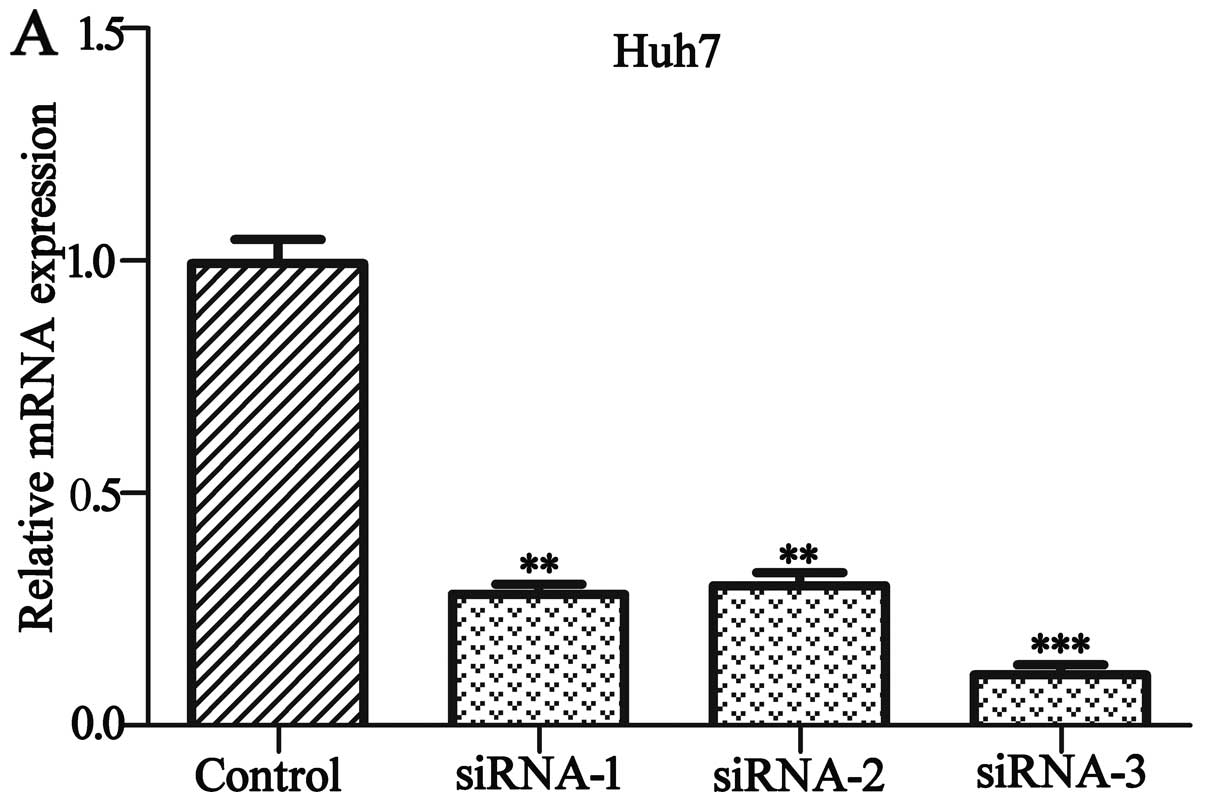
To explore the role of HACE1, we knocked down the
HACE1 gene in the human HCC Huh7 cell line and overexpressed HACE1
in the HCC SMCC7721 cell line, respectively. As shown in Fig. 3A and B, by day 2 post infection,
HACE1 protein and mRNA levels of HACE1-siRNA-infected Huh7 cells
were significantly lower compared to levels in the cultures
infected with the NC siRNA by western blot analysis and qRT-PCR.
The highest knockdown efficiency of HACE1 siRNA was siRNA-3 and
thus siRNA-3 was used in the subsequent experiment (P<0.001).
Similarly, HACE1 mRNA and protein levels of pcDNA3.1-HACE1-infected
SMCC7721 cell line were significantly higher compared to levels in
the cultures infected with the mock plamid as a control (P<0.01;
Fig. 3C and D).
Effect of the knockdown and
overexpression of HACE1 on cellular proliferation and migration in
the Huh7 and SMCC7721 cell lines
To evaluate the role of HACE1 in HCC proliferation
and migration, two different types of cells were examined by CCK-8,
Transwell and wound healing assays. The results indicated that
downregulation of HACE1 expression significantly inhibited the
proliferation and migration of the Huh7 cells (P<0.05; Fig. 4A-C), and upregulation of HACE1
expression obviously promoted the proliferation and migration of
the SMCC7721 cells (P<0.05; Fig.
4D-F).
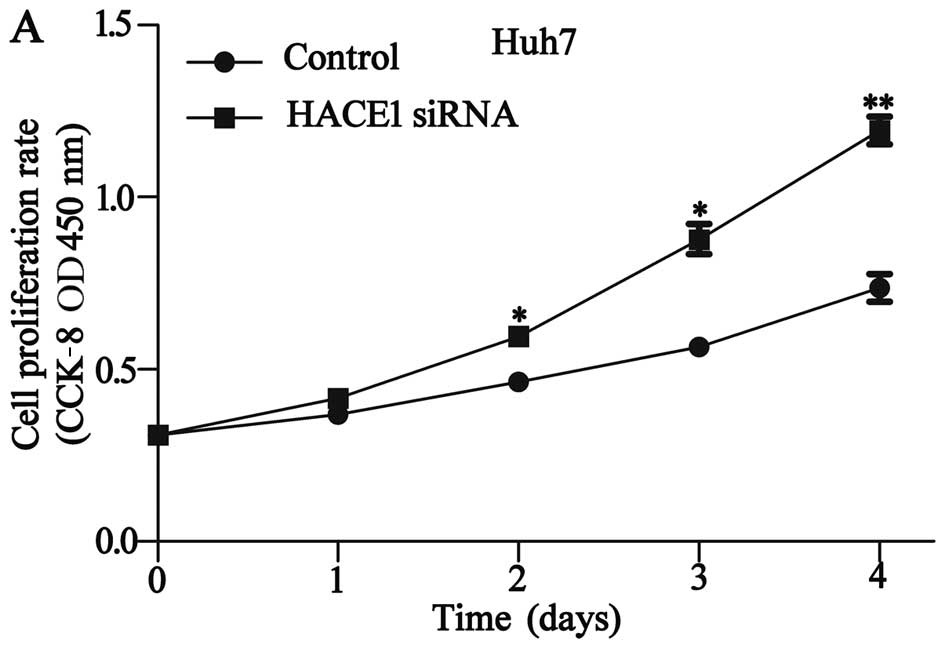 | Figure 4.Effects of the knockdown of HACE1 on
cell proliferation and migration in the Huh7 cell line. (A) The
CCK-8 assay was performed following HACE1 siRNA3 infection, and
cell proliferation of Huh7 cells was measured. (B) The migration of
Huh7 cells was assessed by Transwell assay, (magnification, ×100)
and the number of migratory cells of six random fields was counted.
(C) Wound healing assays of Huh7 cells. HACE1 knockdown had a
measurable inhibitory effect on cell migration. Magnification,
×100. Knockdown of HACE1 promoted the proliferation and migration
of Huh7 cells (*P<0.05, **P<0.01). The histograms depict the
quantification of triplicate results with mean ± SD. HACE1, HECT
domain and ankyrin repeat containing E3 ubiquitin protein ligase 1.
Effect of the overexpression of HACE1 on cell proliferation and
migration in the SMCC7721 cell line. (D) The CCK-8 assay was
performed following pcDNA3.1-HACE1 infection, and cell
proliferation of the SMCC7721 cells was measured. (E) The migration
of SMCC7721 cells was assessed by Transwell assay and the number of
migratory cells for six random fields was counted. (F) Wound
healing assays. HACE1 overexpression had a measurable promoting
effect on the cell migration of the SMCC7721 cells. Magnification,
×100. Overexpression of HACE1 inhibited the proliferation and
migration of SMCC7721 cells (**P<0.01, ***P<0.001). The
histograms depict the quantification of triplicate results with
mean ± SD. HACE1, HECT domain and ankyrin repeat containing E3
ubiquitin protein ligase 1. |
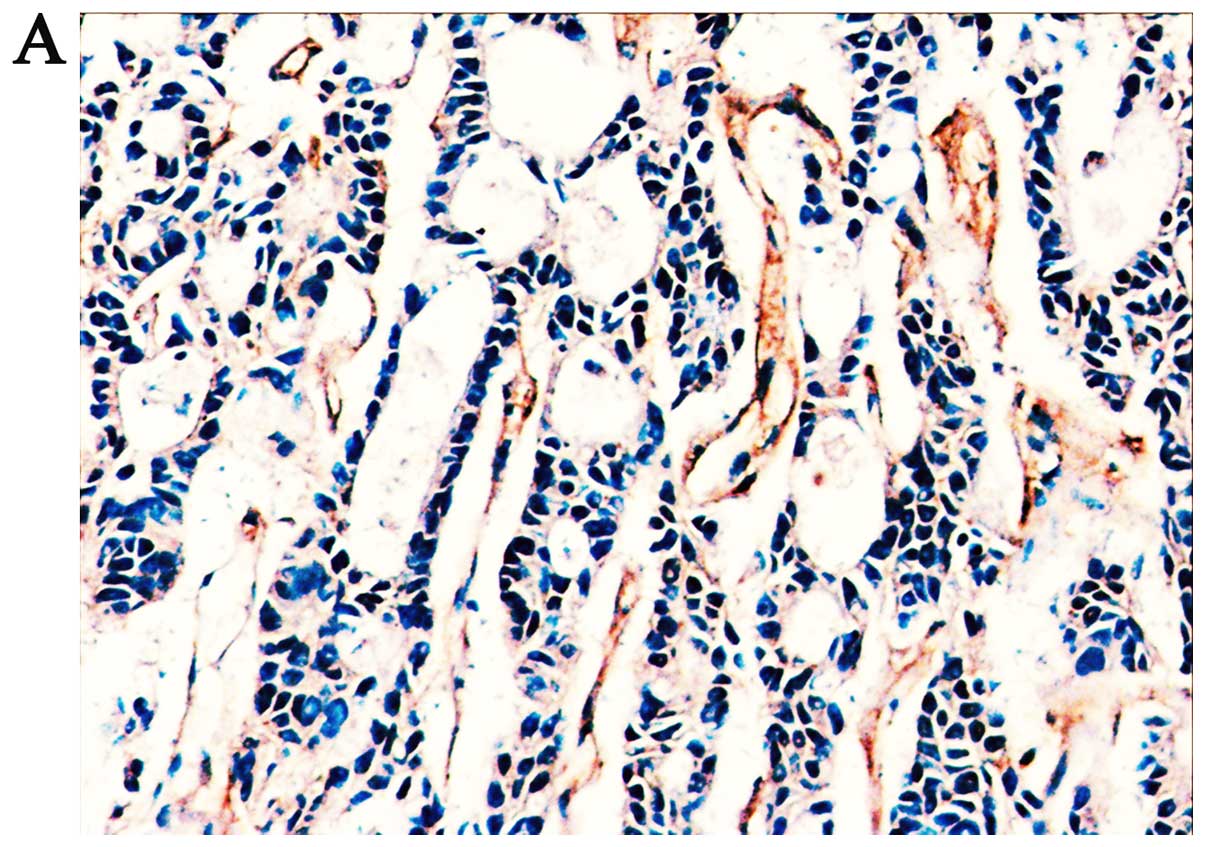
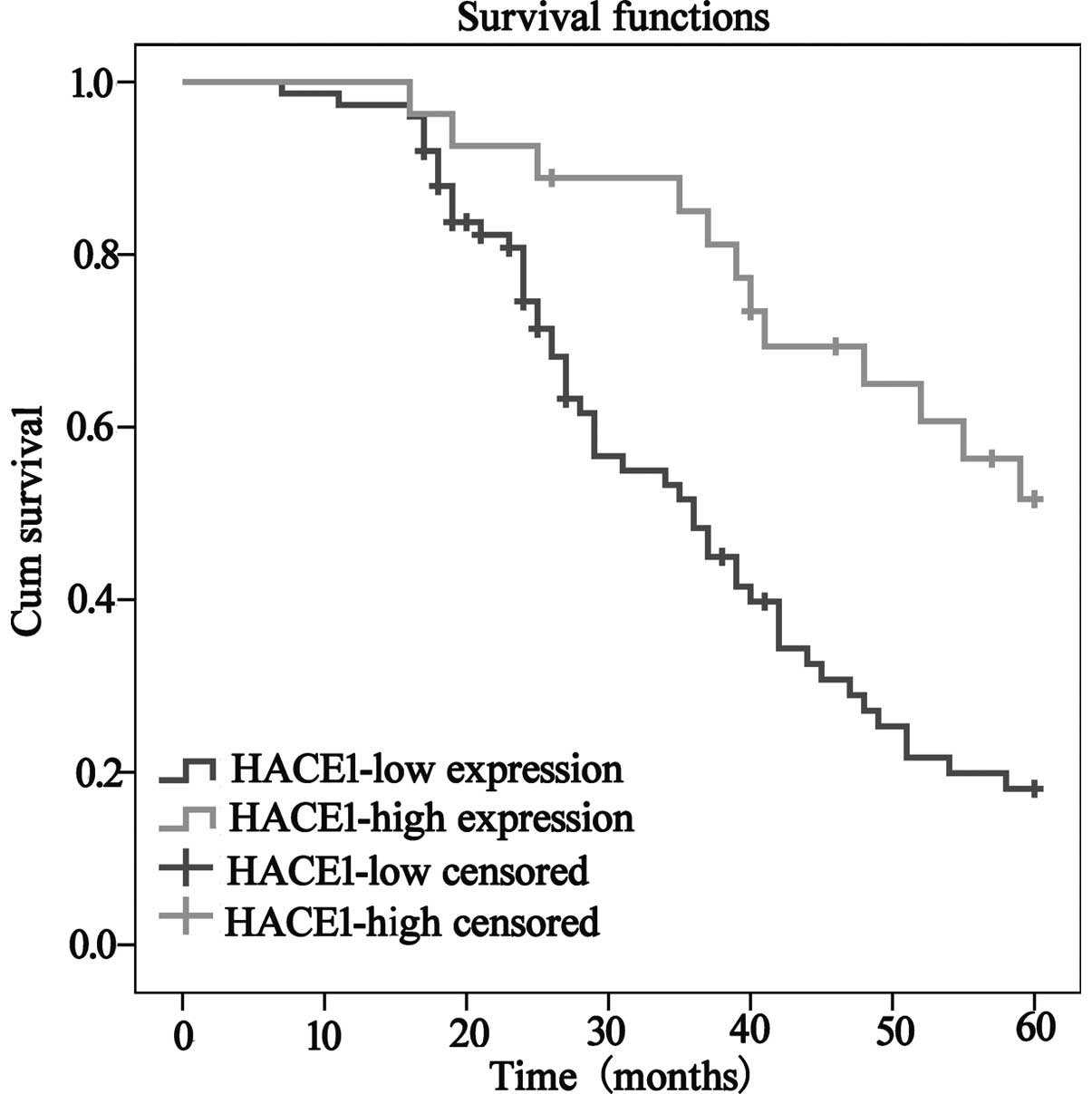
Correlation between HACE1 expression
and clinicopathological characteristics
We explored the correlation of HACE1 expression and
the clinicopathological characteristics of the 102 HCC patients
based on IHC analysis (Fig. 5) and
the results are summarized in Table
I. From the results, we found that 75 samples (73.52%) had low
HACE1 expression, and 27 samples (26.48%) had high HACE1
expression. According to the level of HACE1 expression in the tumor
tissues, we demonstrated that a low level of HACE1 expression was
significantly associated with serum AFP level (P<0.001), tumor
differentiation (P<0.05) and vascular invasion (P<0.05).
Overall survival was significantly decreased in the patients with
low HACE1 expression compared to patients with high HACE1
expression (Fig. 6). In addition,
univariate and multivariate Cox regression analyses demonstrated
that AFP level, tumor differentiation, Child-Pugh and HACE1 level
were significantly associated with overall survival and were
significant prognostic factors for HCC patients (Table II).
 | Table I.Correlation between HACE1 expression
and clinicopathological characteristics of the HCC cases
(n=102). |
Table I.
Correlation between HACE1 expression
and clinicopathological characteristics of the HCC cases
(n=102).
|
| HACE1 |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
indices | Low | High | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
| ≤50 | 38 | 12 | 0.308 | 0.580 |
|
>50 | 37 | 15 |
|
|
| Gender |
|
|
|
|
|
Female | 15 | 5 | 0.028 | 0.868 |
| Male | 60 | 22 |
|
|
| AFP (ng/ml) |
|
|
|
|
| ≤20 | 27 | 20 | 11.583 |
<0.001a |
|
>20 | 48 | 7 |
|
|
| ALT (U/l) |
|
|
|
|
| ≤75 | 31 | 14 | 0.891 | 0.345 |
|
>75 | 44 | 13 |
|
|
| Tumor size (cm) |
|
|
|
|
| ≤5 | 46 | 16 | 0.036 | 0.850 |
|
>5 | 29 | 11 |
|
|
| Tumor number |
|
|
|
|
| 1 | 56 | 20 | 0.004 | 0.952 |
|
>1 | 19 | 7 |
|
|
| Vascular
invasion |
|
|
|
|
| No | 41 | 21 | 4.449 | 0.035a |
| Yes | 34 | 6 |
|
|
| HBsAg |
|
|
|
|
|
Positive | 59 | 17 | 2.578 | 0.108 |
|
Negative | 16 | 10 |
|
|
| Tumor
differentiation |
|
|
|
|
|
I/II | 42 | 22 | 5.515 | 0.019a |
|
III/IV | 33 | 5 |
|
|
| Child-Pugh
score |
|
|
|
|
| A | 27 | 14 | 2.075 | 0.150 |
| B | 48 | 13 |
|
|
| Liver
cirrhosis |
|
|
|
|
|
Positive | 60 | 17 | 3.115 | 0.078 |
|
Negative | 15 | 10 |
|
|
 | Table II.Univariate and multivariate analyses
of the overall survival of 102 patients with HCC. |
Table II.
Univariate and multivariate analyses
of the overall survival of 102 patients with HCC.
|
|
| Univariate
analysis |
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 0.976 | 0.542–1.572 | 0.784 |
|
|
|
| Gender | 0.624 | 0.338–1.334 | 0.337 |
|
|
|
| AFP (ng/ml) | 3.028 | 1.760–6.075 |
<0.001a | 3.432 | 1.831–6.433 |
<0.001a |
| ALT (U/l) | 0.951 | 0.531–1.543 | 0.762 |
|
|
|
| Tumor size
(cm) | 1.295 | 0.881–2.018 | 0.575 |
|
|
|
| Tumor number | 1.058 | 0.610–1.823 | 0.658 |
|
|
|
| Vascular
invasion | 1.032 | 0.595–1.818 | 0.779 |
|
|
|
| HBsAg | 1.135 | 0.635–1.943 | 0.643 |
|
|
|
| Tumor
differentiation | 2.126 | 1.350–3.586 | 0.011a | 1.892 | 1.214–2.721 | 0.039a |
| Child-Pugh
score | 2.051 | 1.319–2.974 | 0.013a | 1.987 | 1.285–3.768 | 0.027a |
| Liver
cirrhosis | 1.259 | 0.796–1.986 | 0.581 |
|
|
|
| HACE1 level | 2.432 | 1.486–4.571 | 0.009a | 2.174 | 1.385–4.090 | 0.013a |
Discussion
Genomic losses or low expression in relation to
human chromosome 6q21 have been described for a wide spectrum of
tumor types including lung or breast cancer, as well as ovarian and
gastric cancers (6,17,18),
suggesting that this region encompasses one or more major
tumor-suppressor genes. The HACE1 gene encodes a 103-kDa protein
containing six N-terminal ankyrin repeats connected via a linker
region to a C-terminal HECT domain (19). The expression of the 6q21 HACE1 gene
is downregulated in multiple human tumor types involving Wilms'
tumor, breast cancer and lung carcinoma (20–23).
Findings of previous studies have demonstrated that HACE1 lower
expression or deletion, as caused by HACE1 ubiquitination or
methylation, is related to the occurrence and invasion in different
types of carcinomas (10,24,25).
It was revealed that HACE1 is a new type of candidate anti-oncogene
and perhaps a therapeutic target for several types of human
cancers. However, to the best of our knowledge, the expression of
HACE1 and its possible role in the anticancer effects on
hepatocellular carcinoma have not been discussed to date.
In the present study, we firstly found that the
HACE1 expression level was downregulated at both the mRNA and
protein levels in HCC tissues and HCC cell lines when compared with
these levels in adjacent non-tumor tissues and a normal cell line
L02 as analyzed by qRT-PCR and western blot analysis. Thus, we
assumed that HACE1 plays an important role in tumor proliferation
and migration. To explore this issue, CCK-8, Transwell and wound
healing assays were performed. The result demonstrated that
overexpression of HACE1 by transfection with pcDNA3.1-HACE1 in
SMCC7721 cells led to lower proliferation and migration ability
compared to the control group. In addition siRNA-mediated HACE1
knockdown in Huh7 cells significantly accelerated cell
proliferation and migration. The downregulation of HACE1 expression
correlated with the serum AFP level, tumor differentiation and
vascular invasion in HCC patients. Survival analyses exhibited that
patients with a low HACE1 expression showed poorer overall survival
than those with a high HACE1 expression, and the status of HACE1
expression was an independent prognostic factor.
In conclusion, we provided strong evidence that
HACE1 was significantly downregulated in HCC cell lines and HCC
tissues. Moreover, the expression of HACE1 in HCC tissues was
associated with the development, progression and metastasis of HCC
or prognosis of the patients by regulating the proliferation and
migration of HCC cells. IHC analysis suggested that the expression
of HACE1 in HCC tissues was associated with serum AFP level, tumor
differentiation and vascular invasion. Survival analyses
demonstrated that patients with low HACE1 expression exhibited
poorer overall survival than those with high HACE1 expression, and
the level of HACE1 expression was an independent prognostic
factor.
In addition, in vitro studies showed that
overexpression of HACE1 decreased the proliferation and migration
ability in the human HCC cell line SMCC7721. On the contrary, HACE1
knockdown in human cell line Huh7 facilitated the proliferation and
migration ability. Yet, further exploration is required to
elucidate the signaling pathways and molecular mechanism of HACE1
in HCC, which may be useful in acquiring better understanding of
the molecular pathogenesis of these tumors. Taken together, the
results suggest that, as a tumor-suppressor, HACE1 can be used to
develop more effective targeted therapeutic strategies and may be a
potential therapeutic target for HCC treatment.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 31270543 and
81101818) and Fundamental Research Funds for the Central
Universities (lzujbky-2013-k21).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waller LP, Deshpande V and Pyrsopoulos N:
Hepatocellular carcinoma: A comprehensive review. World J Hepatol.
7:2648–2663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liccioni A, Reig M and Bruix J: Treatment
of hepatocellular carcinoma. Dig Dis. 32:554–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waghray A, Murali AR and Menon KN:
Hepatocellular carcinoma: From diagnosis to treatment. World J
Hepatol. 7:1020–1029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anglesio MS, Evdokimova V, Melnyk N, Zhang
L, Fernandez CV, Grundy PE, Leach S, Marra MA, Brooks-Wilson AR,
Penninger J, et al: Differential expression of a novel ankyrin
containing E3 ubiquitin-protein ligase, Hace1, in sporadic Wilms'
tumor versus normal kidney. Hum Mol Genet. 13:2061–2074. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Anglesio MS, O'Sullivan M, Zhang
F, Yang G, Sarao R, Mai PN, Cronin S, Hara H, Melnyk N, et al: The
E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor
involved in multiple cancers. Nat Med. 13:1060–1069. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sako N, Dessirier V, Bagot M, Bensussan A
and Schmitt C: HACE1, a potential tumor suppressor gene on 6q21, is
not involved in extranodal natural killer/T-cell lymphoma
pathophysiology. Am J Pathol. 184:2899–2907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Chen P, Gao H, Gu Y, Yang J, Peng
H, Xu X, Wang H, Yang M, Liu X, et al: Ubiquitylation of autophagy
receptor Optineurin by HACE1 activates selective autophagy for
tumor suppression. Cancer Cell. 26:106–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mettouchi A and Lemichez E: Ubiquitylation
of active Rac1 by the E3 ubiquitin-ligase HACE1. Small GTPases.
3:102–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Chen X, Sharma P, Moon M, Sheftel
AD, Dawood F, Nghiem MP, Wu J, Li RK, Gramolini AO, et al:
HACE1-dependent protein degradation provides cardiac protection in
response to haemodynamic stress. Nat Commun. 5:34302014.PubMed/NCBI
|
|
12
|
Deng S and Huang C: E3 ubiquitin ligases
in regulating stress fiber, lamellipodium, and focal adhesion
dynamics. Cell Adhes Migr. 8:49–54. 2014. View Article : Google Scholar
|
|
13
|
Castillo-Lluva S, Tan CT, Daugaard M,
Sorensen PH and Malliri A: The tumour suppressor HACE1 controls
cell migration by regulating Rac1 degradation. Oncogene.
32:1735–1742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goka ET and Lippman ME: Loss of the E3
ubiquitin ligase HACE1 results in enhanced Rac1 signaling
contributing to breast cancer progression. Oncogene. 34:5395–5405.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, de Reyniès A, de Leval L, Ghazi
B, Martin-Garcia N, Travert M, Bosq J, Brière J, Petit B, Thomas E,
et al: Gene expression profiling identifies emerging oncogenic
pathways operating in extranodal NK/T-cell lymphoma, nasal type.
Blood. 115:1226–1237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hibi K, Sakata M, Sakuraba K, Shirahata A,
Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, et
al: Aberrant methylation of the HACE1 gene is frequently detected
in advanced colorectal cancer. Anticancer Res 28 (3A). 1581–1584.
2008.
|
|
17
|
Hudson C, Schwanke C, Johnson JP, Elias
AF, Phillips S, Schwalbe T, Tunby M and Xu D: Confirmation of
6q21-6q22.1 deletion in acro-cardio-facial syndrome and further
delineation of this contiguous gene deletion syndrome. Am J Med
Genet A 164A. 2109–2113. 2014. View Article : Google Scholar
|
|
18
|
Wang XC, Zhang JQ, Shen YQ, Miao FQ and
Xie W: Loss of heterozygosity at 6p21.3 underlying HLA class I
downregulation in gastric cancer. J Exp Clin Cancer Res.
25:115–119. 2006.PubMed/NCBI
|
|
19
|
Scheffner M and Kumar S: Mammalian HECT
ubiquitin-protein ligases: Biological and pathophysiological
aspects. Biochim Biophys Acta. 1843:61–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stewénius Y, Jin Y, Ora I, Panagopoulos I,
Möller E, Mertens F, Sandstedt B, Alumets J, Akerman M, Merks JH,
et al: High-resolution molecular cytogenetic analysis of Wilms
tumors highlights diagnostic difficulties among small round cell
kidney tumors. Genes Chromosomes Cancer. 47:845–852. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Capasso M, Diskin S, Cimmino F, Acierno G,
Totaro F, Petrosino G, Pezone L, Diamond M, McDaniel L, Hakonarson
H, et al: Common genetic variants in NEFL influence gene expression
and neuroblastoma risk. Cancer Res. 74:6913–6924. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capasso M, Diskin SJ, Totaro F, Longo L,
De Mariano M, Russo R, Cimmino F, Hakonarson H, Tonini GP, Devoto
M, et al: Replication of GWAS-identified neuroblastoma risk loci
strengthens the role of BARD1 and affirms the cumulative effect of
genetic variations on disease susceptibility. Carcinogenesis.
34:605–611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Küçük C, Hu X, Iqbal J, Gaulard P,
Klinkebiel D, Cornish A, Dave BJ and Chan WC: HACE1 is a tumor
suppressor gene candidate in natural killer cell neoplasms. Am J
Pathol. 182:49–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gacon G, Mettouchi A and Lemichez E: The
tumor suppressor HACE1 targets Rac1 to ubiquitin-mediated
proteasomal degradatio]. Med Sci (Paris). 28:39–41. 2012.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lachance V, Degrandmaison J, Marois S,
Robitaille M, Génier S, Nadeau S, Angers S and Parent JL:
Ubiquitylation and activation of a Rab GTPase is promoted by a
β2AR-HACE1 complex. J Cell Sci. 127:111–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|