Introduction
Colorectal cancer (CRC) is a malignancy with high
incidence and mortality, placing a heavy economic burden on public
health systems worldwide (1). A
better understanding of the molecular mechanisms involved in CRC
tumorigenesis may provide new approaches for the diagnosis and
treatment of CRC.
Our laboratory previously found that cortactin is
highly expressed in human stage II–III CRC and that the expression
of cortactin is an independent prognostic factor for disease-free
and overall survival (2).
Cortactin, encoded by the CTTN (also known as EMS1)
gene, is an F-actin binding protein that recruits Arp2/3 complex
proteins to F-actin to regulate actin nucleation, and cytoskeletal
assembly and adhesion (3).
Cortactin is mainly located in the cell cortex and contains 4 major
domains: the N-terminal acidic (NTA) domain, a tandem repeat
region, a proline-rich region and an SH3 domain. The proline-rich
domain includes Tyr421, Tyr466 (Tyr470 in humans) and Tyr482
(Tyr486 in humans). It is generally thought to be the signaling end
of the molecule, as it contains phosphorylation sites for a number
of kinases including src (4).
Increased levels of cortactin are involved in the invasion and
metastasis of various types of cancers, including head and neck
squamous cell carcinoma (HNSCC) (5), esophageal (6), gastric (7) and hepatocellular cancer (8), melanoma (9), CRC (2,10) and
ovarian cancer (11). As mentioned
above, cortactin plays a significant role in cancer metastasis, but
little is known concerning its role in cell proliferation. Wei
et al demonstrated that cortactin overexpression in gastric
cancer SGC-7901 cells accelerated tumor growth by promoting EGFR
expression and activating the EGFR signaling pathway (12). In the present study, we found that
cortactin promoted cell proliferation by activating cyclin D1 in
CRC. A mutation of cortactin at the Tyr421 phosphorylation site
impaired its ability to promote CRC cell growth.
Materials and methods
Cell culture and transfection
The human CRC cell lines SW1116 and SW480 were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). These cell lines were cultured in RPMI-1640 medium. All
media were supplemented with 10% fetal calf serum, 100 U/ml
penicillin, and 100 µg/ml streptomycin. They were then cultured at
37°C with 5% CO2. The plasmids for cortactin knockdown
and lentivirus particles for cortactin overexpression were
constructed by Shanghai GenePharma (Shanghai, China). A mutated
form of cortactin (mutation at the Tyr421 phosphorylation site, Tyr
mutant to alanine with flag as tag) was obtained from Shanghai USEN
Biological Technology (Shanghai, China). The plasmids and
lentivirus were transfected according to the manufacturer's
guidelines. Puromycin was used for stable cell screening and
establishment. The transfection efficiency was detected by western
blotting.
Westen blotting
Total cell proteins were ground and lysed in RIPA
lysis buffer with 1% phenylmethylsulfonyl fluoride (PMSF). Total
protein concentration was measured using a BCA assay kit (Pierce,
Rockford, IL, USA). Equal amounts of protein (50 µg) were subjected
to 10% SDS-PAGE and transferred electrophoretically to
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked and then incubated with the primary antibodies overnight at
4°C. Primary antibodies used were as follows: anti-cortactin
(dilution 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-cyclin D1 (dilution 1:1,000), antiflag (dilution 1:1,000)
(both from Cell Signaling Technology, Danvers, MA, USA), and
anti-GAPDH (dilution 1:10,000) (Abcam, Cambridge, MA, USA). After
the proteins were incubated in secondary antibodies for 2 h,
signals were detected by an enhanced chemiluminescence detection
system (Amersham Biosciences, Piscataway, NJ, USA).
Colony formation assay
Equal numbers of cells (1.5×103/well)
were seeded into 6-well plates and cultured for 2 weeks in
RPMI-1640 medium. After being washed with 1% phosphate-buffered
saline (PBS), the cells were fixed with methanol and stained with
0.4% crystal violet. After being carefully washed a second time
with 1% PBS, images were captured and the number of clones was
counted.
Cell Counting Kit-8 (CCK-8) assay
Equal number of cells (100 µl,
4×103/well) were seeded into 96-well plates in
replicates of 6. At the appropriate time (0, 24, 48, 72 and 96 h),
10 µl of CCK-8 (Dojindo, Tokyo, Japan) was added to the cells.
After being incubated for 2 h at 37°C, the cell viability was
measured at a wavelength of 450 nm.
Cell cycle analysis
For cell cycle analysis, cells were fixed with 70%
ethanol and stored overnight at 4°C. After being washed with PBS
twice, they were stained with propidium iodide (PI) solution. Cells
were filtered and analyzed with flow cytometry (Beckman
Instruments, Brea, CA, USA) according to the manufacturer's
instructions.
Xenograft model
BALB/c nude mice at the age of 4 weeks were
purchased from the Institute of Zoology of the Chinese Academy of
Sciences, and housed in a dedicated SPF facility at the Laboratory
Animal Center of Ruijin Hospital. SW480/cortactin/WT cells
(2×106) were resuspended in 100 µl PBS and injected into
the left side of each nude mouse. Equal numbers of
SW480/cortactin/Tyr421A cells were injected into the right sides of
other nude mice. Tumor length (L) and width (W) were measured every
week, and tumor volume (V) was evaluated using the following
formula: V = LW2π/6. Tumors were weighed and
photographed 28 days after injection. Serial 4.0-µm sections were
cut and stained with hematoxylin and eosin. The sections were
further analyzed using immunohistochemistry staining. All
experiments were performed in accordance with the official
recommendations of the Chinese Animal Research Committee.
Immunohistochemistry
Tissues were sectioned and deparaffinized in xylene.
After being rehydrated in graded ethanol, the sections were
incubated in hydrogen peroxide and blocked in 10% goat serum. The
sections were incubated with Ki67 antibody (dilution 1:150; Abcam)
overnight at 4°C, and incubated with the secondary antibody for 1 h
at room temperature. Finally, the sections were incubated with
diaminobenzidine and counterstained with hematoxylin.
Statistical analyses
All data were analyzed by SPSS 17.0 and are
presented as mean ± SD. Statistical differences between two groups
were examined by the Student's t-test. P<0.05 was considered to
indicate a statistically significant result. P<0.01 was
considered highly significant.
Results
Cortactin promotes the proliferation
of CRC cells
To evaluate the role of cortactin in CRC cells,
cortactin expression was stably increased in the SW480 cells by
lentivirus infection and reduced in the SW1116 cells by plasmid
infection (Fig. 1A). The CCK-8
assay showed that overexpression of cortactin significantly
increased the proliferation rate of the SW480 cells, while
depletion of cortactin reduced the proliferation rate of the SW1116
cells (Fig. 1B). Similar results
were observed in the colony formation assays (Fig. 1C and D). Our results indicate that
cortactin plays an important role in promoting CRC proliferation
and growth.
Cortactin promotes the G1/S phase
transition of CRC cells
To explore the mechanism underlying the promotion of
cellular proliferation by cortactin, a flow cytometric analysis was
performed. The number of cells in the G0/G1, S and G2/M phase,
respectively, was analyzed. As shown in Fig. 2A, overexpression of cortactin
induced the increased percentage of G1 phase cells and
significantly decreased the percentage of S phase cells in the
SW480 cell line. We obtained the opposite results after cortactin
knockdown in the SW1116 cells (Fig.
2B). These results indicate that cortactin promotes the
progression of CRC cells in the G1/S phase.
Tyr421 mutation impairs the
growth-promoting activity of cortactin in CRC cells
The proline-rich region of cortactin is a major
regulatory phosphorylation motif that is targeted by a large number
of protein kinases including Src and Erk (13,14).
Tyrosine phosphorylation of cortactin occurs at 3 tyrosine
residues, Tyr421, Tyr466 (Tyr470 in humans) and Tyr482 (Tyr486 in
humans), with Tyr466 and Tyr482 phosphorylation dependent on the
phosphorylation of Tyr421 (15). To
evaluate the impact of the phosphorylation site of cortactin on CRC
cell proliferation, a mutation of cortactin at the Tyr421 site was
constructed (Fig. 3A). As shown in
Fig. 3B, compared with
SW480/cortactin/WT, SW480/cortactin/Tyr421A exhibited a
significantly lower growth rate from day 3 of cell culture.
Furthermore, in the colony formation assay, the mutation of
cortactin in SW480 cells resulted in reduced colony numbers as
compared with the SW480/cortactin/WT cells (Fig. 3C and D). These data suggest that the
Tyr421 site of cortactin is required to promote cell proliferation
and growth.
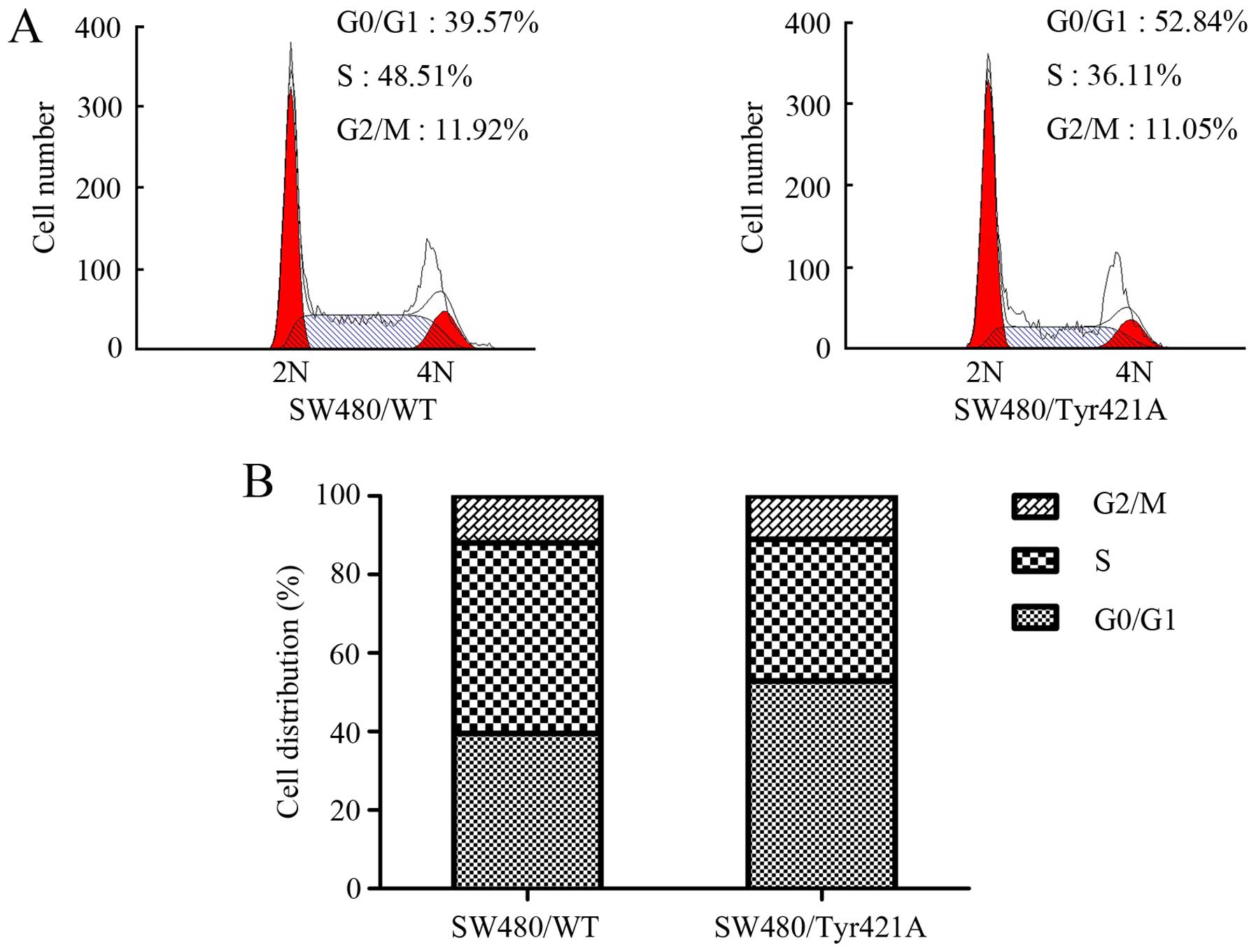
The Tyr421 mutation affects the
ability of cortactin to promote cell cycle progression
To further validate the effects of cortactin on CRC
cell proliferation, we explored the function of Tyr421 in cell
cycle progression. Flow cytometric analysis showed that there was
an increase in the percentage of cells in the G1 phase in the
SW480/cortactin/Tyr421A cells as compared with this percentage in
the SW480/cortactin/WT cells, while the percentage of cells at the
S peak decreased when there was a mutation of cortactin at the
Tyr421 site (Fig. 4A and B). These
results indicate that the mutation of cortactin at the Tyr421 site
caused G1 arrest in the CRC cell cycle.
Cortactin induces cyclin D1
expression
Having found that cortactin promoted the progression
of the G1/S phase, we sought to determine whether this was due to
changes in cell cycle-related protein. Therefore, western blot
analysis was performed to assess the correlation between the
effects of cortactin on the expression of cyclin D1. As shown in
Fig. 5A (in line with the results
of our clone formation assay, CCK-8 assay, and cell cycle analysis)
ectopic cortactin in SW480 cells increased the expression level of
the cell cycle-related protein cyclin D1, while silencing of
cortactin in the SW1116 cells produced the opposite result.
Notably, cyclin D1 was further enhanced by the overexpression of
cortactin/WT in the SW480 cells but not by the mutation of
cortactin at the Tyr421 site (Fig.
5B). These data indicate that the Tyr421 site of cortactin is
required to induce cyclin D1.
The Tyr421 mutation impairs
tumorigenesis in vivo
To further determine the role of Tyr421 in the
tumorigenicity of CRC, equal numbers of SW480/cortactin/WT and
SW480/cortactin/Tyr421A cells were injected subcutaneously into the
flanks of 4-week BALB/c nude mice. Tumor volumes were analyzed
every week, and tumor grafts were excised and weighed after 28
days. As shown in Fig. 6A and B,
tumor growth of the SW480/cortactin/Tyr421A cells was slower than
that of the SW480/cortactin/WT cells. Moreover, the average tumor
weight in the SW480/cortactin/Tyr421A cell group was significantly
lower than that in the SW480/cortactin/WT cell group (Fig. 6B). Immunohistochemical analysis
revealed that SW480/cortactin/WT tumors displayed a higher Ki67
index, whereas SW480/cortactin/Tyr421A tumors showed reduced
numbers of Ki67-positive cells (Fig.
6D). Taken together, these results suggest that cortactin
promotes the tumorigenicity of CRC cells in vivo.
Discussion
Colorectal cancer (CRC) is a malignant tumor of high
morbidity and mortality (1). Cell
proliferation is an obvious characteristic of malignant tumors. The
growth of cells involves a series of mechanisms wherein cell cycle
arrest plays an important role, being responsible for the survival
of the cell growth process (16).
Targeting the deregulated cell cycle is a practical strategy by
which to check uncontrolled proliferation in cancer cells. In the
present study, we demonstrated that cortactin regulated the G1/S
checkpoint and promoted the proliferation of CRC cells both in
vitro and in vivo.
Cortactin was first identified as a tyrosine
phosphorylated protein in Src-transformed primary chicken embryo
cells (17). The gene for cortactin
is in the chromosome 11q13 region, which is frequently amplified in
human cancers (18). Cortactin is a
scaffolding protein that activates the Arp2/3 complex through its
NTA region, which promotes the branching of actin filaments and
stabilizes branched networks. The cortactin SH3 domain interacts
with a number of proteins that regulate membrane trafficking and
cell motility (19). Cortactin is
overexpressed in a large number of cancers and appears to be an
attractive target owing to its prominent role in the formation and
function of invadopodia as well as in the promotion of the
migration and invasion of cancer cells (20). Despite the numerous studies that
have been carried out, the role of cortactin in cancer growth still
requires further research. Li et al indicated that compared
with CortF421F466F482, cortactin contributes to the metastasis of
breast cancer, but has no significant effects on the growth of
cancer cells (21). Ni et al
found that cortactin is significantly upregulated in colon cancer
tissues and that the expression of ectopic cortactin in HCT116
cells promoted cell proliferation and tumorigenicity (22). The results presented in the present
study revealed that ectopic expression of cortactin in the SW480
cells promoted cell proliferation and colony formation, while
knockdown of cortactin in SW1116 cells produced the opposite
results.
Tyrosine phosphorylation of cortactin occurs on
Tyr421, Tyr466 (Tyr470 in humans) and Tyr482 (Tyr486 in humans)
residues located within the proline-rich domain in response to Src
and several other tyrosine kinases (4,13,23).
Notably, tyrosine phosphorylation of cortactin is a progressive
process among the 2 main residues, with pTyr421 required for the
phosphorylation of Tyr466 (15).
Recently Radhakrishnan et al demonstrated that pTyr421
cortactin is highly expressed in colon cancer, and that curcumin
significantly reduced the level of pTyr421 cortactin by a direct
physical interaction with PTPN1. This ultimately led to a decrease
in the migration of colon cancer cells (24). To the best of our knowledge, the
role of Tyr421 cortactin in cell proliferation has not yet been
studied. The results presented in the present study showed that the
Tyr421 mutation of cortactin impaired its growth-supporting
activity both in vitro and in vivo. It may be of
great interest to investigate whether the Tyr470 mutation of
cortactin could regulate the proliferation of cancer cells. To
better understand how cortactin promotes the growth of CRC cells,
we analyzed the impact of cortactin on cell cycle progression. We
found that the silencing of cortactin with shRNA resulted in an
accumulation of cells in the G0/G1 phase, leading to G1/S cell
cycle arrest in the SW116 cells. In contrast, overexpression of
cortactin facilitated the G1/S transition in the SW480 cells.
Notably, the mutation of cortactin at the Tyr421 site in the SW480
cells caused a G1 arrest in the cell cycle. Cylin D1 has been
reported to promote cell progression by inducing cell transition
from G0/G1 to S phase (25). Recent
research has shown that cortactin enhances EGFR expression and
stimulates the EGFR-ERK signaling pathway (22). A previous study reported that the
ERK/cyclin D1 pathway is involved in the proliferation of smooth
muscle cells (26). With this
inference, our results indicate that cortactin promotes the G1/S
transition by regulating cylin D1. Mutation of cortactin at the
Tyr421 residue impaired the activation of cyclin D1, indicating
that the cortactin regulation of cyclin D1 is dependent on Tyr421
phosphorylation. However, further studies are required to identify
the detailed interaction between cortactin and cyclin D1. In
conclusion, the present study demonstrated that cortactin plays an
important role in CRC proliferation and tumorigenicity, suggesting
the possibility of a new therapeutic option.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81272751).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai JH, Zhao R, Zhu JW, Jin XL, Wan FJ,
Liu K, Ji XP, Zhu YB and Zhu ZG: Expression of cortactin correlates
with a poor prognosis in patients with stages II–III colorectal
adenocarcinoma. J Gastrointest Surg. 14:1248–1257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Rossum AG, Moolenaar WH and Schuuring
E: Cortactin affects cell migration by regulating intercellular
adhesion and cell spreading. Exp Cell Res. 312:1658–1670. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weaver AM: Cortactin in tumor
invasiveness. Cancer Lett. 265:157–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodrigo JP, García LA, Ramos S, Lazo PS
and Suárez C: EMS1 gene amplification correlates with poor
prognosis in squamous cell carcinomas of the head and neck. Clin
Cancer Res. 6:3177–3182. 2000.PubMed/NCBI
|
|
6
|
Lu P, Qiao J, He W, Wang J, Jia Y, Sun Y,
Tang S, Fu L and Qin Y: Genome-wide gene expression profile
analyses identify CTTN as a potential prognostic marker in
esophageal cancer. PLoS One. 9:e889182014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Cao W, Mo M, Wang W, Wu H and Wang
J: VEGF and cortactin expression are independent predictors of
tumor recurrence following curative resection of gastric cancer. J
Surg Oncol. 102:325–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan BZ, Zhou X, Zimonjic DB, Durkin ME
and Popescu NC: Amplification and overexpression of the EMS 1
oncogene, a possible prognostic marker, in human hepatocellular
carcinoma. J Mol Diagn. 5:48–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu XZ, Garcia MV, Li TY, Khor LY,
Gajapathy RS, Spittle C, Weed S, Lessin SR and Wu H: Cytoskeleton
alterations in melanoma: Aberrant expression of cortactin, an
actin-binding adapter protein, correlates with melanocytic tumor
progression. Mod Pathol. 23:187–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirakawa H, Shibata K and Nakayama T:
Localization of cortactin is associated with colorectal cancer
development. Int J Oncol. 35:1271–1276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li A, Zhang L, Zhang X, Jin W and Ren Y:
Expression and clinical significance of cortactin protein in
ovarian neoplasms. Clin Transl Oncol. 18:220–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei J, Zhao ZX, Li Y, Zhou ZQ and You TG:
Cortactin expression confers a more malignant phenotype to gastric
cancer SGC-7901 cells. World J Gastroenterol. 20:3287–3300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mezi S, Todi L, Orsi E, Angeloni A and
Mancini P: Involvement of the Src-cortactin pathway in migration
induced by IGF-1 and EGF in human breast cancer cells. Int J Oncol.
41:2128–2138. 2012.PubMed/NCBI
|
|
14
|
Martinez-Quiles N, Ho HY, Kirschner MW,
Ramesh N and Geha RS: Erk/Src phosphorylation of cortactin acts as
a switch on-switch off mechanism that controls its ability to
activate N-WASP. Mol Cell Biol. 24:5269–5280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Head JA, Jiang D, Li M, Zorn LJ, Schaefer
EM, Parsons JT and Weed SA: Cortactin tyrosine phosphorylation
requires Rac1 activity and association with the cortical actin
cytoskeleton. Mol Biol Cell. 14:3216–3229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanner SB, Reynolds AB, Vines RR and
Parsons JT: Monoclonal antibodies to individual
tyrosine-phosphorylated protein substrates of oncogene-encoded
tyrosine kinases. Proc Natl Acad Sci USA. 87:3328–3332. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schuuring E: The involvement of the
chromosome 11q13 region in human malignancies: Cyclin D1 and EMS1
are two new candidate oncogenes - a review. Gene. 159:83–96. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weed SA and Parsons JT: Cortactin:
Coupling membrane dynamics to cortical actin assembly. Oncogene.
20:6418–6434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
MacGrath SM and Koleske AJ: Cortactin in
cell migration and cancer at a glance. J Cell Sci. 125:1621–1626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Tondravi M, Liu J, Smith E,
Haudenschild CC, Kaczmarek M and Zhan X: Cortactin potentiates bone
metastasis of breast cancer cells. Cancer Res. 61:6906–6911.
2001.PubMed/NCBI
|
|
22
|
Ni QF, Yu JW, Qian F, Sun NZ, Xiao JJ and
Zhu JW: Cortactin promotes colon cancer progression by regulating
ERK pathway. Int J Oncol. 47:1034–1042. 2015.PubMed/NCBI
|
|
23
|
Boyle SN, Michaud GA, Schweitzer B, Predki
PF and Koleske AJ: A critical role for cortactin phosphorylation by
Abl-family kinases in PDGF-induced dorsal-wave formation. Curr
Biol. 17:445–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Radhakrishnan VM, Kojs P, Young G,
Ramalingam R, Jagadish B, Mash EA, Martinez JD, Ghishan FK and
Kiela PR: pTyr421 cortactin is overexpressed in colon
cancer and is dephosphorylated by curcumin: Involvement of
non-receptor type 1 protein tyrosine phosphatase (PTPN1). PLoS One.
9:e857962014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Resnitzky D and Reed SI: Different roles
for cyclins D1 and E in regulation of the G1-to-S
transition. Mol Cell Biol. 15:3463–3469. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li T, Song T, Ni L, Yang G, Song X, Wu L,
Liu B and Liu C: The p-ERK-p-c-Jun-cyclinD1 pathway is involved in
proliferation of smooth muscle cells after exposure to cigarette
smoke extract. Biochem Biophys Res Commun. 453:316–320. 2014.
View Article : Google Scholar : PubMed/NCBI
|