Introduction
Melanoma is the most aggressive form of skin cancer
(1). Patients with advanced-stage
melanoma have a very low survival rate (15%) and an overall
survival rate of 8–18 months (2).
Both the incidence and mortality rates of melanoma are increasing
worldwide (3), with melanoma as one
of the fastest growing malignancies in North America (4). Melanoma incidence has been increasing
annually by 4% in the United States (3) and by 3–7% in European countries
(5). There are ~20,000 newly
diagnosed cases of melanoma in China every year (6). In addition, melanoma is known to have
a high risk of relapse after resection of the primary tumor.
Metastatic melanoma is difficult to treat due to its resistance to
high-dose IFN-α2b, chemotherapy, radiotherapy, immunotherapy, and
targeted molecular therapy. Several chemotherapeutic agents show
single-agent activity in melanoma at a level of 10–15% (7).
Cells undergoing EMT expand out of and change the
surrounding microenvironment to subsequently migrate from the
primary site (8). When EMT is
ongoing, it was observed that E-cadherin is downregulated and
N-cadherin and vimentin are upregulated (9). It has been reported that calpains play
a role in EMT. Calpains are members of a family of
calcium-dependent intracellular cysteine proteases, which include
µ-calpain and m-calpain (10,11).
Both µ-calpain and m-calpain form heterodimers consisting of a
large catalytic subunit (80 kDa) encoded by the genes Capn1 and
Capn2, respectively, and a small regulatory subunit (28 kDa)
encoded by the gene Capn4 (11).
Calpains, including Capn4, are implicated in several physiological
processes, including cytoskeletal remodeling, cellular signaling,
apoptosis and cell survival (12).
The involvement of µ-calpain and m-calpain in tumor invasion has
been extensively studied during the last decade. In several types
of cancer, tumor cells, such as those from osteosarcomas (13), rhabdomyosarcomas (14), hepatocellular carcinoma (HCC)
(15,16), colorectal (17) or breast cancer (18), present abnormally high activity of
µ-calpain and/or m-calpain.
Capn4 is involved in regulating invasion, migration
and cell survival (19).
Downregulation of Capn4 suppressed the migration and invasion of
glioma cells by reducing the level of vimentin and N-cadherin
expression in glioblastoma multiforme (GBM) cells (20). Capn4 also promoted the migration and
invasion of HCC (16). Capn4
overexpression is implicated in intrahepatic cholangiocarcinoma
(ICC) metastasis/invasion (21).
Capn4 expression was found to be higher in clear cell renal cell
carcinoma (ccRCC) tumor tissues than that noted in adjacent
non-tumor tissues (22). Capn4 is
overexpressed in nasopharyngeal carcinoma (NPC) and its
downregulation in NPC cells is associated with reduced levels of
matrix metalloproteinase 2 (MMP2) and vimentin (23). Capn4 promotes non-small cell lung
cancer (NSCLC) progression via upregulation of MMP2 (24).
Previous studies have reported that Capn4 is
involved in the proliferation and metastasis of solid tumor cells,
but few studies on Capn4 have been performed on melanoma. We
determined that Capn4 was overexpressed in melanoma as determined
by gene chip analyses from the GEO:GSE3189 database (25). The β-catenin signaling pathway is
reported to play an important role in embryogenesis, stem cell
maintenance and tumorigenesis, including melanoma progression
(26). New research suggests that
the NF-κB/p65 signaling pathway plays an important role in the EMT
pathway (27). The present studies
identified cross-talk among the β-catenin, NF-κB and EMT pathways.
Therefore, we hypothesized that Capn4 promotes the migration and
invasion of human melanoma cells through the EMT or β-catenin
signaling pathways.
Materials and methods
Overexpression of Capn4 in melanoma was determined
by gene chip analyses from the GEO:GSE3189 database (Fig. 1) (25). There was a statistically significant
difference in Capn4 mRNA expression between the melanoma tissues
and normal tissues (P<0.01).
Immunohistochemistry (IHC) of clinical
specimens
All of the clinical specimens used in this study
were obtained from the Department of Pathology, Chongqing Medical
University (Chongqing, China). The clinical specimens included 120
resected melanoma tumor tissues and 34 normal tissues obtained from
patients who underwent surgery for melanoma or other diseases. The
most representative tumor area in each biopsy was carefully
selected and marked on the hematoxylin and eosin-stained
(H&E-stained) slides. IHC was performed on the targets of
interest. None of the enrolled patients had received chemotherapy
or radiation therapy before surgical resection. An anti-Capn4
antibody (diluted 1:300; Proteintech, Wuhan, China) was used for
IHC for Capn4 expression in melanoma tissues and normal tissues.
IHC was performed as previously described (21).
Ethics statement
The clinical processes in this study were approved
by the Ethics Committees of the Chongqing Medical University, and
the patients who participated in the studies provided informed
consent. The animal studies described here were approved by the
Institutional Animal Care and Use Committee of Chongqing Medical
University (Chongqing, China).
Cell culture and reagents
The human keratinocyte cell line HaCaT (HaCaT) and
human melanoma cell lines M14, SK-MEL-1 and MV3 were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA). The
human melanoma cell line A375, obtained from the Shanghai Institute
of Cell Biology in the Chinese Academy of Sciences (Shanghai,
China) was cultured in a growth medium of DMEM (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% FBS, 2000 U/ml
penicillin G (1,000,000 U=1 g) and 500 µg/ml streptomycin solution.
The HaCaT cell line and human melanoma cell lines M14, SK-MEL-1 and
MV3 were cultured in RPMI-1640 (Thermo Fisher Scientific, Inc.)
containing 10% FBS, 2000 U/ml penicillin G and 500 µg/ml
streptomycin. All cells were grown in a humidified incubator at
37°C and 5% CO2.
Capn4 targeting short hairpin RNA
(shRNA) and cell transfection
An shRNA plasmid directed against Capn4 was
purchased from Genomeditech Co., Ltd. (Shanghai, China) and was
used to downregulate Capn4 expression in the A375 and M14 cells.
The shRNA targeting sequences for Capn4 were as follows shRNA,
CCGGCGCCACAGAACTCATGAACATCTCGAG ATGTTCATGAGTTCTGTGGCGTTTTTG and
control shRNA, CCGGTTCTCCGAACGTGTCACGTTTCAAGA
GAACGTGACACGTTCGGAGAATTTTTG.
We generated human melanoma A375 and
M14 cells that were stably transfected with Capn4 shRNA
(shRNA-infected cells)
The shRNA-infected cells (shRNA) were compared with
the control cells (con) in which Capn4 expression had not been
knocked down. The shRNA-infected A375 and M14 cells were assessed
via western blot analysis.
Western blot (WB) analysis
The expression levels of Capn4, cleaved-caspase-3,
β-catenin, E-cadherin, N-cadherin, and vimentin in the
shRNA-infected A375, M14, SK-MEL-1, MV3 and HaCaT cells were
determined by WB. The WB samples were prepared from whole-cell
lysates. Total protein was quantified, and equal amounts of protein
were separated on SDS-PAGE gels and electrotransferred onto PVDF
membranes. The blots were subsequently probed with primary
antibodies as follows: rabbit anti-Capn4 (Proteintech),
anti-cleaved-caspase-3 (Abcam, Cambridge, MA, USA), anti-β-catenin,
anti-E-cadherin, anti-N-cadherin, anti-vimentin,
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz
Biotechnology, Inc., USA) and anti-cyclin-dependent kinase 4 (CDK4;
diluted 1:100; LifeSpan Biosciences, Seattle, WA, USA). The primary
antibodies were detected with peroxidase-conjugated goat
anti-rabbit immunoglobulin antibodies as secondary antibodies that
were diluted 1:2,000 in blocking solution for 2 h at 37°C.
Antibody-bound proteins normalized to GAPDH or CDK4 were used as
internal references to quantitatively analyze protein content and
were detected and analyzed using the Quantity One WB analysis
system.
MTT assay
The metabolic activity in each well, which indicated
the effect of Capn4 on the proliferation and viability of the A375
and M14 cells, was determined using a standard MTT assay. In this
assay, both shRNA-infected cells and control cells were seeded into
96-well plates at low cell concentrations (1.5×105
cells/well) and cultured for 72 h. The medium was replaced with 10
µl MTT solution (5 mg/ml). After being cultured for 1–5 days in the
dark, 150 µl dimethyl sulfoxide (DMSO) was added to each well, and
the plates were incubated for 4 h. The absorbance at 450 nm was
measured using an enzyme-linked immunosorbent assay (ELISA) reader.
The relative number of cells was calculated using comparable
standard curves from the obtained optical density values.
Apoptosis assay
shRNA-infected A375 and M14 cells along with their
comparable controls were seeded onto glass coverslips in 6-well
plates and incubated overnight. Apoptotic A375 or M14 cells were
quantified using an in situ apoptosis detection kit. The
cells were then fixed in 4% formaldehyde solution before being
permeabilized with proteinase K for 25 min at room temperature.
Endogenous peroxidase was blocked with 2%
H2O2 for 5 min at room temperature, and the
sections were incubated with 100 µl TUNEL solution (Beyotime
Institute of Biotechnology, Shanghai, China) for 1 h at 37°C in the
dark. The stained cells were analyzed by fluorescence microscopy.
Six microscopic fields were randomly selected from each sample, and
100 cells were randomly selected from every field. The apoptotic
rate was calculated as follows: (TUNEL-positive cells) = (number of
total apoptotic cells/100) × 100%. The A375 and M14 cells were
washed with phosphate-buffered saline (PBS) and 400 µl of DAPI
(Beyotime Institute of Biotechnology) staining solution was added
to each well. The staining solution consisted of a mixture of
methanol and DAPI as follows: 1 ml methanol and 2 µl DAPI (from a
stock solution of 1 mg/ml). The cells were incubated for 10 min in
the dark with this staining solution and afterwards washed with
PBS. The stained cells were analyzed by fluorescence microscopy.
The TUNEL assay and DAPI staining were merged in situ. The
expression of cleaved-caspase-3 was assayed by WB.
Xenograft tumorigenicity
Five-week-old BALB/c nude mice were obtained from
the Shanghai Laboratory Animal Center of the Chinese Academy of
Sciences (Shanghai, China) and maintained in a sterile animal
facility. shRNA-infected and control A375 cells were harvested,
centrifuged, and washed thoroughly with PBS and adjusted to
appropriate concentrations. A total of 5×106
shRNA-infected A375 cells, suspended in 0.1 ml of PBS, were
subcutaneously injected into the left flank regions of 5-week-old
BALB/c nude mice (n=3). Control BALB/c nude mice were injected with
the untreated A375 control cells (n=3). All animal studies were
approved by the Institutional Animal Care and Use Committee of
Chongqing Medical University. After 10 days of treatment, tumor
sizes were monitored weekly. At the end of treatment, the tumor
tissues were harvested and weighed.
Migration and invasion assays
The Cell Invasion Assay kit (Merck Millipore,
Darmstadt, Germany) was used to measure cell migration and
invasion. For Transwell migration analysis, 3×105 cells
(shRNA-infected or control cells) were suspended in medium with
growth factors and plated in the top chamber lined with a
non-coated membrane. For invasion analysis, the chamber inserts
were coated with 200 mg/ml Matrigel and dried overnight under
sterile conditions. Then, 3×105 cells (shRNA-infected or
control cells) in medium with growth factors were plated in the top
chamber. For both assays, medium-containing serum was added in the
lower chamber as a chemoattractant. After incubation at 37°C for
48–96 h, the top chambers were wiped with cotton wool to remove the
non-migratory or non-invasive cells. The migrating and invading
cells on the underside of the membrane were fixed in 100% methanol
for 10 min, stained with crystal violet, and counted using light
microscopy.
Statistical analysis
In our study, the values are represented as the
means ± SD (standard deviations) for three independent experiments.
Statistical analysis was performed using SPSS software version
20.0. The differences between the groups were analyzed using
t-tests. P values <0.05 (P<0.05) were considered
statistically significant.
Results
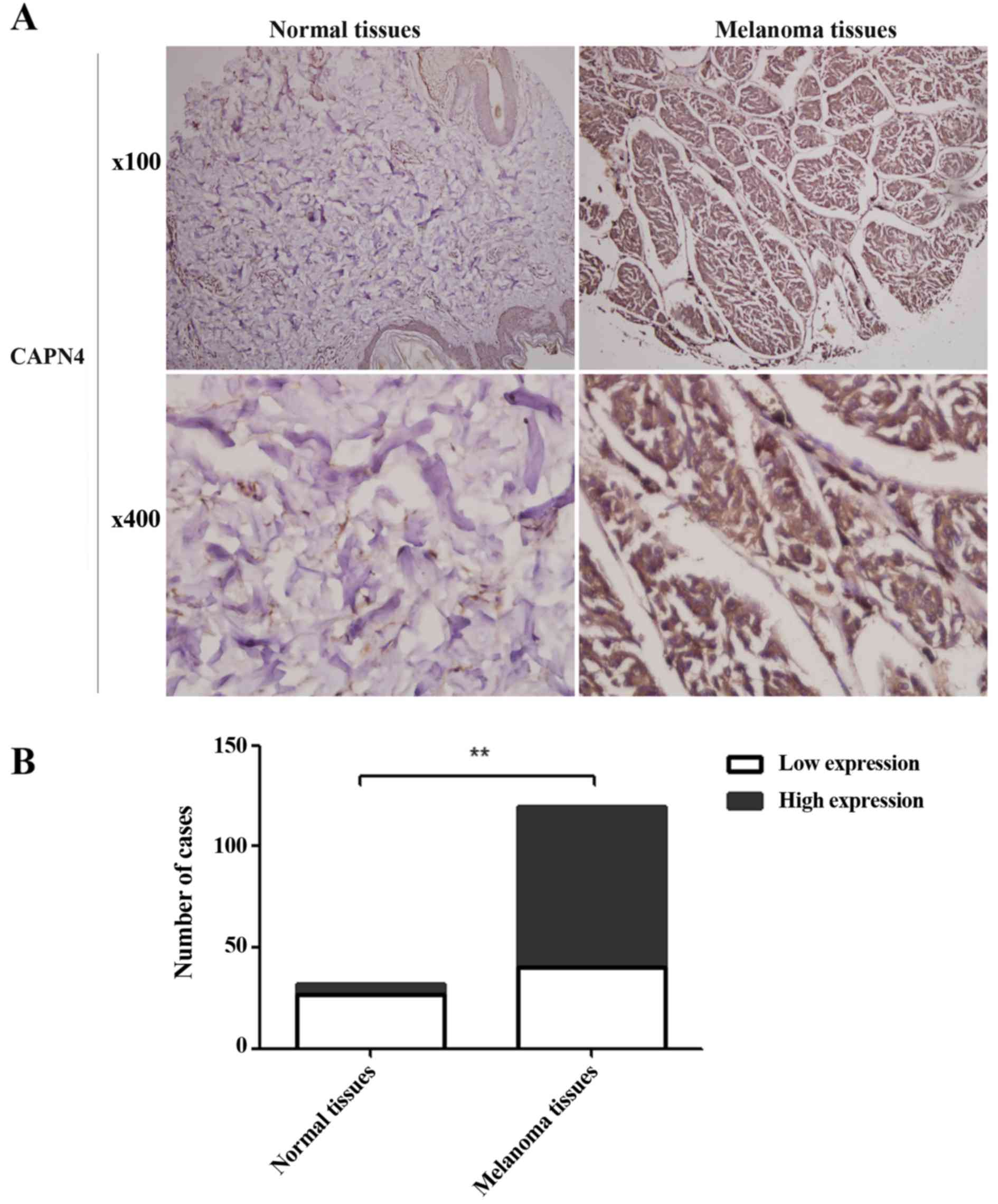
IHC of the clinical specimens
Cells were considered positive for Capn4 expression
when brown granules distributed in the nucleus or cytoplasm could
be observed under a microscope. Cell staining intensity was graded
on a 0–3 scale as follows: 0 (absence of staining), 1 (weakly
stained), 2 (moderately stained) and 3 (strongly stained). The
percentage of positive tumor cells ranged from 0–3 as follows: 0
(absence of positive cells), 1 (<33% positive tumor cells), 2
(33–66% positive tumor cells) and 3 (>66% positive tumor cells).
The staining score, which ranged from 0–9, was calculated by
multiplying the intensity score by the percentage score. The
expression of Capn4 in melanoma tissues vs. normal tissues was
described as low (IHC, 0–4) or high (IHC, 5–9). The Capn4
expression in the melanoma tissues was higher compared than the
expression in the normal tissues (Fig.
2A). The percentage of cases with high Capn4 expression was
~66.67% (80/120) in the melanoma tissues, compared with 20.59%
(7/34) in the normal tissues (Fig.
2B) (P<0.01).
Capn4 protein expression in melanoma
and HaCaT cells
WB analysis showed that the protein expression of
Capn4, normalized to that of GAPDH in all the studied human
melanoma cells (A375, M14, SK-MEL-1 and MV3) was higher than that
in the HaCaT cells (Fig. 3A) and
there was a statistically significant difference between the
melanoma cells when compared with the HaCaT cells (Fig. 3B) (P<0.01.
shRNA targeting Capn4 and cell
transfection
A375 and M14 cells were stably transfected with
Capn4 (shRNA-infected cells, knockdown of Capn4) and compared with
the control cells (con, controls). The protein expression of Capn4
in the shRNA-infected cells was downregulated. WB analysis showed
that the protein expression of Capn4 in the shRNA-infected cells
(A375, M14), normalized to that of GAPDH, was lower than that noted
in the controls (Fig. 4A)
(P<0.05).
MTT assay
The proliferative ability of the shRNA-infected
cells was less than that of the controls. The difference in cell
viability was statistically significant between the shRNA-infected
and the control cells after 4 or 5 days, both in the A375 and M14
cells (Fig. 4B) (P<0.05).
Apoptosis assay by immunofluorescence
and cleaved-caspase-3 expression
DNA fragmentation, as assessed by a TUNEL assay
(green: fragmented DNA), was increased in the shRNA-infected cells
than that noted in the control cells for both the A375 and M14
cells (Fig. 4C). The percentage of
TUNEL-positive cells in the shRNA-infected cells was higher than
that in the controls for both the A375 and M14 cells (Fig. 4D) (P<0.01). In addition, in
situ DAPI staining was performed in the shRNA-infected cells
and controls. Cell nuclei were stained blue allowing them to be
observed using fluorescence microscopy. A number of cells exhibited
nuclear fragmentation, condensed chromatin filaments or nuclear
condensation, which are signs of cell membrane integrity loss.
These characteristics were observed more frequently in the
shRNA-infected cells than that noted in the controls (Fig. 4C). The expression of
cleaved-caspase-3 in the shRNA-infected cells was higher than that
in the controls for both the A375 and M14 cells (Fig. 4E) (P<0.01).
Xenograft tumorigenicity
To evaluate the effect of Capn4 on melanoma tumor
formation in nude mice, a xenograft tumorigenicity assay was
performed. shRNA-infected and control A375 cells were injected into
5-week-old BALB/c nude mice (n=3/group). The tumors grew slowly in
the nude mice injected with the shRNA-infected cells compared with
this rate in the controls. Nude mice treated with the
shRNA-infected A375 cells generated smaller tumors in contrast to
the control group (Fig. 5A). The
volume of the tumors produced by the shRNA-infected cells was
significantly lower than that of the controls (Fig. 5B) (P<0.05).
Knockdown of Capn4 reduces both
migration and invasion of A375 and M14 cells in vitro
To determine the functional role of Capn4 in the
metastasis of human melanoma cells, stably shRNA-infected cells
(Capn4 knockdown) were generated. Capn4 knockdown in the
shRNA-infected cells was verified by WB as previously described
above. Transwell assays were then performed to assess the migration
and invasion of the shRNA-infected and control A375 and M14 cells.
Notably, shRNA-infected cells displayed a statistically significant
lower rate of migration and invasion than did the controls for both
the A375 and M14 cells (Fig. 6)
(P<0.01, P<0.05, respectively).
Capn4 decreases E-cadherin and
increases the expression of β-catenin-N (β-catenin in the nucleus),
N-cadherin and vimentin
To determine the effects of Capn4 expression on the
EMT pathway, the protein levels of EMT markers were detected by WB
in the A375 and M14 cells. The expression of E-cadherin was
increased in the shRNA-infected cells compared with that of the
controls (Fig. 7A and B)
(P<0.01). The expression of β-catenin-N, N-cadherin and vimentin
was decreased in the shRNA-infected cells compared with the
controls (Fig. 7A and B)
(P<0.05). No changes were observed in the expression of
β-catenin-T (total β-catenin, included in the nucleus and in the
cytoplasm) in the shRNA-infected cells compared with the controls
(Fig. 7A and B). These results
suggest that Capn4 plays an important role in the EMT pathway in
human melanoma cells.
 | Figure 7.The expression of E-cadherin,
β-catenin-N, β-catenin-T, N-cadherin and vimentin in Capn4
shRNA-infected cells. (A) Western blot analysis (WB), normalized to
that of GAPDH or anti-cyclin-dependent kinase 4 (CDK4), showed the
expression levels of E-cadherin, β-catenin-N, β-catenin-T,
N-cadherin and vimentin. (B) E-cadherin was increased in the Capn4
short hairpin RNA (shRNA)-infected cells when compared with the
controls. The expression of Capn4, β-catenin-N, N-cadherin and
vimentin were decreased in the shRNA-infected cells compared with
the controls, and no changes were observed in the level of
β-catenin-T; *P<0.05, **P<0.01; Capn4, calpain small subunit
1. |
Discussion
Previous studies have reported that Capn4 is
overexpressed in tumor tissues, including ICC (21), ccRCC (22) and NPC (23). Our study confirmed that Capn4
expression was increased in both melanoma tissues and cells in
vitro. Lower levels of Capn4 expression were detected in the
shRNA-infected cells when compared with the controls and the
viability of the shRNA-infected cells was lower when compared with
the control cells by MTT assay. The TUNEL assay, DAPI staining and
cleaved-caspase-3 level assay proved that apoptosis was enhanced in
the shRNA-infected cells compared with the controls. In other
words, Capn4 enhanced the activity, inhibited apoptosis and
promoted proliferation in human melanoma cells. In vivo, the
tumor cells grew more slowly in nude mice injected with the
shRNA-infected cells than in those injected with the control cells.
In vitro, the Transwell assays demonstrated that the
shRNA-infected cells displayed a statistically significant lower
rate of migration and invasion than did the controls. Our study
demonstrated that Capn4 promotes the migration and invasion of
human melanoma cells in vitro and tumor growth in
vivo.
The migration and invasion of tumor cells are
regulated by several pathways, such as the Wnt/β-catenin, NF-κB/p65
and EMT pathways. β-catenin influences many cellular processes,
including cell adhesion, growth and differentiation (28). High levels of β-catenin have been
reported in tumor cells (29).
β-catenin is also ubiquitously referred to as an ‘oncogenic’
pathway that promotes tumor progression (30) and is reported to play an important
role in melanoma tumorigenesis (26). In addition, the NF-κB pathway
exhibits tumor-promoting functions in different models of
carcinogenesis. The NF-κB pathway is constitutively and highly
activated in melanoma cells (31).
Inhibiting the NF-κB/p65 signaling pathway also promotes melanoma
cell death (32). The EMT
hyperactivated pathways regulate the expression of genes targeting
the initiation of the metastatic cascade. Upregulation of
E-cadherin and dowregulation of N-cadherin and vimentin expression
leads to reduced migration and invasion of bladder carcinoma cells
(9). Evidence has accumulated that
these pathways cross-talk and regulate each other's activities and
functions. β-catenin interacts with NF-κB subunits in colon cancer
cells (33) and also regulates the
activation of NF-κB (34).
NF-κB/p65 promotes lung cancer proliferation through Wnt/β-catenin
signaling (35). For instance, the
NF-κB pathway directly regulates EMT through the transcription of
EMT-associated gene products (3).
Inactivity of β-catenin signaling in lung adenocarcinoma cells
suppressed tumor cell growth and EMT in vitro and in
vivo (36). Downregulation of
Capn4 suppressed the migration and invasion of glioma cells and
reduced the level of vimentin and N-cadherin (20). It has also been reported that
upregulation of Capn4 promotes migration of hepatoma cells via the
NF-κB/p65 pathway (37). In our
experiments, the protein levels of EMT markers were detected by WB
assay. The levels of β-catenin-N, N-cadherin and vimentin were
decreased, and the level of E-cadherin was increased in
Capn4-knockdown cells. There was no change in the level of total
β-catenin expression in the shRNA-infected cells compared with that
of the controls. Evidence has accumulated in support of the theory
that Wnt/β-catenin signaling promotes EMT in cancer cells (38–40).
Therefore, we concluded that Capn4 contributes to the EMT pathway
through the promotion of β-catenin nuclear entry but not through
the increase of total β-catenin expression.
In conclusion, our findings revealed that Capn4 was
overexpressed in melanoma tumor tissues and melanoma cells.
Moreover, Capn4 promoted the migration and invasion of human
melanoma cells in vitro and tumor growth in vivo.
Furthermore, we also unveiled a potential mechanism by which Capn4
promoted the migration and invasion of human melanoma cells through
the activation of the EMT pathway via the increase of β-catenin-N
expression. We presume that Capn4 is an underlying target for
melanoma treatment, and we will continue to study Capn4 in regards
to the prognosis in melanoma in the future.
Acknowledgements
This study was supported by the General Program of
the National Natural Science Foundation of China (no. 81171365) and
the Natural Science Foundation of Chongqing in China
(cstc2013jcyjA10136).
References
|
1
|
Petrella T, Verma S, Spithoff K, Quirt I
and McCready D: Melanoma disease site group: Adjuvant interferon
therapy for patients at high risk for recurrent melanoma: An
updated systematic review and practice guideline. Clin Oncol (R
Coll Radiol). 24:413–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin K, Baritaki S, Militello L, Malaponte
G, Bevelacqua Y and Bonavida B: The role of B-RAF mutations in
melanoma and the induction of EMT via dysregulation of the
NF-κB/Snail/RKIP/PTEN circuit. Genes Cancer. 1:409–420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng H, Gao L, Feng Y, Yuan L, Zhao H and
Cornelius LA: Down-regulation of Rap1GAP via promoter
hypermethylation promotes melanoma cell proliferation, survival,
and migration. Cancer Res. 69:449–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larkin JM, Fisher RA and Gore ME: Adjuvant
interferon therapy for patients at high risk for recurrent
melanoma: An updated systematic review. Clin Oncol (R Coll Radiol).
24:410–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu S and Guo J: Interpretation of clinical
practice guidelines for management of melanoma in China (new
version of 2011). Chin Clin Oncol. 2:159–171. 2012.
|
|
7
|
Yang AS and Chapman PB: The history and
future of chemotherapy for melanoma. Hematol Oncol Clin North Am.
23:583–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:172016. View Article : Google Scholar
|
|
9
|
Tsui KH, Lin YH, Chung LC, Chuang ST, Feng
TH, Chiang KC, Chang PL, Yeh CJ and Juang HH: Prostate-derived ets
factor represses tumorigenesis and modulates
epithelial-to-mesenchymal transition in bladder carcinoma cells.
Cancer Lett. 375:142–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cong J, Goll DE, Peterson AM and Kapprell
HP: The role of autolysis in activity of the
Ca2+-dependent proteinases (µ-calpain and m-calpain). J
Biol Chem. 264:10096–10103. 1989.PubMed/NCBI
|
|
11
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Storr SJ, Carragher NO, Frame MC, Parr T
and Martin SG: The calpain system and cancer. Nat Rev Cancer.
11:364–374. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan DG, Dai JY, Tang J, Wu MM, Sun SG,
Jiang JL and Fan QY: Silencing of calpain expression reduces the
metastatic potential of human osteosarcoma cells. Cell Biol Int.
33:1263–1267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roumes H, Leloup L, Dargelos E, Brustis
JJ, Daury L and Cottin P: Calpains: Markers of tumor
aggressiveness? Exp Cell Res. 316:1587–1599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen B, Tang J, Guo YS, Li Y, Chen ZN and
Jiang JL: Calpains are required for invasive and metastatic
potentials of human HCC cells. Cell Biol Int. 37:643–652. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, You X, Wang Q, Zhang T, Du Y, Lv
N, Zhang Z, Zhang S, Shan C, Ye L, et al: Hepatitis B virus X
protein drives multiple cross-talk cascade loops involving NF-κB,
5-LOX, OPN and Capn4 to promote cell migration. PLoS One.
7:e314582012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lakshmikuttyamma A, Selvakumar P, Kanthan
R, Kanthan SC and Sharma RK: Overexpression of m-calpain in human
colorectal adenocarcinomas. Cancer Epidemiol Biomarkers Prev.
13:1604–1609. 2004.PubMed/NCBI
|
|
18
|
Shiba E, Kambayashi JI, Sakon M, Kawasaki
T, Kobayashi T, Koyama H, Yayoi E, Takatsuka Y and Takai SI:
Ca2+-dependent neutral protease (calpain) activity in
breast cancer tissue and estrogen receptor status. Breast Cancer.
3:13–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pamonsinlapatham P, Gril B, Dufour S,
Hadj-Slimane R, Gigoux V, Pethe S, Lhoste S, Camonis J, Garbay C,
Raynaud F, et al: Capns1, a new binding partner of RasGAP-SH3
domain in K-RasV12 oncogenic cells: Modulation of cell
survival and migration. Cell Signal. 20:2119–2126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai JJ, Qi ZX, Chen LC, Yao Y, Gong Y and
Mao Y: miR-124 suppresses the migration and invasion of glioma
cells in vitro via Capn4. Oncol Rep. 35:284–290. 2016.PubMed/NCBI
|
|
21
|
Zhang C, Bai DS, Huang XY, Shi GM, Ke AW,
Yang LX, Yang XR, Zhou J and Fan J: Prognostic significance of
Capn4 overexpression in intrahepatic cholangiocarcinoma. PLoS One.
8:e546192013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhuang Q, Qian X, Cao Y, Fan M, Xu X and
He X: Capn4 mRNA level is correlated with tumour progression and
clinical outcome in clear cell renal cell carcinoma. J Int Med Res.
42:282–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng PC, Chen X, Zhu HW, Zheng W, Mao LH,
Lin C, Liu JN and Zheng M: Capn4 is a marker of poor clinical
outcomes and promotes nasopharyngeal carcinoma metastasis via
nuclear factor-κB-induced matrix metalloproteinase 2 expression.
Cancer Sci. 105:630–638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu J, Xu FK, Zhao GY, Lu CL, Lin ZW, Ding
JY and Ge D: Capn4 promotes non-small cell lung cancer progression
via upregulation of matrix metalloproteinase 2. Med Oncol.
32:512015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Talantov D, Mazumder A, Yu JX, Briggs T,
Jiang Y, Backus J, Atkins D and Wang Y: Novel genes associated with
malignant melanoma but not benign melanocytic lesions. Clin Cancer
Res. 11:7234–7242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chin L, Garraway LA and Fisher DE:
Malignant melanoma: Genetics and therapeutics in the genomic era.
Genes Dev. 20:2149–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uchibori R, Tsukahara T, Mizuguchi H, Saga
Y, Urabe M, Mizukami H, Kume A and Ozawa K: NF-κB activity
regulates mesenchymal stem cell accumulation at tumor sites. Cancer
Res. 73:364–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tarapore RS, Siddiqui IA, Saleem M, Adhami
VM, Spiegelman VS and Mukhtar H: Specific targeting of
Wnt/β-catenin signaling in human melanoma cells by a dietary
triterpene lupeol. Carcinogenesis. 31:1844–1853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan G, Zhang B, Yang S, Jin L, Datta A,
Bae S, Chen X and Datta PK: Novel role of STRAP in progression and
metastasis of colorectal cancer through Wnt/β-catenin signaling.
Oncotarget. 7:16023–16037. 2016.PubMed/NCBI
|
|
30
|
Lucero OM, Dawson DW, Moon RT and Chien
AJ: A re-evaluation of the ‘oncogenic’ nature of Wnt/β-catenin
signaling in melanoma and other cancers. Curr Oncol Rep.
12:314–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu S, Luo Q, Cun B, Hu D, Ge S, Fan X and
Chen F: The pharmacological NF-κB inhibitor BAY11-7082 induces cell
apoptosis and inhibits the migration of human uveal melanoma cells.
Int J Mol Sci. 13:15653–15667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shan X, Tian LL, Zhang YM, Wang XQ, Yan Q
and Liu JW: Ginsenoside Rg3 suppresses FUT4 expression through
inhibiting NF-κB/p65 signaling pathway to promote melanoma cell
death. Int J Oncol. 47:701–709. 2015.PubMed/NCBI
|
|
33
|
Du Q, Zhang X, Cardinal J, Cao Z, Guo Z,
Shao L and Geller DA: Wnt/β-catenin signaling regulates
cytokine-induced human inducible nitric oxide synthase expression
by inhibiting nuclear factor-κB activation in cancer cells. Cancer
Res. 69:3764–3771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schön S, Flierman I, Ofner A, Stahringer
A, Holdt LM, Kolligs FT and Herbst A: β-catenin regulates NF-κB
activity via TNFRSF19 in colorectal cancer cells. Int J Cancer.
135:1800–1811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li D, Beisswenger C, Herr C, Hellberg J,
Han G, Zakharkina T, Voss M, Wiewrodt R, Bohle RM, Menger MD, et
al: Myeloid cell RelA/p65 promotes lung cancer proliferation
through Wnt/β-catenin signaling in murine and human tumor cells.
Oncogene. 33:1239–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qu J, Li M, An J, Zhao B, Zhong W, Gu Q,
Cao L, Yang H and Hu C: MicroRNA-33b inhibits lung adenocarcinoma
cell growth, invasion, and epithelial-mesenchymal transition by
suppressing Wnt/β-catenin/ZEB1 signaling. Int J Oncol.
47:2141–2152. 2015.PubMed/NCBI
|
|
37
|
Zhang F, Wang Q, Ye L, Feng Y and Zhang X:
Hepatitis B virus X protein upregulates expression of calpain small
subunit 1 via nuclear factor-κB/p65 in hepatoma cells. J Med Virol.
82:920–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang T, Zhang H, Qiu H, Li B, Wang J, Du
G, Ren C and Wan X: EFEMP1 is repressed by estrogen and inhibits
the epithelial-mesenchymal transition via Wnt/β-catenin signaling
in endometrial carcinoma. Oncotarget. 7:25712–25725.
2016.PubMed/NCBI
|
|
39
|
Bernaudo S, Salem M, Qi X, Zhou W, Zhang
C, Yang W, Rosman D, Deng Z, Ye G, Yang B, et al: Cyclin G2
inhibits epithelial-to-mesenchymal transition by disrupting
Wnt/β-catenin signaling. Oncogene. 35:4816–4827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han JW, Lyu J, Park YJ, Jang SY and Park
TK: Wnt/β-catenin signaling mediates regeneration of retinal
pigment epithelium after laser photocoagulation in mouse eye.
Invest Ophthalmol Vis Sci. 56:8314–8324. 2015. View Article : Google Scholar : PubMed/NCBI
|