Introduction
Hepatocellular carcinoma (HCC) is a highly malignant
tumor and its incidence is ranked fifth in malignant tumors
(1). Epithelial-mesenchymal
transition (EMT) contributes to the promotion of HCC progression
and metastasis (2). In addition,
chemotherapy is utilized as an aid to prevent the recurrence and
metastasis of HCC (3). However, a
wide range of resistance often occurs in the treatment of HCC
resulting from aberrant gene expression (4). Therefore, it is necessary to identify
novel therapeutic target for HCC.
PEG10 is a new genetic imprinting gene which is
identified in hepatocellular carcinoma tissues (5), it contains two open reading frames
(ORFs) which code two proteins (PEG10-ORF1 and PEG10-ORF2) through
the mechanism of ribosomal shift. A previous study showed that
PEG10-ORF1 which acts as the main functional protein is modulated
by various genes, such as c-myc (6). Recent studies have proved that the
expression level of PEG10 is specifically higher in HCC tissues
than in normal tissues (7).
Moreover, PEG10 was confirmed to play an important role in the
progression, proliferation, metastasis and prognosis in human lung
and breast cancer (8,9) and increase the progression of
neuroendocrine prostate cancer (10). PEG10 promoted the migration of human
Burkitt's lymphoma cells by increasing the expression of matrix
metalloproteinase-2 and −9 (11).
These results suggest that PEG10 display functional roles in many
disease, especially in tumors. However, the precise roles of PEG10
in facilitating metastasis of HCC still remain unknown.
It is well accepted that aberrant EMT contributes to
tumor progression, in which cells lose their epithelial cell-like
characteristics and alternatively adopt a mesenchymal phenotype,
thus, initiating tumor metastasis. This process is thus considered
to be a vital process increasing the invasion of cancers (12). Many components in tumor
microenvironment such as TGF-β1 is able to induce EMT, which has
been proved to facilitate a highly invasive and metastatic
phenotype in various tumors (2,13,14).
In addition, TGF-β1 polymorphism has been proved to contribute to
pulmonary metastasis of hepatocellular carcinoma in a mouse model
(15). However, it remains to be
determined whether PEG10 is involved in EMT and the TGF-β1
signaling also include the regulation of PEG10 expression in HCC
metastasis.
To address these problems, we established an HCC
culture model in which TGF-β1 induced migration, invasion and EMT.
In the present study, we constructed a recombinant adenovirus
vector of PEG10 and PEG10 shRNAs and infected them into HepG2 cells
to investigate the promotive roles in HCC migration and invasion
and possible mechanisms of PEG10 on TGF-β1-induced EMT. In
addition, we further explored the effect of PEG10 on the
chemotherapy resistance of HCC. To the best of our knowledge, the
roles of PEG10 validated here have not yet been reported.
Accordingly, these results might provide novel insights for
ameliorating or finding new therapeutic target for the HCC
progression.
Materials and methods
Patient samples
Thirty-nine HCC tissues and adjacent normal tissues
were obtained from patients who underwent surgery at the Eastern
Hepatobiliary Surgery Hospital from June 2014 to November 2016, and
all 39 cases presented metastasis. In addition, 31 HCC tissues with
lymph node metastasis were obtained at the same time. The present
study was approved by the Eastern Hepatobiliary Surgery Hospital
Research Ethics Committee.
Cell culture
Human normal hepatic cell line, L02 and hepatic
cancer cell lines (HepG2, HCCLM3 and HUH7) were purchased from the
Cell Bank in Chinese Academy of Sciences of China (Shanghai,
China). Cells were cultured in RPMI-1640 medium (Gibco, Grand
Island, NY, USA) supplemented with 100 mg/ml streptomycin, 100
IU/ml penicillin and 10% fetal bovine serum (FBS; Gibco) at 37°C
under humidified air with 5% CO2.
Adenovirus vectors construction
We thank Hanbio, Inc., (Shanghai, China) for
constructing the adenovirus vector containing the coding area of
PEG10 (Ad-PEG10) and adenovirus vector of PEG10 shRNAs (Ad-shRNA1
and Ad-shRNA2), which was verified by DNA sequencing.
Quantitative real-time PCR
(qRT-PCR)
Firstly, total RNA was prepared from the cells using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the
manufacturer's instructions. Secondly, total RNA was reverse
transcribed into cDNA using M-MLV (Promega, Madison, WI, USA)
according to standard protocols. To examine the mRNA expression
levels, following the protocols of SYBR Premix Ex Taq™ kit (Takara
Bio, Inc., Dalian, China), qRT-PCR was carried out on an ABI Prism
7500 detection system (Applied Biosystems, Inc., Foster City, CA,
USA). The primers for EMT markers and GAPDH were previously
described (12). The primers for
PEG10 are: PEG10: forward, 5′-CCAACGACAAGAACGATTA-3′ and reverse,
5′-TTTTGCCAGTTTGAAAAAC-3′. The specificity of the amplified
products was confirmed by using melting curve analysis. GAPDH
served as an endogenous control. Finally, the 2−∆∆ct
method was utilized for analyzing relative gene expression.
Immunohistochemistry
The detailed procedures were described previously
(16). Immunohistochemistry of
hepatic, normal and hepatic tissues with lymph node metastasis for
PEG10 was performed manually using an anti-PEG antibody (cat. no.
ab181249; Abcam).
Western blot analysis
The whole HepG2 cell extracts were obtained using
RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China)
following the standard protocols. The concentration of the total
protein was detected by Bradford assay. A total of 30 µg samples of
protein extracts were separated using sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto
polyvinylidene fluoride (PVDF; Millipore, Billerica, MA, USA)
membranes for western blotting. The membranes were blocked with 5%
milk solution in TBST (Tris-HCl-buffered saline supplemented with
0.5% Tween-20), and incubated with the primary antibodies against
PEG10, E-cadherin (ab1416; Abcam), vimentin (ab8978; Abcam), Bcl-2
(ab32124; Abcam) Bax (ab32503; Abcam) and β-actin (ab8226; Abcam)
in 5% bovine serum albumin (BSA) in TBST at 4°C overnight, followed
by incubation with the corresponding horseradish peroxidase-linked
secondary antibodies (Beyotime Institute of Biotechnology). Blots
were washed for 15 min, three times and the signals were detected
using an enhanced chemiluminescence detecting reagent (Thermo
Fisher Scientific, Waltham, MA, USA).
Would healing assay
Detailed procedure was described elsewhere (17).
Transwell migration and invasion
Transwell migration and invasion assays were
performed via using 24-well Millicell Hanging Cell Culture inserts
(8 mm) PET (Millipore), and the PETs coated with Matrigel matrix
gel (BD Biosciences, San Jose, CA, USA) were used for invasion
assays. Briefly, 24 h after infection with adenovirus vectors,
8×104 cells in 200 µl serum-free medium were plated onto
the upper chamber, while complete medium containing 10% FBS was
added to lower chamber, which was used as a chemoattractant. The
time for migration assays was 36 h and 48 h for invasion assays.
Then cells on the upper chamber were removed with a cotton swab,
and migrated cells on the lower chamber were fixed using methanol
for 30 min, following staining with crystal violet solution for 30
min and washed with phosphate-buffer solution (PBS) several times.
Six random fields from each of the triplicate migration and
invasion assays were counted by using phase contrast microscopy.
The dye was dissolved with acetic acid, and crystal violet
absorbance was measured at 570 nm using a microplate reader.
Cell adhesion assays
The detailed procedure was described previously
(17). Briefly, 96-wells plate was
coated with matrix gel at 37°C for 3.5 h and blocked with 5% BSA
for another 1.5 h. Cells infected with adenovirus vectors were
suspended at a final concentration of 105 cells/well in
serum-free medium. Non-adherent cells were washed away with PBS for
1 h adhesion.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT)-assay was used to examine the number of adherent cells.
MTT assays
MTT assays were used to determine the cell
viability. Cells (4×103/well) were planted in 96-well
plates and were treated with the IC50 of doxorubicin
after the adhesion, followed by 24, 48 and 72 h of observation. MTT
was added in the medium at 0.25 mg/ml concentration and the
absorbance at 570 nm was measured using a microplate reader.
Cell cycle analysis
HepG2 cells were infected with Ad-PEG10 or Ad-shRNAs
and then treated with doxorubicin for 48 h. The detailed procedure
for cell cycle analysis ws described previously (18).
Apoptosis assay
HepG2 cells were infected with Ad-PEG10 construct or
Ad-shRNAs and then doxorubicin was added in the medium. After
culturing for 48 h apoptotic cells were analyzed by flow cytometry
(BD Biosciences) using Annexin V-FITC apoptosis detection kit
(Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China) following the
manufacturers protocol.
Statistical analysis
All data were obtained from at least three
independent experiments (n≥3) and presented as mean ± SD. The
differences between the groups were analyzed with the Students
t-test except for qRT-PCR in which one-way analysis of variance
(ANOVA) was used, and P≤0.05 were considered to indicate a
statistically significant result.
Results
PEG10 expression associates with the
lymph node metastasis and OS of HCC
To determine the relationship between PEG10
expression and HCC OS or metastasis PEG10 expression was detected
in 39 HCC tissues with or without lymph node metastasis and
adjacent normal tissues by qRT-PCR analyses. As shown in Fig. 1A, PEG10 mRNA level is significantly
higher in HCC tissues than in the adjacent normal tissues.
Moreover, compared to the HCC tissues without lymph node
metastasis, PEG10 mRNA level is much higher in HCC tissues with
lymph node metastasis (Fig. 1A) and
high PEG10 expression is associated with poorer OS of the patients
(Fig. 1B). PEG10 protein expression
was further examined using immunohistochemical analyses, as
expected, the results were consistent with the qRT-PCR results
(Fig. 1C). In addition, the mRNA
level of PEG10 was also detected in hepatic tumor cell lines
(HepG2, HCCLM3 and HUH7) and the normal hepatic cell line L02
(Fig. 1D). The results showed that
PEG10 expression level is remarkably higher in hepatic cell lines
than in L02 cells, especially in HepG2 cells which were used for
additional study. Therefore, our results suggest that PEG10
associates with the lymph node metastasis and OS of HCC.
Ectopic expression of PEG10 confers
mesenchymal-like phenotypes in HepG2 cells and promotes cell
migration and invasion
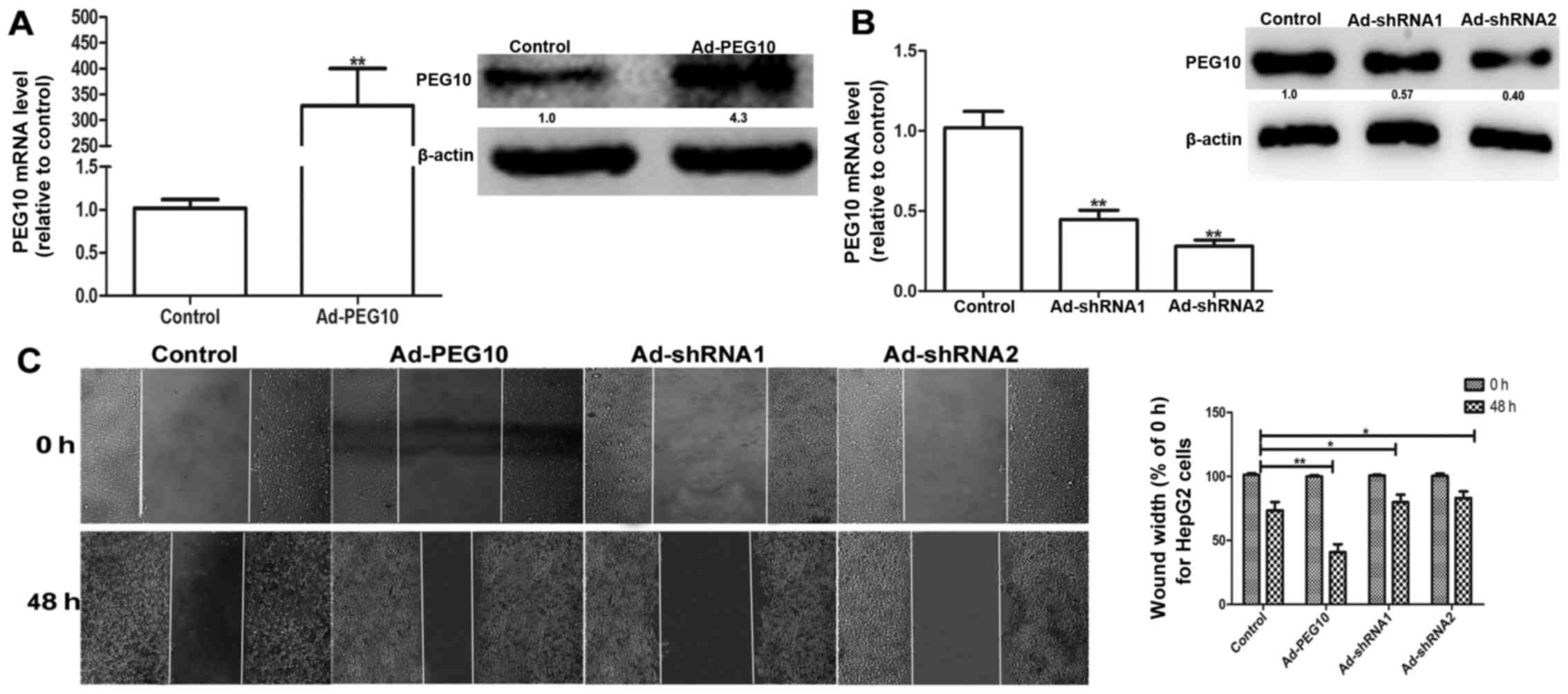
Based on the above observations, we speculated that
ectopic expression of PEG10 could enhance the migration, invasion
and EMT in hepatic tumor cells. We constructed the adenovirus
vectors with PEG10 coding area (Ad-PEG10) or shRNAs against PEG10
(Ad-shRNA1 and Ad-shRNA2) which were further infected into HepG2
cells. The efficiency of infection was examined via qRT-PCR and
western blot assays. As shown in Fig.
2A, the mRNA and protein levels of PEG10 in HepG2 cells
infected with Ad-PEG10 is significantly higher than the control
group. In contrast, the Ad-shRNAs-infected cells showed lower
expression levels (Fig. 2B). In
addition, the wound-healing, Transwell migration and invasion
assays showed that HepG2 cells infected with Ad-PEG10 exhibited
greater migration and invasion ability than the control cells,
while Ad-shRNAs inhibited this effects (Figs. 2C and 3A
and B). The adhesion ability is essential for the invasion of
tumor cells (12). As shown in
Fig. 3C, cells overexpressing PEG10
markedly enhanced the adhesive ability, while infection with
Ad-shRNAs remarkably decreased cell adhesion. The expression of EMT
markers (E-cadherin and vimentin) were further examined in HepG2
cells infected with Ad-PEG10 or Ad-shRNAs. As shown in Fig. 3D and E, overexpression of PEG10 in
HepG2 cells increased the expression levels of mesenchymal marker
protein, vimentin and decreased the expression levels of epithelial
marker protein, E-cadherin. In contrast, Ad-shRNAs could reduce the
expression of vimentin and promote the expression of E-cadherin.
Overall, our results indicate that PEG10 overexpression could
promote EMT, migration and invasion of HepG2 cells.
PEG10 is essential in TGF-β1-induced
EMT in vitro
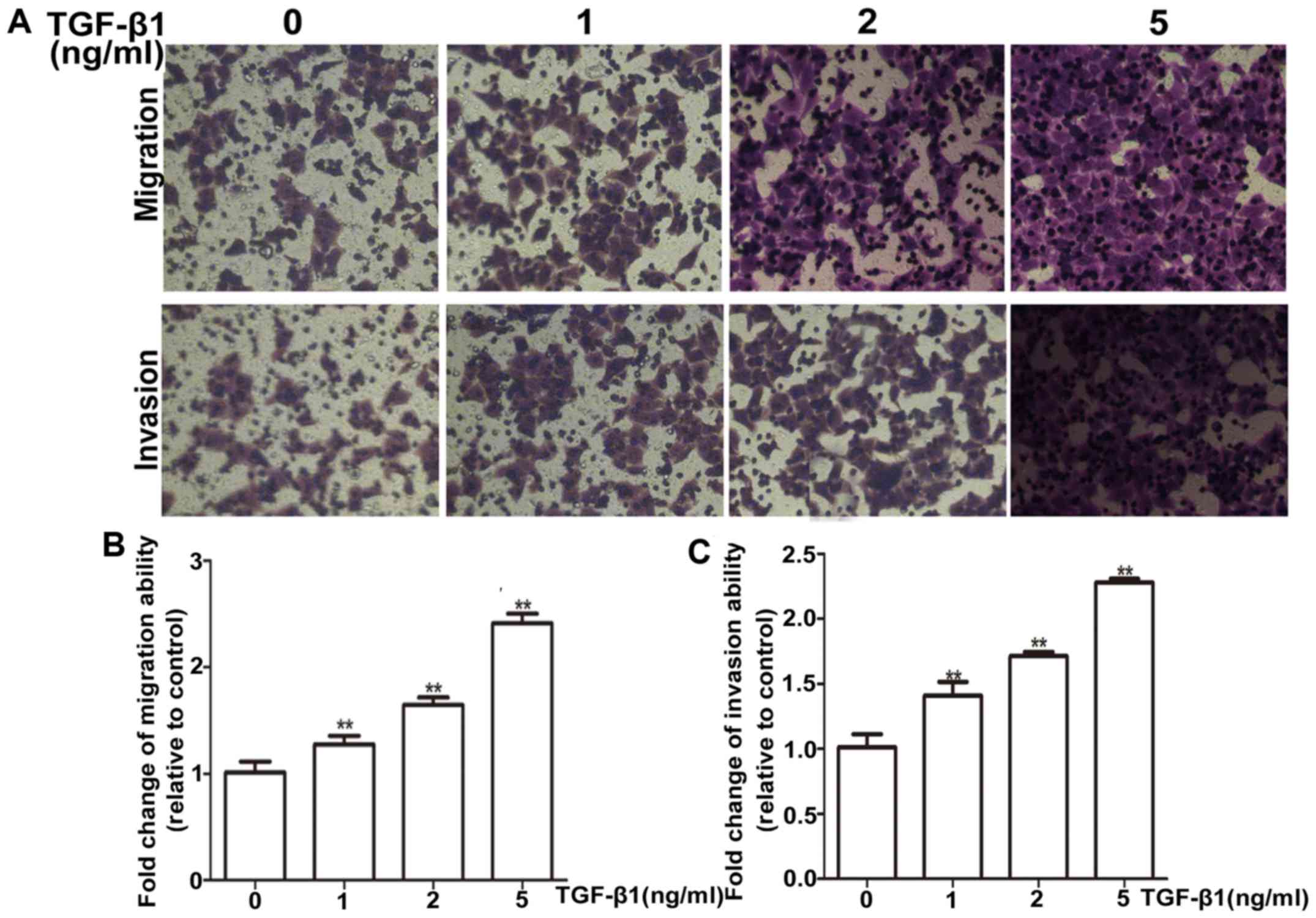
Firstly, the suitable condition for TGF-β1 induction
was tested, HepG2 cells were treated with different concentrations
(1, 2 and 5 ng/ml) of TGF-β1 for 72 h. HepG2 cells exhibited a
dose-dependent increase in mRNA and protein levels of vimentin
(Fig. 4A and B). Most importantly,
the PEG10 mRNA and protein levels were also increased
concentration-dependently on TGF-β1 (Fig. 4C and D). Moreover, the TGF-β1
treatment was confirmed to promote migration and invasion ability
of HepG2 cells via wound-healing and Transwell migration and
invasion assays (Figs. 4E and F and
5A and B), indicating the TGF-β1
pathway was activated. To further examine whether PEG10 is involved
in TGF-β1-induced EMT, HepG2 cell- infected Ad-PEG10 or Ad-shRNAs
were treated with 2 ng/ml TGF-β1. As shown in Fig. 6A-C, Ad-PEG10 infected cells
exhibited higher levels of migration and invasion relative to cells
treated TGF-β1 alone, while decreased levels of migration and
invasion were observed in Ad-shRNAs-infected cells. In addition,
Ad-PEG10 infected cells exhibited higher levels of vimentin
relative to cells treated TGF-β1 alone, while decreased levels of
vimentin were observed in Ad-shRNA-infected cells (Fig. 6D and E). Therefore, these results
suggest that PEG10 expression is essential for TGF-β1-induced
EMT.
Overexpression of PEG10 confers
doxorubicin resistance in HepG2 cells
A previous study has shown that EMT possessed a
promotive role in chemoresistance in HCC (19). Here, we further investigated whether
ectopic expression of PEG10 lead to doxorubicin resistance in HepG2
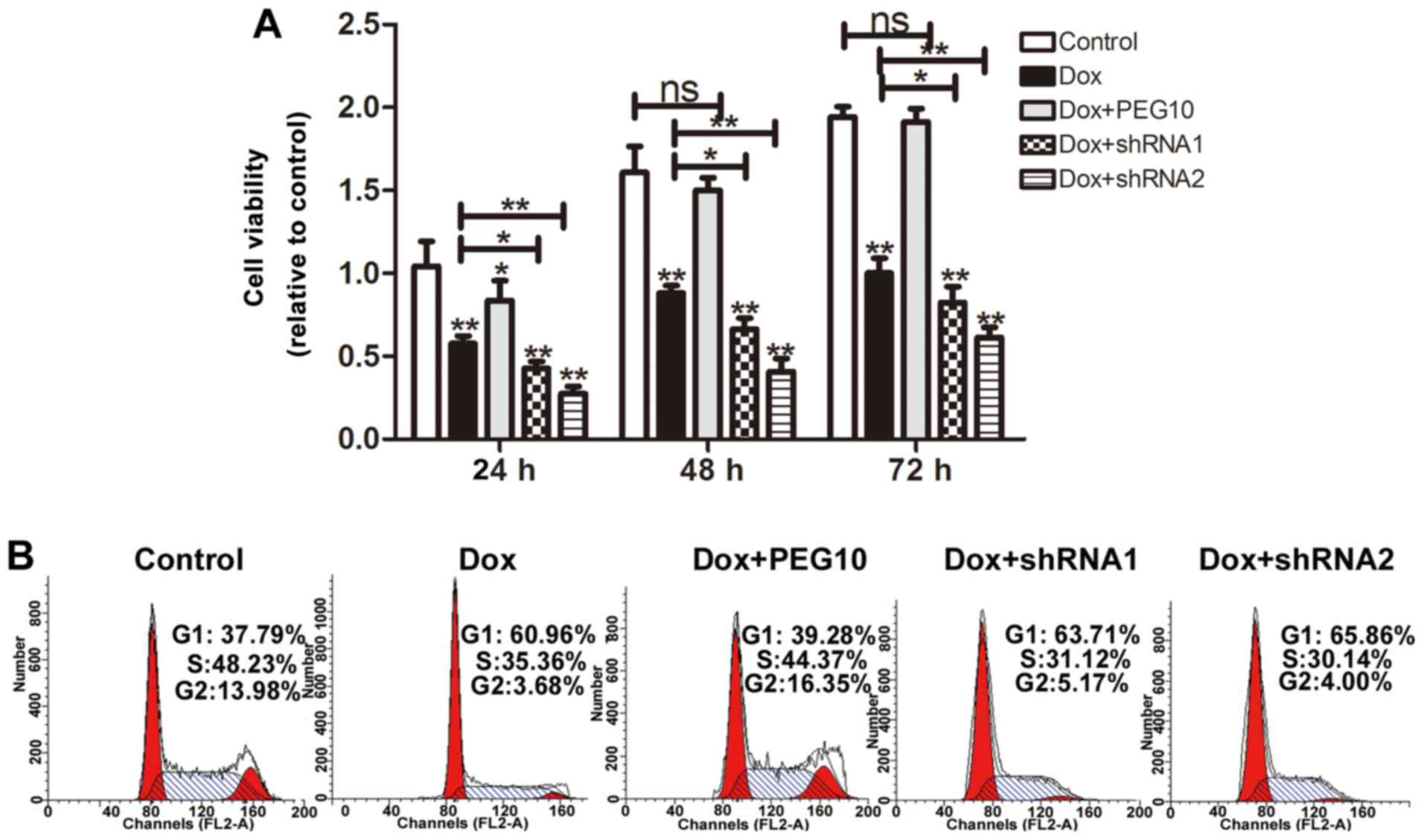
cells. MTT assays were performed to detect the cell viability, as
shown in Fig. 7A, HepG2 cells
overexpressing PEG10 displayed low doxorubicin sensitivity and
knockdown of PEG10 increased doxorubicin sensitivity. Furthermore,
HepG2 cell viability was also examined by cell cycle assays. As
expected, when treated with doxorubicin, HepG2 cells showed a
decrease of cells in S phase, while HepG2 cells overexpressing
PEG10 showed litte change compare to the control. Noteworthy, HepG2
cells treated with doxorubicin plus shRNAs of PEG10 exhibited a
stronger decrease compared to the group treated with doxorubicin
alone (Fig. 7B). In addition, HepG2
cells were analyzed by apoptosis assays. Results showed that
upregulation of PEG10 in HepG2 cells inhibited apoptosis induced by
doxorubicin treatment and knockdown of PEG10 enhanced the effect
(Fig. 8A). The protein level of
apoptotic markers (Bcl-2 and Bax) were examined by western blot
analysis. As shown in Fig. 8B,
exposure of the Ad-PEG10 infected cells to doxorubicin decreased
the expression of Bax and increased Bcl-2 expression. Conversely,
treatment with Ad-shRNAs promoted the expression of Bax but
decreased Bcl-2 expression. Hence, these results indicate that
enforced expression of PEG10 was able to confer doxorubicin
resistance in HepG2 cells.
Discussion
Although previous studies have indicated that PEG10
might be a critical factor in gallbladder adenocarcinoma (20), immune response (21), breast (9) and lung cancer (8), the related studies of PEG10 on the EMT
and chemoresistace of HCC are rare and the mechanism of PEG10 in
the progression of HCC is still unclear. EMT has been implicated in
several tumor types, including breast (12) and human non-small cell lung cancer
(22). TGF-β1 is a well-known
trigger and plays a vital role in the EMT process (16). Thus, in the present study, a
recombinant adenovirus vector of PEG10 and its shRNAs were
constructed and transferred into HepG2 cells to explore the
promotive effects on HCC migration, invasion and the possible roles
in TGF-β1-induced EMT.
In the present study, 39 HCC tissues with or without
lymph node metastasis and adjacent normal tissues were used to
explore the relationship between the expression level of PEG10 and
OS or lymph node metastasis of HCC. Our results showed that high
PEG10 expression is associated with poorer OS of the patients,
which is consistent with previous studies (23,24)
and makes our results even more reliable. Although recent studies
have suggested that EMT is not required for lung and pancreatic
cancer metastasis (25,26), our results provide substantial
evidence that overexpression of PEG10 enhances EMT and migration,
invasion and adhesion in HCC, which is consistent with the
functional roles of PEG10 in breast cancer (9). However, further studies are necessary
for investigating the mechanisms of PEG10 in EMT and whether PEG10
promotes the metastasis of HCC via EMT. Most importantly, as TGF-β1
is a key factor involved in the induction of EMT, stimulation with
TGF-β1 led to the transition of HCC to a mesenchymal phenotype,
which is proved in this study. However, following stimulation of
TGF-β1 with knockdown of PEG10 exhibited a significant abrogation
in TGF-β1-induced EMT, migration and invasion in HCC and
upregulation of PEG10 enhanced these effects. To the best of our
knowledge, effects of PEG10 on EMT and TGF-β1-induced EMT,
migration and invasion have not been previously reported.
Evidence has confirmed that EMT plays a promotive
role in chemoresistance of various cancers and targeting EMT with
Met inhibitors has been proved to reverse chemoresistance in small
cell lung cancer (27,28). In addition, doxorubicin resistance
is one reason for HCC recurrence (19). Subsequently, we further investigated
whether PEG10 is associated with doxorubicin resistance in HCC. We
demonstrated that overexpression of PEG10 conferred doxorubicin
resistance in HepG2 cells. However, cells with knockdown of PEG10
displayed greater sensitivity to doxorubicin. These results might
provide evidence for the fact that patients with high expression of
PEG10 have poor survival, and tumor recurrence in HCC (7).
In conclusion, our results present that PEG10
promoted migration, invasion and EMT in HCC and enhanced
TGF-β1-induced EMT. In addition, PEG10 aids in chemoresistance in
HepG2 cells. These results may provide a new potential
therapeutical target for HCC metastasis and chemoresistance.
However, further study is needed to explain the action of PEG10 in
HCC and the mechanisms of PEG10 in TGF-β1-induced EMT, and thus,
resolve the issue of HCC chemoresistance.
Acknowledgements
We thank Professor Song Li from the Second Military
Medical University for providing valuable advice and critically
reviewing this manuscript.
References
|
1
|
Papatheodoridis GV, Dalekos GN, Yurdaydin
C, Buti M, Goulis J, Arends P, Sypsa V, Manolakopoulos S, Mangia G,
Gatselis N, et al: Incidence and predictors of hepatocellular
carcinoma in Caucasian chronic hepatitis B patients receiving
entecavir or tenofovir. J Hepatol. 62:363–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WX, Zhang ZG, Ding ZY, Liang HF, Song
J, Tan XL, Wu JJ, Li GZ, Zeng Z, Zhang BX, et al: MicroRNA-630
suppresses tumor metastasis through the TGF-β-miR-630-Slug
signaling pathway and correlates inversely with poor prognosis in
hepatocellular carcinoma. Oncotarget. 7:22674–22686.
2016.PubMed/NCBI
|
|
3
|
Xu MY, Chen R, Yu JX, Liu T, Qu Y and Lu
LG: AZGP1 suppresses epithelial-to-mesenchymal transition and
hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways. Cancer
Lett. 374:241–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferroudj S, Yildiz G, Bouras M, Iscan E,
Ekin U and Ozturk M: Role of fanconi anemia/BRCA pathway genes in
hepatocellular carcinoma chemoresistance. Hepatol Res. Feb
16–2016.(Epub ahead of print). doi: 10.1111/hepr.12675. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ono R, Kobayashi S, Wagatsuma H, Aisaka K,
Kohda T, Kaneko-Ishino T and Ishino F: A retrotransposon-derived
gene, PEG10, is a novel imprinted gene located on human chromosome
7q21. Genomics. 73:232–237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li CM, Margolin AA, Salas M, Memeo L,
Mansukhani M, Hibshoosh H, Szabolcs M, Klinakis A and Tycko B:
PEG10 is a c-MYC target gene in cancer cells. Cancer Res.
66:665–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bang H, Ha SY, Hwang SH and Park CK:
Expression of PEG10 is associated with poor survival and tumor
recurrence in hepatocellular carcinoma. Cancer Res Treat.
47:844–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng X, Hu Y, Ding Q, Han R, Guo Q, Qin J,
Li J, Xiao R, Tian S, Hu W, et al: PEG10 plays a crucial role in
human lung cancer proliferation, progression, prognosis and
metastasis. Oncol Rep. 32:2159–2167. 2014.PubMed/NCBI
|
|
9
|
Li X, Xiao R, Tembo K, Hao L, Xiong M, Pan
S, Yang X, Yuan W, Xiong J and Zhang Q: PEG10 promotes human breast
cancer cell proliferation, migration and invasion. Int J Oncol.
48:1933–1942. 2016.PubMed/NCBI
|
|
10
|
Akamatsu S, Wyatt AW, Lin D, Lysakowski S,
Zhang F, Kim S, Tse C, Wang K, Mo F, Haegert A, et al: The
placental gene PEG10 promotes progression of neuroendocrine
prostate cancer. Cell Rep. 12:922–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong J, Qin J, Zheng Y, Peng X, Luo Y and
Meng X: PEG10 promotes the migration of human Burkitts lymphoma
cells by up-regulating the expression of matrix metalloproteinase-2
and −9. Clin Invest Med. 35:E117–E125. 2012.PubMed/NCBI
|
|
12
|
Li X, Zheng L, Zhang F, Hu J, Chou J, Liu
Y, Xing Y and Xi T: STARD13-correlated ceRNA network inhibits EMT
and metastasis of breast cancer. Oncotarget. 7:23197–23211.
2016.PubMed/NCBI
|
|
13
|
Da C, Liu Y, Zhan Y, Liu K and Wang R:
Nobiletin inhibits epithelial-mesenchymal transition of human
non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3
signaling pathway. Oncol Rep. 35:2767–2774. 2016.PubMed/NCBI
|
|
14
|
Dong Z, Tai W, Lei W, Wang Y, Li Z and
Zhang T: IL-27 inhibits the TGF-β1-induced epithelial-mesenchymal
transition in alveolar epithelial cells. BMC Cell Biol. 17:72016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li GC, Ye QH, Dong QZ, Ren N, Jia HL and
Qin LX: TGF beta1 and related-Smads contribute to pulmonary
metastasis of hepatocellular carcinoma in mice model. J Exp Clin
Cancer Res. 31:932010. View Article : Google Scholar
|
|
16
|
Ma H, Gao L, Li S, Qin J, Chen L, Liu X,
Xu P, Wang F, Xiao H, Zhou S, et al: CCR7 enhances TGF-β1-induced
epithelial-mesenchymal transition and is associated with lymph node
metastasis and poor overall survival in gastric cancer. Oncotarget.
6:24348–24360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Li T, Gao C, Lv X, Liu K, Song H,
Xing Y and Xi T: FOXO1 3UTR functions as a ceRNA in repressing the
metastases of breast cancer cells via regulating miRNA activity.
FEBS Lett. 588:3218–3224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng L, Li X, Meng X, Chou J, Hu J, Zhang
F, Zhang Z, Xing Y, Liu Y and Xi T: Competing endogenous RNA
networks of CYP4Z1 and pseudogene CYP4Z2P confer tamoxifen
resistance in breast cancer. Mol Cell Endocrinol. 427:133–142.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu T, Zhang J, Chen W, Pan S, Zhi X, Wen
L, Zhou Y, Chen BW, Qiu J, Zhang Y, et al: ARK5 promotes
doxorubicin resistance in hepatocellular carcinoma via
epithelial-mesenchymal transition. Cancer Lett. 377:140–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu DC, Yang ZL and Jiang S:
Identification of PEG10 and TSG101 as carcinogenesis, progression,
and poor-prognosis related biomarkers for gallbladder
adenocarcinoma. Pathol Oncol Res. 17:859–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng W, Zhao G, Ma Y, Yu H and Wang X:
Dendritic cells transfected with PEG10 recombinant adenovirus
elicit anti-tumor immune response in vitro and in vivo. Vaccine.
29:3501–3506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu K, Guo L, Guo Y, Zhou B, Li T, Yang H,
Yin R and Xi T: AEG-1 3-untranslated region functions as a ceRNA in
inducing epithelial-mesenchymal transition of human non-small cell
lung cancer by regulating miR-30a activity. Eur J Cell Biol.
94:22–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jie X, Lang C, Jian Q, Chaoqun L, Dehua Y,
Yi S, Yanping J, Luokun X, Qiuping Z, Hui W, et al: Androgen
activates PEG10 to promote carcinogenesis in hepatic cancer cells.
Oncogene. 26:5741–5751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ip WK, Lai PB, Wong NL, Sy SM, Beheshti B,
Squire JA and Wong N: Identification of PEG10 as a progression
related biomarker for hepatocellular carcinoma. Cancer Lett.
250:284–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bharti R, Dey G and Mandal M: Cancer
development, chemoresistance, epithelial to mesenchymal transition
and stem cells: A snapshot of IL-6 mediated involvement. Cancer
Lett. 375:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cañadas I, Rojo F, Taus Á, Arpí O,
Arumí-Uría M, Pijuan L, Menéndez S, Zazo S, Dómine M, Salido M, et
al: Targeting epithelial-to-mesenchymal transition with Met
inhibitors reverts chemoresistance in small cell lung cancer. Clin
Cancer Res. 20:938–950. 2014. View Article : Google Scholar : PubMed/NCBI
|