Introduction
Prostate cancer (PCa), a clinically heterogeneous
multifocal disease, remains a major health concern in men, with an
estimated 180,890 new cases and 26,120 deaths in the US in 2016
(1). Although strategies such as
lifestyle modifications offer opportunities to reduce the risk of
PCa, the incidence of PCa has continued to increase in recent years
(2). To date, the early detection
of PCa by PSA screening has been controversial for many years.
Therefore, optimization for prostate-specific antigen (PSA)
screening strategies and finding new biomarkers may minimize
overdiagnosis related with PSA screening and improve the detection
rate of PCa. Even after removal, >20% of patients suffer a
recurrence, mostly detected by a rise in the serum level of PSA
(3). Facing this global health
issue, the identification of new biomarkers and potential oncogenes
involved in PCa may provide more sophisticated ways for the early
diagnosis and further treatment.
The SOX family, consisting of a number of
transcription factors that contain a highly conserved high-mobility
group (HMG) DNA-binding domain, plays critical roles in regulating
cell fate decisions during development (4). The SOX family is comprised of >20
members identified by homology-based analysis of the HMG
DNA-binding domain (5). In recent
years, accumulating research has confirmed the link between
SOX genes and various human diseases including cancer
(6). The expression of SOX
genes was found to differ in various types of cancer, and their
functions in cancer development are also heterogeneous (7). For instance, SOX2 was found to be
overexpressed in adenoid cystic carcinoma (ACC) and is associated
with the poor prognosis in patients with ACC (8). However, overexpression of SOX2 was
found to indicate a favorable prognosis in patients with non-small
cell lung cancer (9). SOX10, SOX8
and SOX9 are overexpressed in hepatocellular carcinoma (HCC) and
SOX10 is an oncogene promoting the progression of HCC (10). In the case of the sex determining
region Y (SRY)-box 18 (SOX18), it has been determined that it
participates in the process of angiogenesis and lymphangiogenesis,
and loss of SOX18 is responsible for
hypotrichosis-lymphodema-teleangiectasia syndrome (11–13). A
growing number of studies suggest that SOX18 is overexpressed in
various types of tumors and may play an important role in tumor
occurrence and progression (14).
It has been documented that SOX18 is overexpressed in HCC, ovarian
and non-small cell lung cancer and ductal breast and cervical
carcinoma compared with normal tissues and is related with a poor
prognosis in patients with these types of cancer (15–18).
Furthermore, SOX18 may regulate the progression of tumors via the
activation of its downstream transcription factors such as MMP-7
and endothelial-specific claudin-5 (19,20).
However, the expression level and biological function of SOX18 in
PCa remain unclear, and the potential mechanisms involved still
need to be addressed.
In the present study, we observed a frequent
overexpression of SOX18 in PCa tissues. Silencing of SOX18
significantly impaired the proliferation, migration and invasion
ability of PCa cells in vitro and also supressed tumor
growth in vivo. Statistical analysis demonstrated that a
high expression of SOX18 was correlated with the poor clinical
features of patients with PCa. Markedly, knockdown of SOX18 in PC3
cells induced decreased expression of TCF1, c-Myc, cyclin D1 and
MMP-7, which establishes it as an oncogene.
Materials and methods
Tissue microarray
A tissue microarray with 179 spots of human PCa and
adjacent normal tissues, or benign hyperplasia (BPH) tissues (98
PCa and 81 normal/BPH tissues) was obtained from Shanghai Outdo
Biotech (Shanghai, China).
Cell lines, siRNAs and lentivirus
Three PCa cell lines PC3, DU145 and LNcaP were
directly obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA) for no more than 6 months. Cells were cultured
in RPMI-1640 (HyClone, Logan, UT, USA) medium supplemented with 10%
fetal bovine serum (FBS) (Gibco, Sydney, Australia) and 1%
penicillin/streptomycin (Gibco, Grand Island, NY, USA), and
incubated at 37°C in a humidified atmosphere with 5%
CO2.
Small interfering RNAs (siRNA) targeting SOX18 were
synthesized by GenePharma (Suzhou, China). The following sequences
were used: siRNA1, 5′-GGGUUACAUUUUUGAAGCATT-3′ (sense) and
5′-UGCUUCAAAAAUGUAACCCTT-3′ (antisense); siRNA2,
5′-CUCUCUCAUACGCGUGUAUTT-3′ (sense) and 5′-AUACACGCGUAUGAGAGAGTT-3′
(antisense); and negative control (siNC)
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′
(antisense). The siRNAs were transfected into PC3 and DU145 cells
using Lipofectamine 3000 Transfection kit (Invitrogen, Carlsbad,
CA, USA) according to the manufacturer's instructions. The
efficiency of SOX18 silencing was determined using western
blotting.
Lentiviruses carrying the silencing sequence
(LV-SOX18) and the negative control sequence (LV-NC) were packaged
by the Shanghai GeneChem Co., Ltd. (Shanghai, China). The silencing
sequence was the same as the siRNA2 and the control sequence as the
siNC. The PC3 and DU145 cell lines were infected with the
lentiviruses at a multiplicity of infection (MOI) of 100 for 24 h.
Then, puromycin (Calbiochem, La Jolla, CA, USA) was used to screen
the stably infected cells. The efficiency of infection was assessed
by western blotting.
RT-PCR
The preparation of total RNA and the performance of
RT-PCR were as previously described (21). The primers used were as follows:
SOX18 (forward, 5′-CGCGTGTATGTTTGGTTC-3′ and reverse,
5′-ATGTAACCCTGGCAACTC-3′); and GAPDH (forward,
5′-CACCCACTCCTCCACCTTTG-3′ and reverse,
5′-CCACCACCCTGTTGCTGTAG-3′).
Immunohistochemical analysis
After being dewaxed, rehydrated and blocked with 30%
normal goat serum for 30 min, the microarray was incubated with
rabbit monoclonal antibody against SOX18 (1:100, Abcam, Cambridge,
MA, USA) in a moist chamber at 4°C overnight. Immunodetection was
conducted using the Envision ABC kit (Gene Tech Co., Ltd. Shanghai,
China). After staining with hematoxylin, the microarray was
dehydrated and mounted. The intensity and extent of SOX18 staining
were evaluated by two experienced pathologists without the clinical
data, respectively. The method for calculating the score of SOX18
staining was as follows: the extent of staining in an ×200 field
was scored as 0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–100%. The intensity
of staining was scored as 0, no signal; 1, light brown; 2, brown;
3, dark brown. The final score of each field was the average
obtained from the two pathologists from multiplying the extent
score by the intent score. The scores of SOX18 staining were
categorized as follows: low expression (−/+), when scores were 0–1
(−) and 2–3 (+); high expression (++/+++), when scores were 4–6
(++) and 7–9 (+++). All evaluations were performed using a Leica
DM4000 M microscope.
Western blotting
The cells were lysed on ice in RIPA buffer
(Solarbio, Beijing, China) supplemented with a 1% protease
inhibitor cocktail (Thermo Scientific, Rockford, IL, USA). The
protein concentration was assessed using BCA protein assays
(Solarbio, Beijing, China). Equal amounts of proteins were
separated using 10% SDS-PAGE gels, and were then transferred onto
nitrocellulose membranes (Millipore, Bedford, MA, USA). The
membranes were blocked in rapid blocking liquid (Promoton,
Shanghai, China) for 10 min and incubated with a primary antibody
at 4°C overnight. After being incubated with the secondary antibody
for 1 h at room temperature, the immunoreactive bands were detected
by Chemiluminescent and Fluorescent Imaging System (Sagecreation,
Hangzhou, China). The primary antibodies were SOX18 rabbit
monoclonal antibody (1:1,000; Abcam), GAPDH rabbit monoclonal
antibody (1:3,000) and β-catenin/TCF1/c-Myc/cyclin
D1/c-Jun/MMP-7/MMP-2/MMP-9 rabbit monoclonal antibodies [1:1,000;
all from Cell Signaling Technology (CST), Inc., Danvers, MA, USA].
The secondary antibodies were goat anti-rabbit IgG/HRP (Bioss,
Beijing, China) and the intensity of the target proteins was
normalized to the intensity of GAPDH.
Cell Counting Kit-8 (CCK-8)
assays
After transfection for 24 h, 1,500 cells were seeded
into 96-well plates in 100 µl conditioned medium with 10% FBS. For
quantitation of cell proliferation, CCK-8 assays were performed.
Briefly, 10 µl of CCK-8 reagent (Dojindo, Kunamoto, Japan) was
added to each well and incubated at 37°C, and then the absorbance
of each well at 450 nm was assessed after 1.5 h. Each experiment
was performed in triplicate.
Cell migration and invasion
assays
Twenty-four hours after transfection with siRNAs,
the PC3 and DU145 cells were starved for 6 h. Then,
5×104 cells in 200 µl serum-free media from each group
were added into the upper chamber of a 24-well Transwell or
invasion chamber (Corning, Corning, New York, USA) with a
polycarbonate filter (8-µm pore size). The bottom chamber contained
the conditioned medium with 10% FBS. After a 24-h incubation, the
non-migrated or non-invaded cells in the upper chamber were scraped
off using a cotton swab, and the migrated or invaded cells on the
bottom were fixed with methanol and stained with hematoxylin. The
number of cells was counted in five randomly chosen fields
(magnification, ×100). Each experiment was performed in triplicate,
and the results were obtained from three individual
experiments.
Tumor xenograft model in nude
mice
All protocols for the animal experiments were
approved by the Peking University Institutional Animal Care and Use
Committee. Twenty-four female BALB/c nude mice (4–6 weeks old,
weighing 18–22 g) were obtained from Peking University Animal
Center, and were randomly divided into 4 groups for tumor
xenografts as LV-SOX18 PC3, LV-NC PC3, LV-SOX18 DU145 and LV-NC
DU145 groups. Cells (5×106) in 80 µl PBS were
subcutaneously injected into the left and right flanks of nude mice
for each time-point (day 0). The mice were raised in a germ-free
environment in the animal facility. The tumor diameter was measured
every 5 days, and the tumor volume was calculated by: length ×
width2 × 0.5. After 5 weeks, the mice were sacrificed by
carbon dioxide narcosis (day 35), and then the tumors were
measured, weighed and photographed.
Statistical analyses
Data are expressed as the mean ± SEM. Statistical
analysis was performed using a rank-sum test and a Student's
t-test. Values of P<0.05 were considered as statistically
significant differences. All statistical evaluations were carried
out by SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
SOX18 is overexpressed in PCa
tissues
To investigate the expression of SOX18 in PCa and
adjacent non-tumor tissues, we measured the SOX18 protein level in
a tissue microarray by immunohistochemistry. Compared to the
adjacent non-tumor tissues (28/81, 34.6%), high expression of SOX18
was found in 72 of the 98 (73.5%) PCa tissues, and was frequently
located in the nuclei of the cells (Fig. 1A). SOX18 was also confirmed in
prostate cancer cell lines by western blotting and RT-PCR analyses
(Fig. 1B and C).
Expression of SOX18 is significantly
correlated with the clinical features of the patients with PCa
According to the results obtained from
immunohistochemistry, we analyzed whether the expression of SOX18
was correlated with the clinical features of the PCa cases. The
rate of high SOX18 staining in the tissues with histological grades
III–IV (25/30, 83.3%) was higher than that in the tissues of grades
I–II (47/68, 69.1%). The difference between the cases with a high
and low histological grade was significant (P<0.05). When
comparing the positive frequency between a higher Gleason (≥8) and
lower scores (≤7), we found that the high SOX18 expression rate of
cases with a higher Gleason score was 88.2% (30/34), which had a
higher frequency than cases with a lower score (65.6%, 42/64)
(P<0.05). More importantly, expression of SOX18 was
significantly related to the clinical stage of PCa. A strong
positive rate was observed in 77.4% (24/31) of the tissues with
stages III–IV, while the strong positive rate was 71.6% (48/67) in
tissues with stages I–II (P<0.05). However, no relationship
between SOX18 expression and age was observed (P=0.762) (Table I).
 | Table I.Clinicopathological variables and
evaluation of SOX18 immunostaining in prostate cancer tissues. |
Table I.
Clinicopathological variables and
evaluation of SOX18 immunostaining in prostate cancer tissues.
|
|
| Scores for SOX18
staining |
|
|---|
|
|
|
|
|
|---|
| Classification | No. of pts. | (−) | (+) | (++) | (+++) | P-value |
|---|
| Type |
|
|
|
|
| 0.000 |
|
Non-tumor | 81 | 20 | 33 | 24 | 4 |
|
|
PCa | 98 | 9 | 17 | 40 | 32 |
|
| Age (years) |
|
|
|
|
| 0.762 |
|
>70 | 58 | 5 | 12 | 22 | 19 |
|
|
≤70 | 40 | 4 | 5 | 18 | 13 |
|
| Clinical stage |
|
|
|
|
| 0.048 |
|
I–II | 67 | 6 | 13 | 32 | 16 |
|
|
III–IV | 31 | 3 | 4 | 8 | 16 |
|
| Histological
grade |
|
|
|
|
| 0.010 |
|
I–II | 68 | 7 | 14 | 31 | 16 |
|
|
III–IV | 30 | 2 | 3 | 9 | 16 |
|
| Gleason score |
|
|
|
|
| 0.005 |
| ≤7 | 64 | 6 | 16 | 27 | 15 |
|
| ≥8 | 34 | 3 | 1 | 13 | 17 |
|
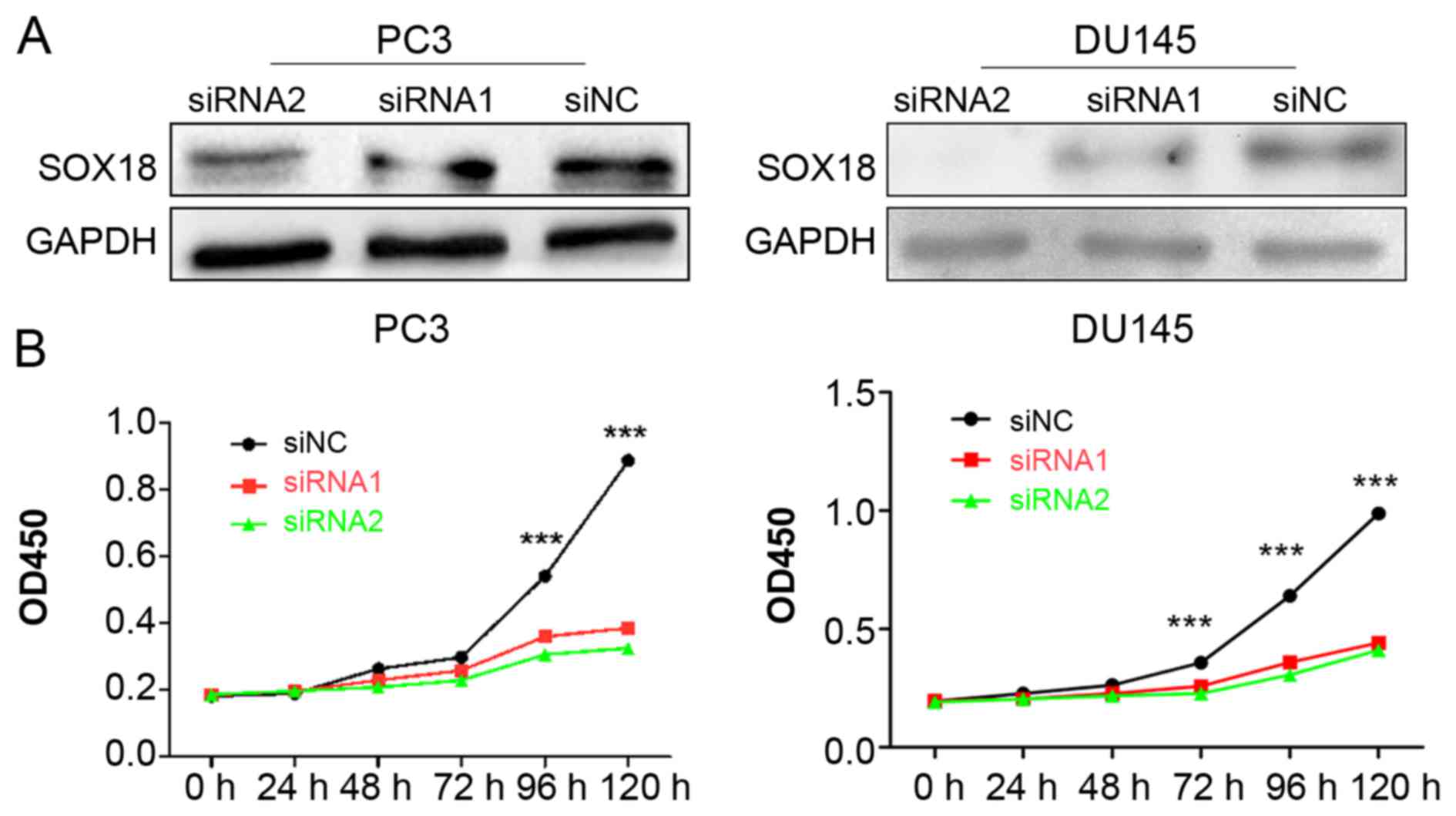
Knockdown of SOX18 significantly
impacts the proliferation of both PC3 and DU145 cells
To explore the potential role of SOX18 in PCa cell
proliferation, we knocked down the expression of SOX18 in both PC3
and DU145 cell lines using two siRNAs, and analyzed the cell
proliferation ability using CCK-8 assays. The silencing efficiency
of SOX18 in PCa cell lines was confirmed by western blotting
(Fig. 2A). The results revealed
that the cell proliferation of both cell lines transfected with
siRNAs was significantly impaired compared with those transfected
with siNC (Fig. 2B).
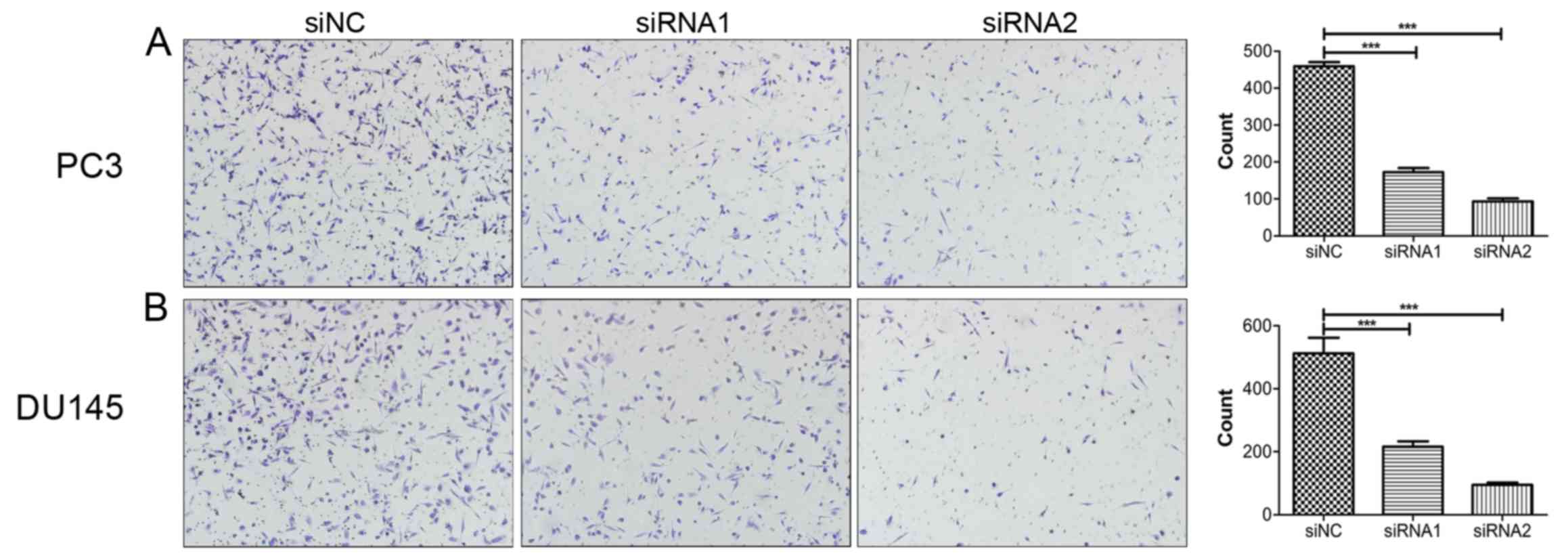
Knockdown of SOX18 notably suppresses
the migration of PCa cells
To investigate the impact of SOX18 on PCa cell
migration, Transwell assays were conducted with the same number of
PC3 and DU145 cells transfected with siRNAs. Compared with their
respective controls, knockdown of SOX18 notably suppressed the
number of cells that crossed over the filter (Fig. 3).
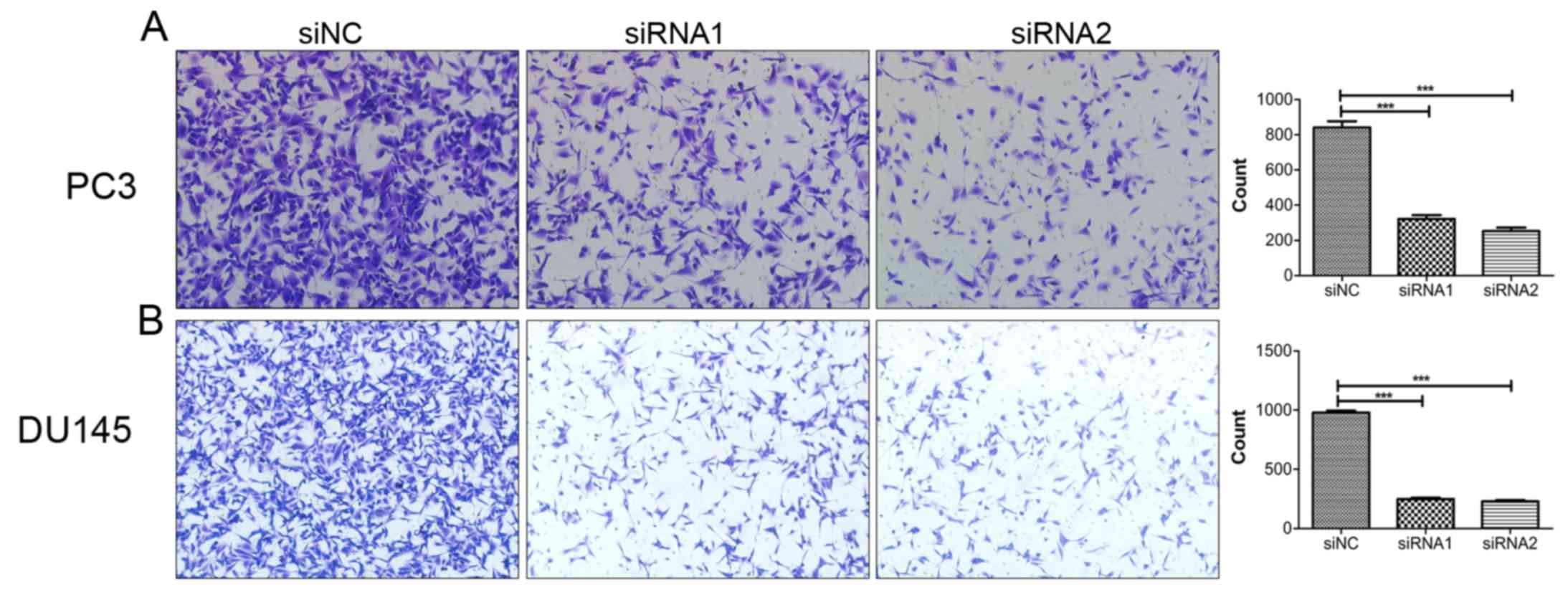
Downregulation of SOX18 reduces the
invasion ability of PCa cells
After establishing the role of SOX18 in the
migration of PCa cells, we aimed to explore whether SOX18 is
involved in the invasion of PCa cells. Therefore, we performed
invasion assays using the same number of PC3 and DU145 cells
transfected with siRNAs and siNC. As a result, downregulation of
SOX18 significantly decreased the number of invaded cells compared
with the controls (Fig. 4).
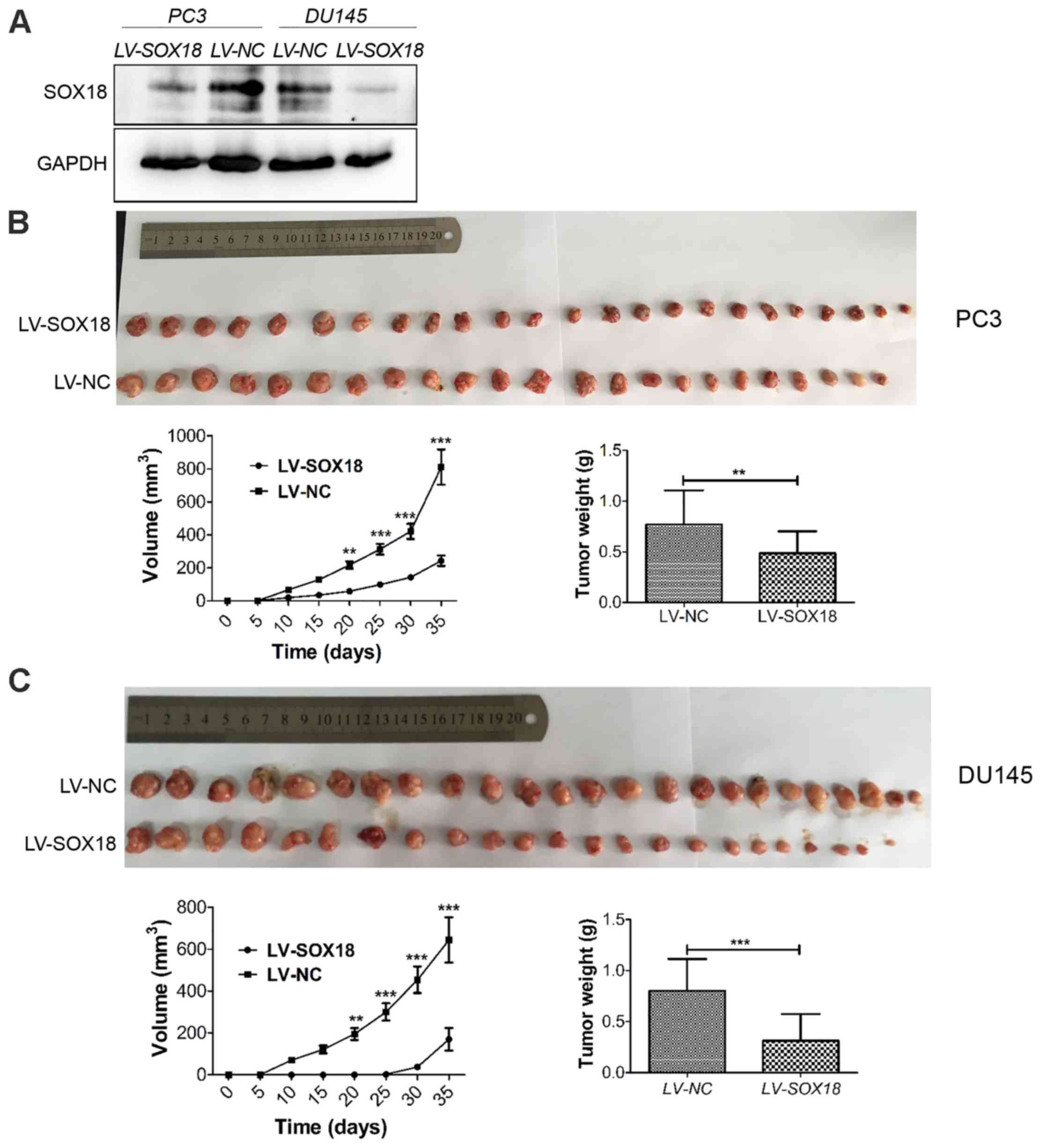
Knockdown of SOX18 suppresses tumor
growth in nude mice
After the demonstration of the proliferation
inhibition of SOX18 silencing in vitro, we subsequently used
xenograft models in nude mice to investigate whether SOX18
silencing inhibited tumor growth in vivo. The same number of
PC3 and DU145 cells transfected with LV-SOX18 or LV-NC were
injected into each group of nude mice. The efficiency of SOX18
silencing was detected by western blotting (Fig. 5A). The tumor volume and weight in
mice receiving cells transfected with LV-SOX18 were significantly
decreased than those transfected with LV-NC (Fig. 5B and C).
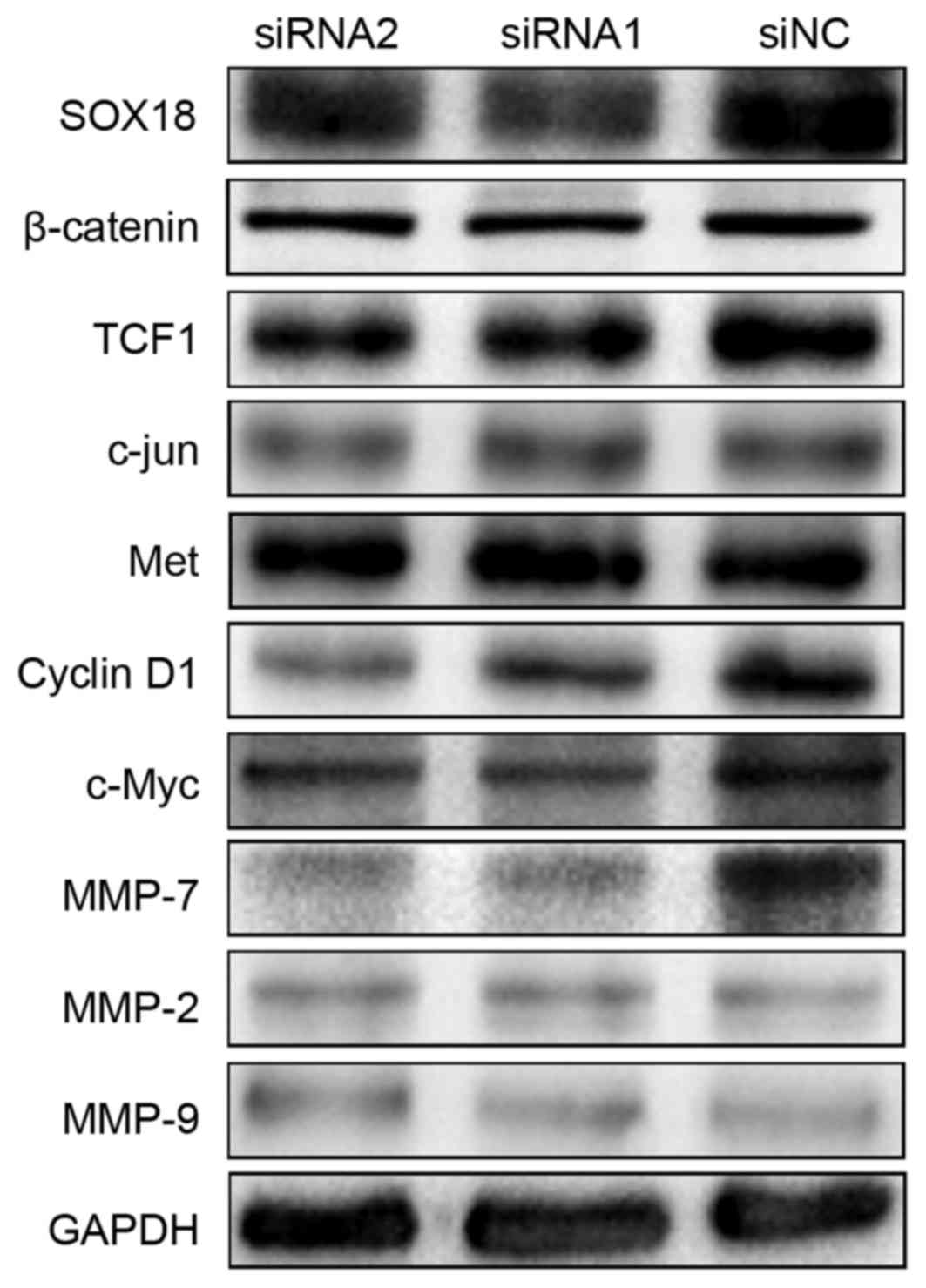
SOX18 regulates the expression of
TCF1, c-Myc, cyclin D1 and MMP-7
To explore the molecular mechanisms of SOX18
underlying the proliferation, migration and invasion in PCa cells,
we first assessed using western blotting several proteins that may
be involved in the progression of PCa in PC3 cells after a 48-h
transfection with siRNAs and siNC. There were notably decreased
protein levels of TCF1, c-Myc, cyclin D1 and MMP-7 in the SOX18
silenced cells compared with the controls. Considering these
signals are involved in the Wnt signaling pathway, we detected
several other Wnt family members (including β-catenin, c-Jun and
Met), but found no differences. In addition, we detected two other
members of the MMP family (MMP-2 and MMP-9), but also found no
differences (Fig. 6).
Discussion
Recently, a growing number of studies suggest that
SOX18 is overexpressed in various types of tumors, and plays an
important role in tumor occurrence and progression (13). The role and the potential function
of SOX18 in various types of cancer have been revealed. Since the
SOX18 gene behaves as an oncogene in various types of human
cancer, its targeting has great therapeutic potential. In the
present study, we found that SOX18 was significantly overexpressed
in prostate cancer (PCa) tissues using immunohistochemistry, which
was further confirmed with the expression of SOX18 at both the mRNA
and protein level by western blotting and RT-PCR. A high expression
of SOX18 was related to poor clinical characteristics (including a
higher Gleason score, a higher histological grade and an advanced
clinical stage) of patients with PCa. In addition, SOX18 was
confirmed to be involved in the proliferation, migration and
invasion of PCa cell lines in vitro and involved in tumor
growth in vivo. As for the mechanisms of SOX18 underlying
the progression of PCa, we found that TCF1, c-Myc, cyclin D1 and
MMP-7 were decreased when SOX18 was silenced by siRNAs in the PC3
cell line. Our data indicated that SOX18 may be of diagnostic and
therapeutic value for PCa.
Previous studies demonstrated that SOX18 promoted
the cell proliferation of HepG2 hepatocellular carcinoma (HCC) and
MCF-7 breast cancer cells (15,17).
Consistent with these findings, knockdown of SOX18 in PCa cell
lines notably suppressed cell growth in vitro. Moreover, the
role of SOX18 silencing in the suppression of cell proliferation
was also confirmed in vivo in the present study. Notably,
in vivo, the tumor size was almost equal to that of the
control group, but inside the tumor, the tumor was full of the
hydrops, which indicated that knockdown of SOX18 promoted apoptosis
in the PCa cells (data not shown). In a study of SOX18 in HCC,
SOX18 knockdown was found to induce G1 phase arrest and apoptosis
of HCC cells, indicating that SOX18 may contribute to cell
proliferation via the promotion of cell cycle progression from the
G1 to the S phase and the suppression of apoptosis. In the present
study, we discovered that cyclin D1 was reduced in the
SOX18-knockdown cells at the protein level. Cyclin D1, a
transcription factor, is associated with cell proliferation via the
promotion of the cell cycle from G1 to the S transition (22,23).
Previous studies found that cyclin D1 is overexpressed and
contributes to the androgen-dependent DNA damage repair in PCa
cells (24). Although we had a lack
of data from the trials to address the changes in the PCa cell
cycle after SOX18 silencing, the decreased level of cyclin D1
expression caused by SOX18 silencing indicated that SOX18 may
promote cell cycle transition from the G1 to the S phase via the
regulation of the expression of cyclin D1 to accelerate the
aggressiveness of PCa.
Matrix metalloproteinases (MMPs), a family of
transcription factors, can regulate the tumor microenvironment
mainly through the degradation of the extracelluar matrix, and were
found to be increased in expression and activation in almost all
human types of cancer including PCa compared with normal tissues.
The upregulation of MMPs was found to be related to the enhanced
invasion ability of PCa cells in vitro (25). Consistent with previous research,
our data showed that SOX18 silencing also impaired the migration
and invasion abilities of PCa cells. Markedly, we found a decreased
protein level of MMP-7 in the SOX18-silenced PCa cells. Grindel
et al reported that MMP-7 acts as a switch altering PCa cell
behavior and favoring cell dispersion and invasiveness (26). MMP-2 and MMP-9 are also involved in
the mobility of PCa (27), but our
results showed no differences when comparing the siRNA and siNC
groups. Hoeth et al reported that SOX18 regulated the
expression of MMP-7 in human endothelial cells by directly
combining to the promoter of MMP-7 and activating its transcription
(19). Although our results did not
confirm whether SOX18 could combine to the promoter of MMP-7, we
did demonstrate that SOX18 may regulate the mobility of PC3 cells
via the regulation of MMP-7, but not that of MMP-2 or MMP-9.
Whether or not the process of SOX18 regulation of MMP-7 in PCa is
roughly analogous to that in endothelial cells needs further
study.
However, cyclin D1, MMP-7, TCF1 and c-Myc also
exhibited a decreased protein level in the SOX18-silencing cells.
TCF1 and c-Myc are also transcriptional factors, which are involved
in tumor progression (28). Cyclin
D1, MMP-7, TCF1 and c-Myc were also found to be involved in the Wnt
signaling pathway, which is an important pathway in tumorigenesis
and tumor progression (29), and
TCF1 is located in the upstream of c-Myc, cyclin D1 and MMP-7. We
detected other Wnt members, but found no differences among the
siRNA and siNC groups. Previous research has reported that SOX7
decreases the expression of c-Myc and cyclin D1 via the
downregulation of Wnt/β-catenin transcription through the HMG-box
which is the common domain of all SOX family members (30). Whether a simple link between SOX18
and the Wnt signaling pathway exists warrants further study.
The research on transcription factors and signaling
pathways related with cancer has gradually become a ‘hot spot’ in
the field of cancer research. Controlling the expression levels of
certain transcription factors or some key points in signaling
pathways to regulate the epofenetic characteristics of cells are
promising therapeutic approaches. Transcription factor SOX18 is
overexpressed in PCa, and the expression of SOX18 is notably
correlated with both the clinical characteristics of patients and
the malignant biological behavior of PCa cells. SOX18 may promote
PCa progression via the upregulation of various transcription
factors, such as TCF1, c-Myc, cyclin D1 and MMP-7. Therefore,
further research on SOX18 is of potential value for the early
diagnosis, risk evaluation and therapeutic approaches of PCa.
Acknowledgements
We thank Professor Gongwei Wang and Chenglong Zhao
(Department of Pathology, Peking University People's Hospital) for
their technological support in the immunohistochemistry and
staining evaluation.
Glossary
Abbreviations
Abbreviations:
|
SOX18
|
sex determining region Y (SRY)-box
18
|
|
MMP-7
|
matrix metalloproteinase-7
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cuzick J, Thorat MA, Andriole G, Brawley
OW, Brown PH, Culig Z, Eeles RA, Ford LG, Hamdy FC, Holmberg L, et
al: Prevention and early detection of prostate cancer. Lancet
Oncol. 15:e484–e492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: European Association of Urology: EAU guidelines
on prostate cancer. Part 1: Screening, diagnosis, and local
treatment with curative intent-update 2013. Eur Urol. 65:124–137.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarkar A and Hochedlinger K: The sox
family of transcription factors: Versatile regulators of stem and
progenitor cell fate. Cell Stem Cell. 12:15–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
She ZY and Yang WX: SOX family
transcription factors involved in diverse cellular events during
development. Eur J Cell Biol. 94:547–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castillo SD and Sanchez-Cespedes M: The
SOX family of genes in cancer development: Biological relevance and
opportunities for therapy. Expert Opin Ther Targets. 16:903–919.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thu KL, Becker-Santos DD, Radulovich N,
Pikor LA, Lam WL and Tsao MS: SOX15 and other SOX family members
are important mediators of tumorigenesis in multiple cancer types.
Oncoscience. 1:326–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai W, Tan X, Sun C and Zhou Q: High
expression of SOX2 is associated with poor prognosis in patients
with salivary gland adenoid cystic carcinoma. Int J Mol Sci.
15:8393–8406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao W, Chen H and He J: The role of SOX-2
on the survival of patients with non-small cell lung cancer. J
Thorac Dis. 7:1113–1118. 2015.PubMed/NCBI
|
|
10
|
Zhou D, Bai F, Zhang X, Hu M, Zhao G, Zhao
Z and Liu R: SOX10 is a novel oncogene in hepatocellular carcinoma
through Wnt/β-catenin/TCF4 cascade. Tumour Biol. 35:9935–9940.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
François M, Caprini A, Hosking B, Orsenigo
F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M,
et al: Sox18 induces development of the lymphatic vasculature in
mice. Nature. 456:643–647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duong T, Koltowska K, Pichol-Thievend C,
Le Guen L, Fontaine F, Smith KA, Truong V, Skoczylas R, Stacker SA,
Achen MG, et al: VEGFD regulates blood vascular development by
modulating SOX18 activity. Blood. 123:1102–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wünnemann F, Kokta V, Leclerc S, Thibeault
M, McCuaig C, Hatami A, Stheneur C, Grenier JC, Awadalla P,
Mitchell GA, et al: Aortic dilatation associated with a de novo
mutation in the SOX18 gene: Expanding the clinical spectrum of
hypotrichosis-lymphedema-telangiectasia syndrome. Can J Cardiol.
32:135.e1–135.e7. 2016. View Article : Google Scholar
|
|
14
|
Saitoh T and Katoh M: Expression of human
SOX18 in normal tissues and tumors. Int J Mol Med. 10:339–344.
2002.PubMed/NCBI
|
|
15
|
Wang G, Wei Z, Jia H, Zhao W, Yang G and
Zhao H: Knockdown of SOX18 inhibits the proliferation, migration
and invasion of hepatocellular carcinoma cells. Oncol Rep.
34:1121–1128. 2015.PubMed/NCBI
|
|
16
|
Pula B, Kobierzycki C, Solinski D,
Olbromski M, Nowak-Markwitz E, Spaczynski M, Kedzia W, Zabel M and
Dziegiel P: SOX18 expression predicts response to platinum-based
chemotherapy in ovarian cancer. Anticancer Res. 34:4029–4037.
2014.PubMed/NCBI
|
|
17
|
Pula B, Olbromski M, Wojnar A,
Gomulkiewicz A, Witkiewicz W, Ugorski M, Dziegiel P and
Podhorska-Okolow M: Impact of SOX18 expression in cancer cells and
vessels on the outcome of invasive ductal breast carcinoma. Cell
Oncol. 36:469–483. 2013. View Article : Google Scholar
|
|
18
|
Petrovic I, Milivojevic M, Popovic J,
Schwirtlich M, Rankovic B and Stevanovic M: SOX18 is a novel target
gene of Hedgehog signaling in cervical carcinoma cell lines. PLoS
One. 10:e01435912015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoeth M, Niederleithner H, Hofer-Warbinek
R, Bilban M, Mayer H, Resch U, Lemberger C, Wagner O, Hofer E,
Petzelbauer P, et al: The transcription factor SOX18 regulates the
expression of matrix metalloproteinase 7 and guidance molecules in
human endothelial cells. PLoS One. 7:e309822012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fontijn RD, Volger OL, Fledderus JO,
Reijerkerk A, de Vries HE and Horrevoets AJ: SOX-18 controls
endothelial-specific claudin-5 gene expression and barrier
function. Am J Physiol Heart Circ Physiol. 294:H891–H900. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng Z, Liu Y, Qin C, Liu Z, Yuan Y, Hu
F, Du Y, Yin H, Qiu X and Xu T: IgG is involved in the migration
and invasion of clear cell renal cell carcinoma. J Clin Pathol.
69:497–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tu K, Liu Z, Yao B, Xue Y, Xu M, Dou C,
Yin G and Wang J: BCL-3 promotes the tumor growth of hepatocellular
carcinoma by regulating cell proliferation and the cell cycle
through cyclin D1. Oncol Rep. 35:2382–2390. 2016.PubMed/NCBI
|
|
23
|
Lee HR, Mitra J, Lee S, Gao SJ, Oh TK, Kim
MH, Ha T and Jung JU: Kaposi's sarcoma-associated herpesvirus viral
interferon regulatory factor 4 (vIRF4) perturbs the G1-S
cell cycle progression via deregulation of the cyclin D1 gene. J
Virol. 90:1139–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casimiro MC, Di Sante G, Ju X, Li Z, Chen
K, Crosariol M, Yaman I, Gormley M, Meng H, Lisanti MP, et al:
Cyclin D1 promotes androgen-dependent DNA damage repair in prostate
cancer cells. Cancer Res. 76:329–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Gong LH, Zhang HQ, Du Q, You JF,
Tian XX and Fang WG: Extracellular ATP enhances in vitro invasion
of prostate cancer cells by activating Rho GTPase and upregulating
MMPs expression. Cancer Lett. 293:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grindel BJ, Martinez JR, Pennington CL,
Muldoon M, Stave J, Chung LW and Farach-Carson MC:
Matrilysin/matrix metalloproteinase-7 (MMP7) cleavage of
perlecan/HSPG2 creates a molecular switch to alter prostate cancer
cell behavior. Matrix Biol. 36:64–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kato T, Fujita Y, Nakane K, Mizutani K,
Terazawa R, Ehara H, Kanimoto Y, Kojima T, Nozawa Y, Deguchi T, et
al: CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3
prostate cancer cells by increasing secretion of MMPs 2/9 and by
activating ERK and Rac signaling. Cytokine. 64:251–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan L, Peng G, Sahgal N, Fazli L, Gleave
M, Zhang Y, Hussain A and Qi J: Regulation of c-Myc expression by
the histone demethylase JMJD1A is essential for prostate cancer
cell growth and survival. Oncogene. 35:2441–2452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohammed MK, Shao C, Wang J, Wei Q, Wang
X, Collier Z, Tang S, Liu H, Zhang F, Huang J, et al: Wnt/β-catenin
signaling plays an ever-expanding role in stem cell self-renewal,
tumorigenesis and cancer chemoresistance. Genes Dis. 3:11–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao T, Yang H, Tian Y, Xie Q, Lu Y, Wang
Y, Su N, Dong B, Liu X, Wang C, et al: SOX7 is associated with the
suppression of human glioma by HMG-box dependent regulation of
Wnt/β-catenin signaling. Cancer Lett. 375:100–107. 2016. View Article : Google Scholar : PubMed/NCBI
|