Introduction
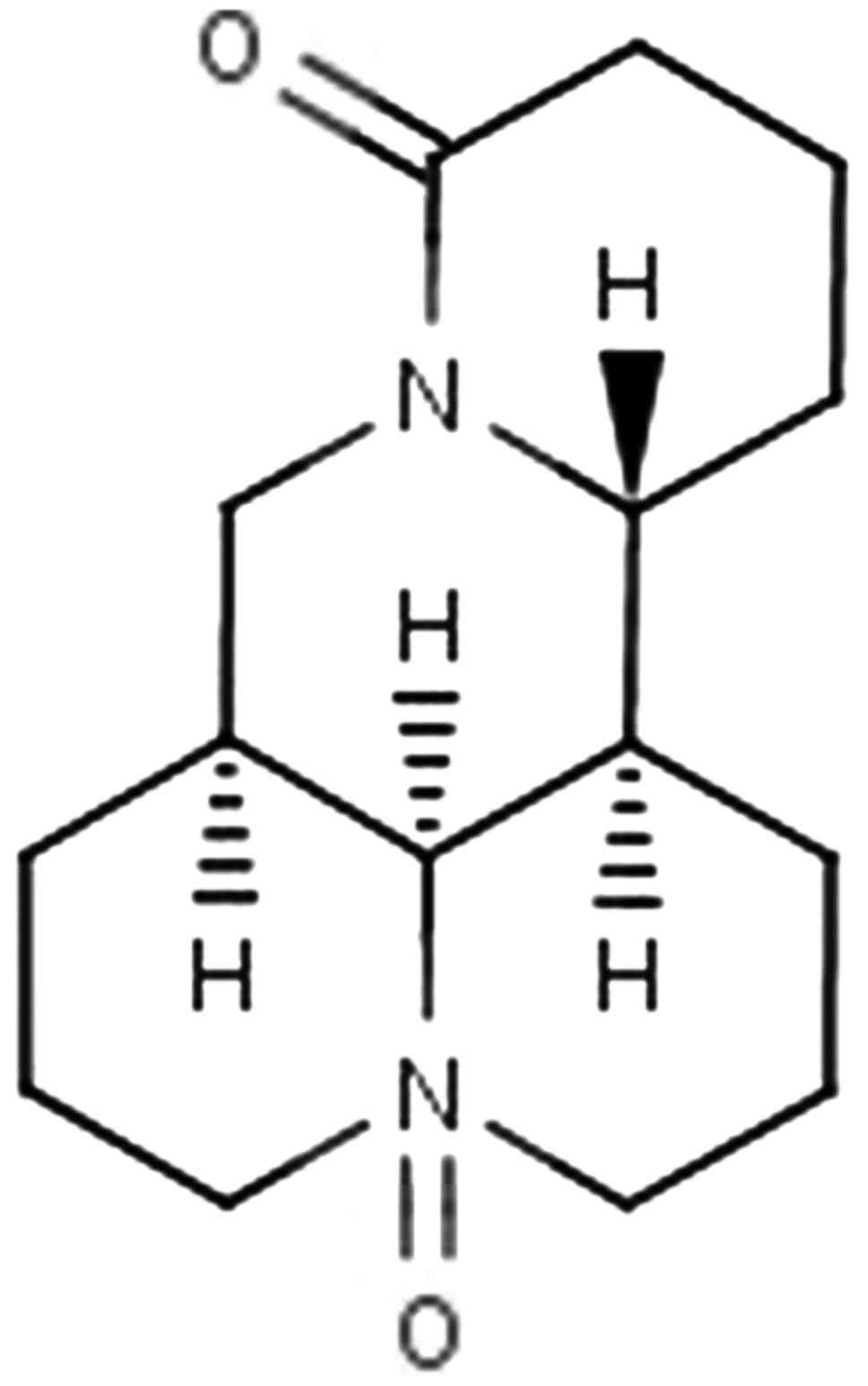
Oxymatrine (OM) (structure shown in Fig. 1) is the principal component of
Sophora flavescens Ait, which is frequently prescribed in
traditional Chinese medicine. OM has been reported to have immune
regulation, anti-inflammatory and diuretic effects (1–6).
Moreover, OM inhibits the growth of various types of cancer cells
(7–9). Although there are some studies in
regards to OM concerning the inhibition of malignant tumor cell
growth, studies concerning the molecular mechanisms, signaling
pathways, invasion and metastasis of the anticancer effects of OM
are rare.
Colorectal carcinoma is one of the most common
malignant tumors (10). Invasion
and metastasis are fundamental properties of malignant colon
cancer, which lead to a high recurrence rate after surgery and
therapeutic approaches (11). In
the process of tumor metastasis, epithelial-mesenchymal transition
(EMT) is a key mediator (12). The
molecular mechanisms of EMT involved in tumor metastasis remain
unclear, and current treatments have limited effectiveness. Thus,
in order to develop new effective therapeutic measures for colon
cancer, further investigation of its molecular mechanisms is
required.
Plasminogen activator inhibitor 1 (PAI-1) is a rapid
and specific inhibitor of the plasminogen/plasmin system (13). In recent years, studies have
demonstrated that PAI-1 is a potent regulator of tumor growth in
vivo. Increased PAI-1 has been confirmed in many solid tumor
types and was found to be associated with a poor prognosis
(14–16). Thus, PAI-1 is regarded as a
biochemical marker for poor prognosis and may serve as a
therapeutic target for various types of cancers. There is evidence
that plasma PAI-1 is closely correlated with rectal cancer
metastasis, and tumor tissue PAI-1 is associated with the
histopathology and outcome of rectal cancer (17). Whether PAI-1 may serve as a target
in the antitumor therapy of colorectal carcinoma remains
unclear.
EMT is a process by which epithelial cells lose
their orientation and cell-cell contact, and acquire migratory and
invasive properties of mesenchymal cells. Transforming growth
factor-β1 (TGF-β1) is known as a key mediator of EMT (18). TGF-β1 promotes EMT via upregulation
of ECM expression and downregulation of transcriptional activity of
matrix-degrading enzyme genes. Increasing studies have shown that
SBE (Smad binding element) exists in the promoter region of genes,
such as PAI-1, regulated by TGF-β (19). SBE in the promoter region of these
genes combines directly or indirectly with the Smad complex. PAI-1
is one of the most important target genes in the TGF-β/Smad
signaling pathway, which can hinder the degradation of ECM
composition and may promote cell invasion and migration (20). It is unclear how TGF-β/Smad controls
PAI-1 in colorectal cancer and the relation between PAI-1 and
colorectal cancer.
In the present study, we investigated the
anti-metastatic and anti-invasive effects of OM in RKO cells.
Simultaneously we detected the effects of OM on the TGF-β1/Smad
signaling pathway and PAI-1 in RKO cells to investigate the
underlying signaling molecular mechanisms by which OM inhibits the
invasion and metastasis of RKO cells.
Materials and methods
Reagents and medicine
OM was obtained from National Institutes for Food
and Drug Control (Beijing, China). The relative molecular mass of
OM is 264.367, and the molecular structural formula is
C15H24N2O2. The purity
of OM was determined by high-performance liquid chromatography
(HPLC) as 98%. OM, a colorless columnar crystal, was dissolved in
PBS and prepared for 20 mg/ml mother solution. The solution was
stored at −20°C in a refrigerator and stored away from light.
Serum-free and antibiotic-free RPMI-1640 medium was diluted to the
required concentration. OM was diluted to the required
concentration by serum-free and antibiotic-free RPMI-1640 medium
for use.
TRIzol reagent (Invitrogen, Carlsbad, CA, USA),
GoScript™ Reverse Transcription system kit (Promega, Madison, WI,
USA), Matrigel (BD Biosciences Inc., Franklin Lakes, NJ, USA), Cell
Counting Kit-8 (ATCC Biosciences Center, USA), RIPA buffer (Kaiji
Biotechnology, Shanghai, China), PVDF membranes (Millipore,
Billerica, MA, USA) primary E-cadherin antibody (rabbit, 1:400,
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), α-SMA
antibody (mouse, 1:400, Sigma-Aldrich, St. Louis, MO, USA), FN
antibody (goat, 1:150, Santa Cruz Biotechnology, Inc.), TGF-β1
antibody (mouse, 1:200, Santa Cruz Biotechnology, Inc.), anti-Smad4
antibody (rabbit, 1:1000, Atlas, Sweden), anti-PAI-1 antibody
(rabbit, 1:1000, American Research Products Inc., Grandville, MI,
USA), phospho-p38 (pP38) MAPK (Tyr182) antibody (rabbit, 1:1000,
Affinity Biosciences, Cincinnati, OH, USA), β-actin antibody
(mouse, 1:400, Santa Cruz Biotechnology, Inc.), phospho-Smad2
(pSmad2) antibody (Ser465/467) (rabbit, 1:150, Cell Signaling
Technology, Inc., Danvers, MA, USA), and horseradish
peroxidase-conjugated secondary antibodies (1:5000, Santa Cruz
Biotechnology, Inc.) were procured for the experiments. All
chemicals were purchased in the purest form available.
Cell culture
Colorectal carcinoma RKO cells, obtained from ATCC
Biosciences Center, were grown in RPMI-1640 medium supplemented
with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad,
CA, USA), 100 µg/ml streptomycin and 100 U/ml penicillin in a
humidified atmosphere containing 5% CO2 at 37°C. Cells
grew as a single cell layer attached to specially treated plastic
surfaces. The human fetal colon FHC cell line was cultured in basal
medium (cat. no. 30-2006) supplemented with 10 mM HEPES, 10 ng/ml
cholera toxin, 0.005 mg/ml insulin, 0.005 mg/ml transferrin, 100
ng/ml hydrocortisone and 10% fetal bovine serum. In order to
maintain the viability and active growth of adherent cells, it is
necessary to subculture them at regular intervals (2 or 3 times a
week). Cells during an exponential growth phase were used in the
experiments. However, in the invasion and migration experiments,
the cells were cultured in a serum-free medium. The cells were
randomly divided into three groups: FHC cell control group (FHC
group), untreated RKO cell group (UR group), and OM-treated RKO
cell group (OR group).
Cell Counting Kit-8 assay
The antiproliferative effects of different
concentrations of OM on the RKO cell line were detected by CCK-8
assay. Cells were seeded at a density of 1×104 per well
in 100 µl of RPMI-1640 medium with 10% FBS in 96-well plates. On
the next day, the medium was replaced with serum-free RPMI-1640
with OM (from 0–20 mg/ml) for the RKO cells. Each concentration was
used in 4 wells. After incubation for 24, 48, 72 h in a humidified
atmosphere containing 5% CO2 at 37°C, respectively, 10
µl CCK (5 mg/l)was added each well, and the plates were incubated
for an additional 4 h. Then the medium was discarded, and the
absorbance (A) of the CCK-8 solution was determined at 450 nm using
a multiwell spectrophotometer (Bio-Tek Instruments, XL-808,
Winooski, VT, USA). The proliferation rate of the cells was
calculated as follows: Proliferation rate (%) = A of the treated
wells/A of the control wells (OM 0 mg/ml) × 100. The assay was
performed in triplicate.
Wound healing assay
The cells were seeded into 6-well plates and when
the cells were grown to confluency, a wound was created by manually
scraping the cells with a pipette tip. Debris was removed from the
culture by washing it twice with PBS. Then the cells were incubated
with serum-free medium to exclude the effect of cell growth
facilitated by serum and the cells were then allowed to migrate
into the wounded area at 37°C. Images were immediately acquired at
0 and 24 h after wounding and measured using Image-Pro Plus 6.0
software. The cell migration rate (%) = (wound width at 0 h - wound
width at 24 h)/wound width at 0 h × 100. The assay was performed in
triplicate.
Western blot analysis
Cells were plated onto culture flasks at a density
of 2×105 cells/ml and cultured at 37°C in 5%
CO2. On the next day, different concentrations of OM (0,
0.25, 0.5, 1 and 2 mg/ml) were added. After 24 h, the levels of
proteins were quantified through western blot analysis. Proteins
were extracted by the addition of 200 µl of lysis buffer [50 mmol/l
Tris-HCl (pH 7.4), 5 mmol/l EDTA, 1% Triton X-100, 150 mmol/l NaCl,
5 mmol/l MgCl2, 2 mmol/l Na3VO4,
1X Complete™ protease inhibitor] to each well. The cell lysates
were incubated on ice for 30 min vortexing every 10 min, followed
by centrifugation at 12000 × g for 30 min at 4°C. An amount 40
µg/µl of protein of the cell lysate was mixed equally with 2X
electrophoresis buffer [50% glycerol, 25% mercaptoethanol, 10% SDS,
0.3 M Tris (pH 6.8), 0.025% bromphenol blue] and boiled for 10 min.
The samples (40 µg of protein) of total cell lysates were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 75
V (the voltage was changed to 100 V when the indicator reached 1.5
cm from the edge of the 10% separation gel) and electrophoretically
transferred onto a polyvinylidene difluoride membrane (Millipore)
in transfer buffer containing 25 mM Tris, 150 mM glycine and 20%
methanol. The membranes were blocked using 5% skim milk at 37°C for
2 h. The membranes were then incubated with primary antibodies for
16–18 h at 4°C. The membranes were washed in 1X TBST 3 times (10
min each time) at room temperature. The membranes were subsequently
probed with an anti-rabbit IgG antibody with the HRP-conjugated
secondary antibody (1:1000) for 2 h. Control blots were performed
using anti-actin antibodies. The membranes were washed in 1X TBST
for 3 times (10 min each time) at room temperature, and detection
was achieved by measuring the chemiluminescence of the blotting
agent after exposure of the filters to X-omat films. The densities
of the bands were quantified using a computerized densitometer
(ImageJ Launcher, Broken Symmetry Software).
Reverse transcription PCR
Different concentrations of OM (0, 0.25, 0.5, 1 and
2 mg/ml) were added into the cells, respectively. After 24 h, the
levels of mRNA were quantified through reverse transcription-PCR
(RT-PCR). RNA was isolated from the treated RKO cells using TRIzol
reagent. Following the manufacturer's protocol, cDNA was generated
from the total RNA using the GoScript™ Reverse Transcription system
kit. Subsequently, PCR was conducted using the following primer
sequences: sense primer: β-actin (used as the input control),
forward primer, 5′-ACCACCATGTACCCAGGCAT-3′ and reverse primer
5′-CCGGACTCATCGTACTCCTG-3′; TGF-β1 (400 bp NM 021578.2), forward
primer 5′-AAGGCTCGCCAGTCCCCCGA-3′ and reverse primer
5′-AGTGGGGGTCAGCAGCCGT-3′; PAI (1400 bp), forward primer
5′-CGGAGCACGGTCAAGCAAGTG-3′ and reverse primer
5′-GGTGAGGGCAGAGAGAGGCAC-3′. Reaction conditions consisted of: 95°C
for 5 min (initial denaturation), followed by 35 cycles of 95°C for
30 sec, 58°C for 60 sec and 72 °C for 60 sec. This was followed by
a final 8-min extension period at 72°C. The 5 µl amplified
fragments, to which 1 µl 6X loading buffer was added, were
visualized on 1.5% agarose gel electrophoresis at 120 V for 20 min.
The results were investigated under a transilluminator. The PCR
product was subjected to electrophoresis and the absorbance (A) was
analyzed by gel imaging and an analysis system. The relative mRNA
level (%) = A of the treated PCR product/A of the β-actin PCR
product.
Statistical analysis
All assays were performed in triplicate. Statistical
analyses were performed using the SPSS 19.0 software program (SPSS,
USA). All data are presented as the mean ± standard deviation (SD).
Statistical differences were determined by the Student's t-test.
Statistical significance of differences was accepted at
P<0.05.
Statement of ethics
The present study was approved by the Institutional
Review Board (CWO) of Liaoning University of Traditional Chinese
Medicine.
Results
Effects of oxymatrine on the
proliferation of RKO cells
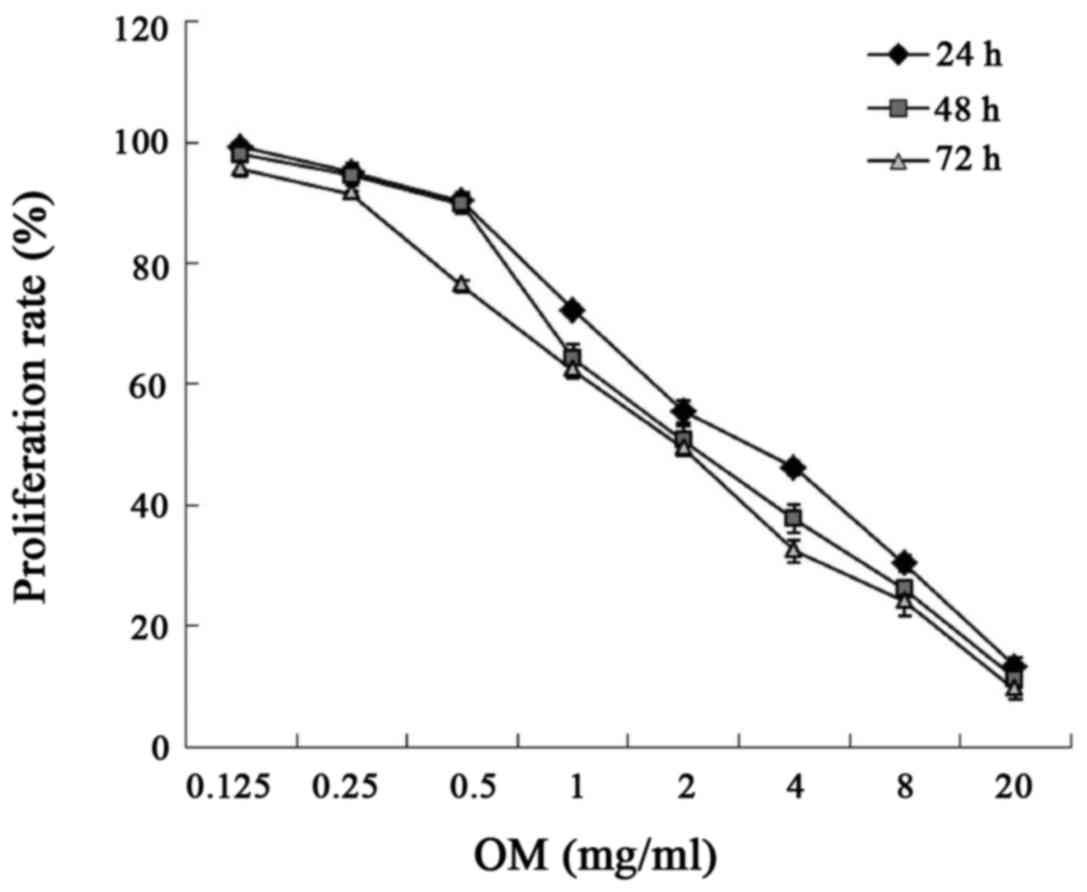
The cytotoxicity of OM was evaluated using the Cell
Counting Kit-8 assay. As illustrated in Fig. 2 and Table I, after RKO cells were treated with
different concentrations of OM for 24, 48 and 72 h, OM inhibited
the proliferation of the RKO cells in concentration-dependent and
time-dependent manner.
 | Table I.Effects of OM on the proliferation of
RKO cells. |
Table I.
Effects of OM on the proliferation of
RKO cells.
|
| Proliferation rate
(%) |
|---|
|
|
|
|---|
|
| 24 h | 48 h | 72 h |
|---|
| OM (mg/ml) |
|
|
|
| 0.125 | 99.209±0.492 | 97.944±0.955 | 95.598±1.129 |
| 0.25 | 95.089±1.384 | 94.621±1.737 | 91.490±0.462 |
| 0.5 | 90.261±1.331 |
89.766±1.599a |
76.267±0.987a |
| 1 |
72.223±0.990a |
64.231±2.406a |
62.221±1.361a |
| 2 |
55.446±1.842a |
50.662±2.393a |
49.359±0.309a |
| 4 |
46.207±1.098a |
37.748±2.385a |
32.431±1.866a |
| 8 |
0.320±1.329a |
26.146±1.293a |
24.051±2.362a |
| 20 |
30.366±1.520a |
11.265±1.341a |
9.469±1.550a |
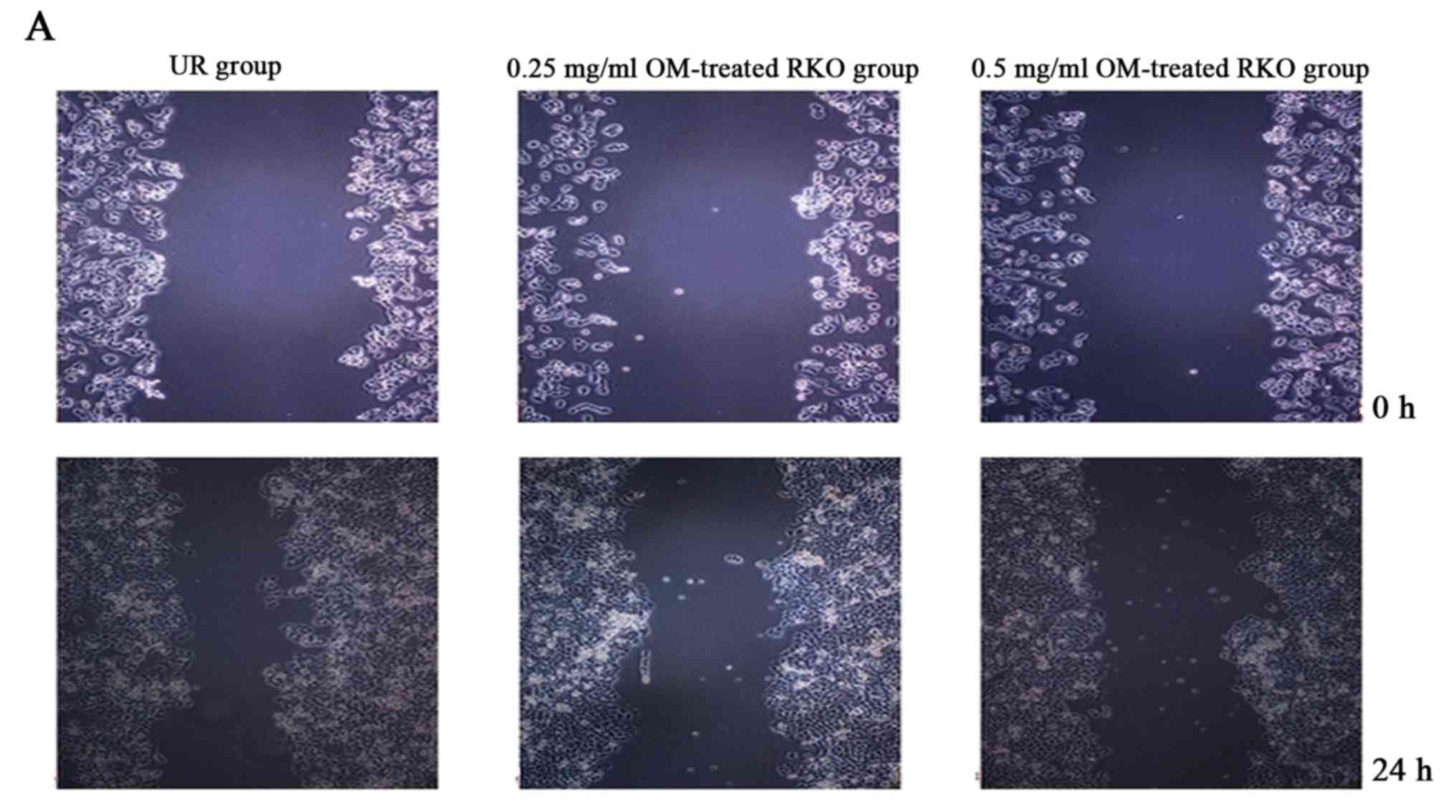
OM inhibits the migration of RKO
cells
OM was used to examine the effect on the migratory
ability of RKO cells by wound healing assay. As a result, treatment
of OM obviously reduced the migratory potential of the RKO cells
(Fig. 3). The treated cells
survived up to 2 days, indicating that the inhibitory changes were
not due to the cytotoxic effect of OM. These findings suggest a
critical role of OM in inhibiting the migration of RKO cells.
Effect of OM on the EMT of colorectal
cancer cells
To evaluate the effect of OM on RKO cells, the
results of the CCK-8 assay, in dose-dependent and time-dependent
trials, confirmed that a dose of 0.50 mg/ml for 24 h was unlikely
to exert a significant toxic effect on the cells. Therefore, 0.50
mg/ml OM was used in the subsequent tests. To determine whether OM
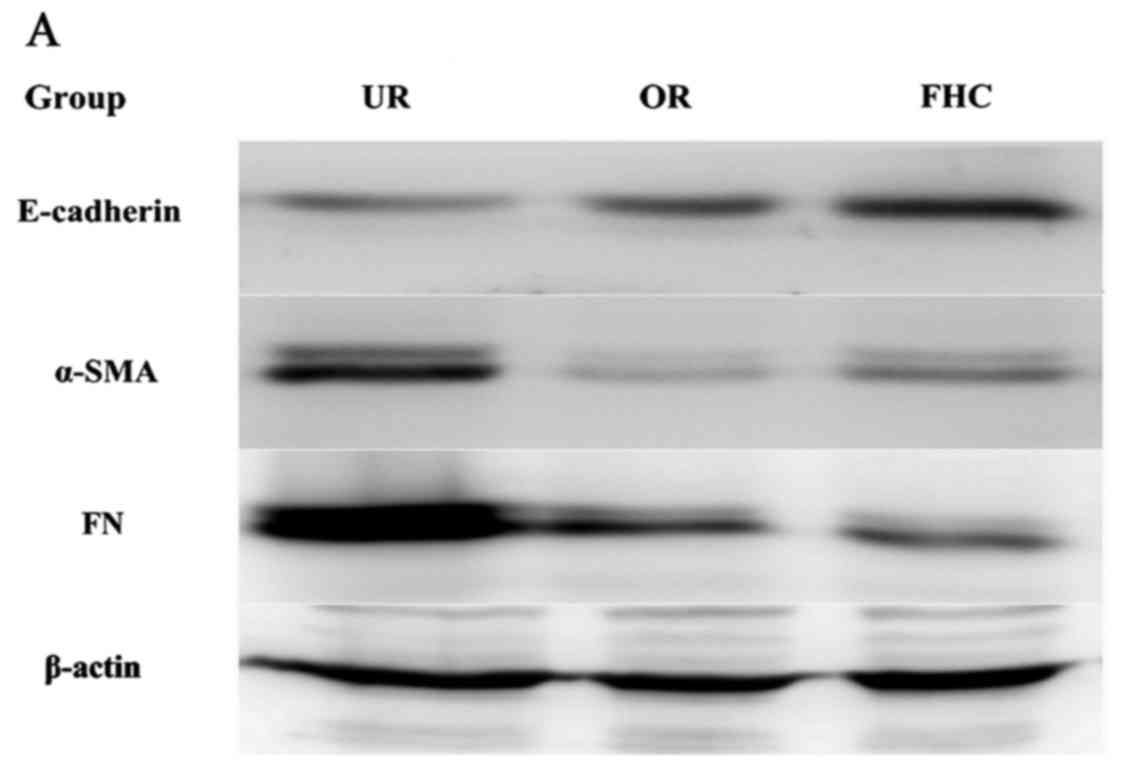
affected EMT, related marker expression levels of E-cadherin
(marker for epithelial cells) and α-SMA (marker for mesenchymal
cells) were examined by western blotting. The results showed that
E-cadherin was significantly reduced and α-SMA was markedly
increased in the RKO cells as compared to the FHC cells. After the
RKO cells were treated with 0.50 mg/ml OM, OM obviously upregulated
E-cadherin expression and downregulated α-SMA expression (Fig. 4). Taken together, these results
demonstrated that OM reversed the EMT process in the colorectal
cancer cells.
Effect of OM on fibronectin (FN) in
colorectal cancer cells
One key indicator of tumor cell migration is the
accumulation of ECM proteins such as FN. EMT mainly leads to the
deposition of ECM. To confirm whether OM affects ECM expression, we
examined the expression of FN by western blotting, which showed
that FN was significantly increased in the RKO cells as compared to
the FHC cells. After the RKO cells were treated with 0.50 mg/ml OM,
the expression of FN was significantly decreased in the RKO cells
as compared to the untreated control group (Fig. 4). The results suggest that OM can
alleviate the excessive deposition of FN in colorectal cancer.
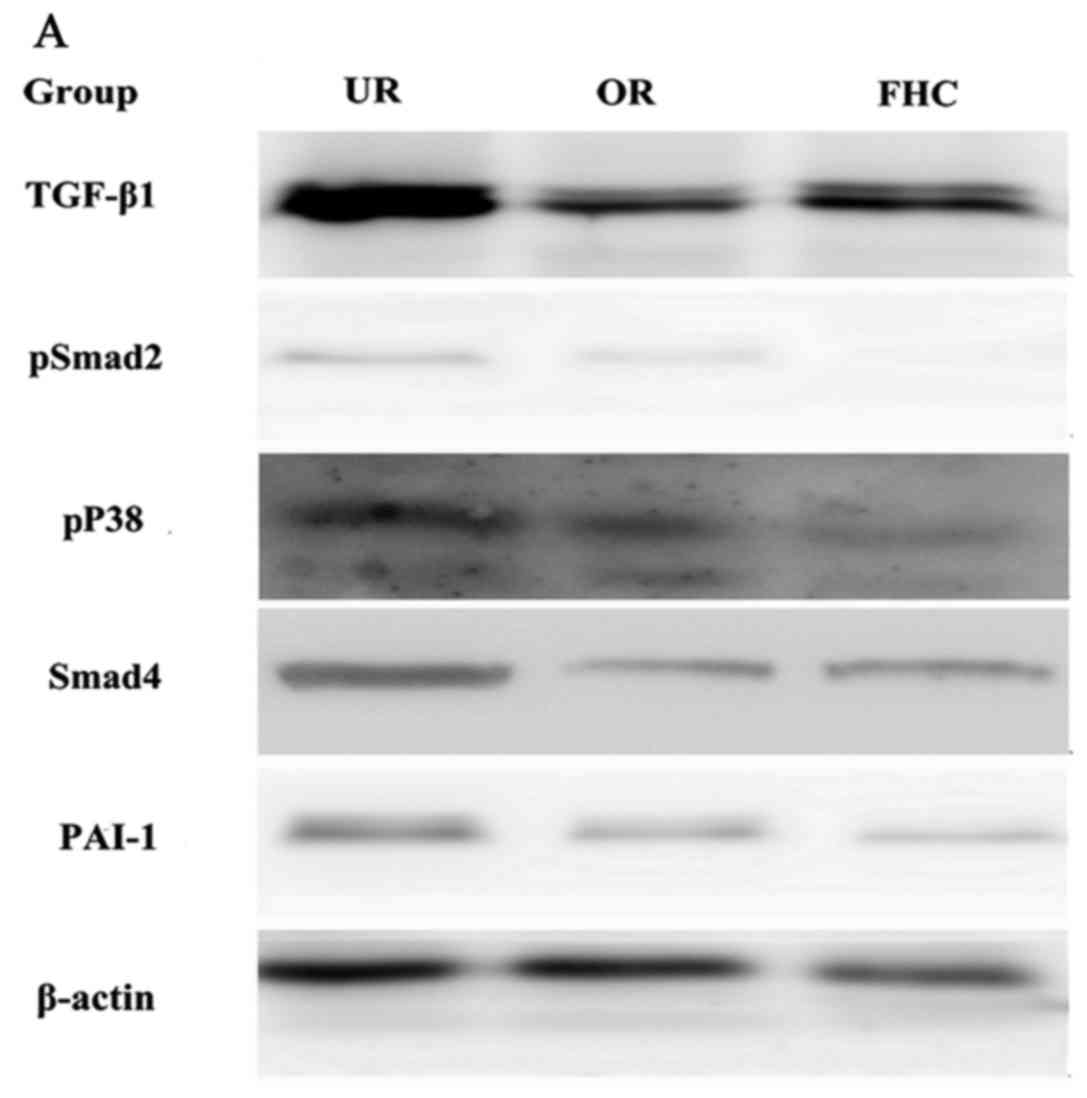
Effect of OM on the expression of
TGF-β1, P38 and PAI-1 in RKO cells
The TGF-β1/Smad signaling pathway plays an important
role in EMT. P38 promoted the TGF-β1/Smad signaling pathway in EMT,
suggesting that P38 has an synergistic effect on colorectal cancer.
To determine whether OM affects the TGF-β1/Smad signaling pathway
in RKO cells and to investigate the probable molecular mechanisms,
we used western blotting and RT-PCR to examine the protein and mRNA
expression levels of TGF-β1 and PAI-1. In addition, Smad4, pSmad2
and pP38 were detected by western blotting. Western blotting showed
that the protein levels of TGF-β1, pP38 and PAI-1 were
significantly increased in the RKO cells as compared to the FHC
cells. Meanwhile, the protein expression levels of TGF-β1 and PAI-1
were significantly decreased in the OR group as compared to the UR
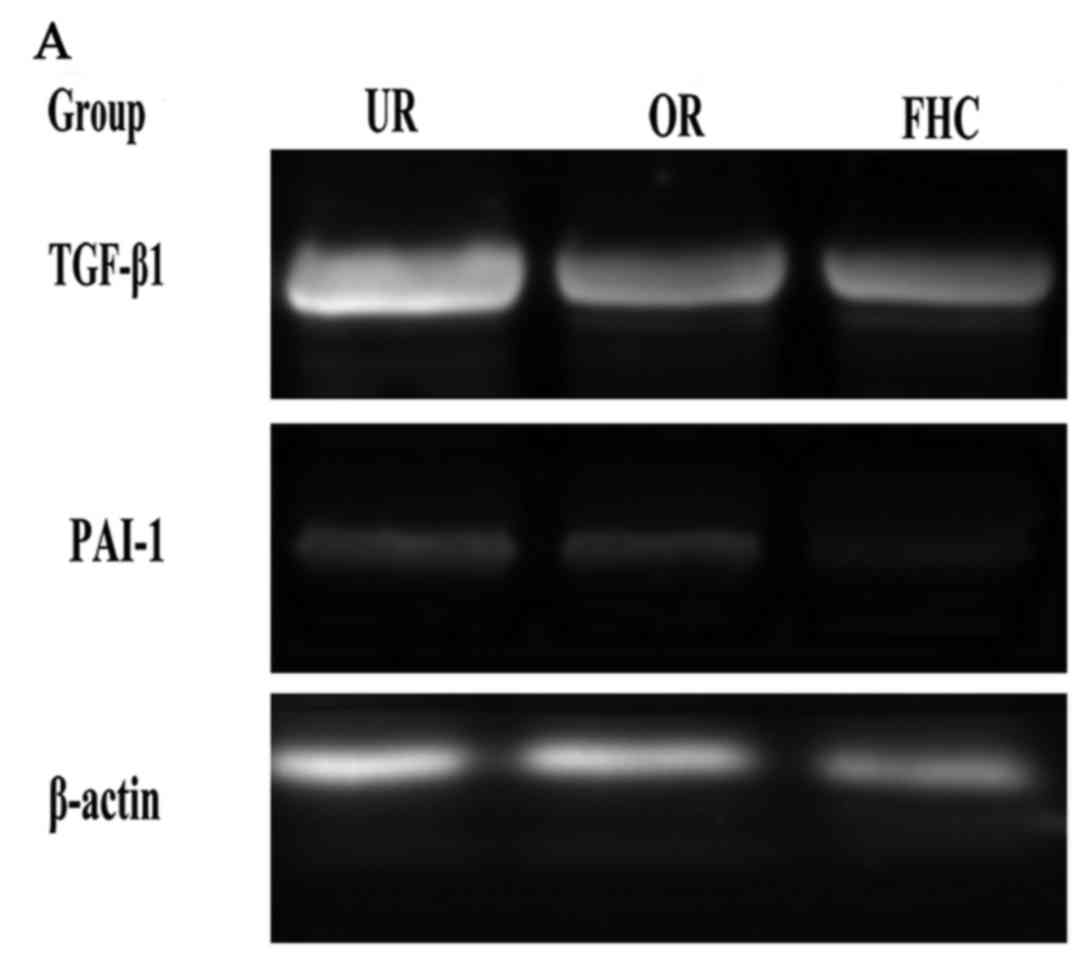
group (Fig. 5). RT-PCR results
showed that TGF-β1 and PAI-1 mRNA levels were markedly increased in
the UR group as compared to the FHC group. After RKO cells were
treated with OM, OM was able to decrease TGF-β1 and PAI-1 mRNA
expression as compared to the UR group (Fig. 6). Of note, the protein levels of
Smad2/3/4 and pSmad2 were significantly increased in the RKO cells
as compared to the FHC cells, which implied that TGF-β1 markedly
induced Smad2 phosphorylation of RKO cells. Meanwhile, the protein
levels of Smad2/3/4 and pSmad2 were significantly decreased in the
OR group as compared to the UR group, which demonstrated that OM
could reverse Smad2 phosphorylation via downregulation of TGF-β1
expression. Taken together, these results demonstrated that OM
markedly inhibited the Smad2 phosphorylation and the formation of
Smad2/3/4 induced by TGF-β1 and eventually reduced the protein and
mRNA levels of PAI-1.
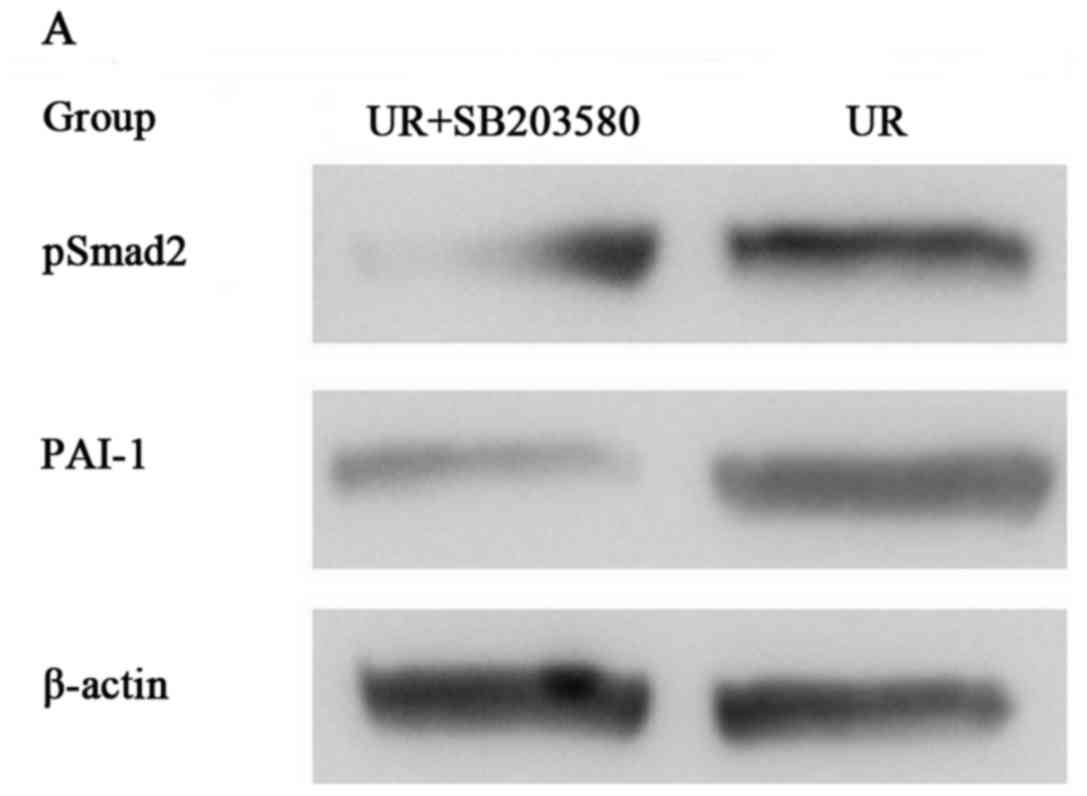
Effect of OM on the expression of P38
in RKO cells
Previous studies have found that P38 is associated
with PAI-1 proteins in their free forms, as well as when they are
bound to TGF-β1. P38 had a positive correlation with PAI-1 between
the UR group and FHC group, which suggests that the RKO cells
exhibited increased PAI-1 expression perhaps through the P38 and
OM-mediated downregulated PAI-1 expression perhaps by inhibiting
P38 expression. To further investigate the molecular mechanisms
involved in OM-inhibited EMT in RKO cells, we treated the RKO cells
with SB203580 (a p38 MAPK inhibitor). Then western blotting was
used to detect the protein levels of pSmad2 and PAI-1. Western
blotting showed that SB203580 inhibited pSmad2 and the expression
of PAI-1 induced by TGF-β1 as compared to the RKO cells (Fig. 7). The results suggest that P38
regulates the phosphorylation of Smad2 and the synthesis of the
Smad2/3/4 compound. Taken together, these results demonstrated that
the protein expression and transcriptional activity of PAI-1 were
regulated by the TGF-β1 signaling pathway via P38. Moreover, these
results suggest that OM inhibits the protein expression and
transcriptional activity of PAI-1 via inhibiting the increase in
P38 induced by the TGF-β1 signaling pathway.
Discussion
Tumor metastasis is the principal cause of the
mortality of colorectal cancer patients, and is also a key factor
determining the extent of colorectal cancer progression (10). TGF-β1, as a fundamental mediator of
ECM, plays a critical role in the EMT process by a Smad-dependent
pathway, resulting in tumor metastasis (18). Smad2 proteins are phosphorylated by
the TGF-β1 receptor and mediate the intracellular signal
transduction of TGF-β1 (21).
PAI-1 is not only a rapid and specific inhibitor of
the plasminogen/plasmin system, but is a potent regulator of tumor
growth in vivo. Increased PAI-1 has been confirmed in many
solid tumors and was found to be associated with a poor prognosis
in several human cancers including colorectal cancer. Thus, PAI-1
has been an attractive potential target for various cancers. Recent
studies have demonstrated that the expression of TGF-β1 was
significantly increased, while the expression of PAI-1 was
significantly increased in colorectal tissues from patients with
colorectal cancer, resulting in the persistent activation of the
TGF-β1/Smad pathway (19,20). This activation leads to the
induction and promotion of EMT in colorectal cancer cells and the
subsequent onset of tumor metastasis. Similarly, these studies have
shown that the expression of E-cadherin (a marker for epithelial
cells) was significantly reduced, while the expression of α-SMA (a
marker for mesenchymal cells) and FN (an important component of
ECM) were significantly increased. The mRNA and protein levels of
TGF-β1 and PAI-1 were markedly increased in the colorectal cancer
cells. These results suggest that EMT was induced and promoted in
colorectal cancer by elevating the expression of TGF-β1 and
PAI-1.
In the present study, the protein and mRNA levels of
PAI-1 were upregulation in the colorectal cancer cells, which
implied that the expression of PAI-1 was regulated at the
transcriptional level, including the P38 gene. P38 is recognized as
an important positive regulating factor of the TGF-β1/Smad
signaling pathway, which promotes activation of TGF-β1 target genes
and enhances the biological effects of the TGF-β1/Smad signaling
pathway by interacting with Smad proteins (22). Furthermore, we performed in
vitro western blotting assay and found that SB203580, a p38
MAPK inhibitor, markedly inhibited the expression of PAI-1.
As an important regulator, P38 modulates signaling
pathways. P38 promotes activation of the TGF-β1 signaling pathway.
Once TGF-β1 signaling is activated, P38 binds to pSmad2/3 and
induces upregulation of Smad2/3/4. In the present study, the
expression of P38, pSmad2 and PAI-1 was significantly increased in
the RKO cells, demonstrating that TGF-β1/Smad promotes the
P38-mediated increased expression of PAI-1 mRNA mediated by
p-Smad2/Smad3.
OM is a traditional Chinese herbal product. As the
main active component of Sophora flavescens Ait, OM has
multiple pharmacological effects and functions. OM was found to
attenuate EMT of hepatocellular carcinoma and pulmonary carcinoma
via inhibiting the TGF-β1/Smad signaling pathway (23). However, it is not known whether OM
can attenuate EMT of colorectal carcinoma in the development of
tumor metastasis. Our findings showed that OM reversed the marked
decrease in E-cadherin and significantly increased α-SMA, FN,
TGF-β1, pSmad2, Smad2/3/4 and P38 and attenuated the P38-dependent
increased expression of PAI-1 induced in colorectal cancer, which
indicates that OM can inhibit EMT in colorectal cancer via
inhibiting the TGF-β1/Smad signaling pathway by reducing
P38-dependent increased expression of PAI-1. Hence, OM could be a
novel therapeutic agent for colorectal cancer.
References
|
1
|
Yuan X, Sun Y, Miao N, Sun S, Wang Y, Hu
Z, Yuan J, Xu M and Liu Z: The synergistic anti-inflammatory effect
of the combination of sodium ferulate and oxymatrine and its
modulation on inflammation-associated mediators in RAW 264.7 cells.
J Ethnopharmacol. 137:1477–1485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen X, Sun R, Hu J, Mo Z, Yang Z, Liao D
and Zhong N: Attenuation of bleomycin-induced lung fibrosis by
oxymatrine is associated with regulation of fibroblast
proliferation and collagen production in primary culture. Basic
Clin Pharmacol Toxicol. 103:278–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan H, Chen R, Shen L, Lv J, Xiong P, Shou
Z and Zhuang X: Oxymatrine improves TNBS-induced colitis in rats by
inhibiting the expression of NF-kappaB p65. J Huazhong Univ Sci
Technolog Med Sci. 28:415–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao P, Zhou R, Li HN, Yao WX, Qiao HQ,
Wang SJ, Niu Y, Sun T, Li YX and Yu JQ: Oxymatrine attenuated
hypoxic-ischemic brain damage in neonatal rats via improving
antioxidant enzyme activities and inhibiting cell death. Neurochem
Int. 89:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guzman JR, Koo JS, Goldsmith JR, Mühlbauer
M, Narula A and Jobin C: Oxymatrine prevents NF-κB nuclear
translocation and ameliorates acute intestinal inflammation. Sci
Rep. 3:16292013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo C, Han F, Zhang C, Xiao W and Yang Z:
Protective effects of oxymatrine on experimental diabetic
nephropathy. Planta Med. 80:269–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Jiang K and Zhao F: Oxymatrine
suppresses proliferation and facilitates apoptosis of human ovarian
cancer cells through upregulating microRNA-29b and downregulating
matrix metalloproteinase-2 expression. Mol Med Rep. 12:5369–5374.
2015.PubMed/NCBI
|
|
8
|
Fei ZW, Qiu MK, Qi XQ, Dai YX, Wang SQ,
Quan ZW, Liu YB and Ou JM: Oxymatrine suppresses proliferation and
induces apoptosis of hemangioma cells through inhibition of HIF-1a
signaling. Int J Immunopathol Pharmacol. 28:201–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moorthy NS Narayana, Ramos MJ and
Fernandes PA: Human ether-a-go-go-related gene channel blockers and
its structural analysis for drug design. Curr Drug Targets.
14:102–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Wang J, Wang Z, Wang Q and Li H:
Dynamic monitoring of plasma amino acids and carnitine during
chemotherapy of patients with alimentary canal malignancies and its
clinical value. Onco Targets Ther. 8:1989–1996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomida C, Aibara K, Yamagishi N, Yano C,
Nagano H, Abe T, Ohno A, Hirasaka K, Nikawa T and Teshima-Kondo S:
The malignant progression effects of regorafenib in human colon
cancer cells. J Med Invest. 62:195–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q,
Jiang J and Zhang S: Baicalein suppresses metastasis of breast
cancer cells by inhibiting EMT via downregulation of SATB1 and
Wnt/β-catenin pathway. Drug Des Devel Ther. 10:1419–1441. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wind T, Jensen JK, Dupont DM, Kulig P and
Andreasen PA: Mutational analysis of plasminogen activator
inhibitor-1. Eur J Biochem. 270:1680–1688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tong Q, Weaver MR, Kosmacek EA, O'Connor
BP, Harmacek L, Venkataraman S and Oberley-Deegan RE: MnTE-2-PyP
reduces prostate cancer growth and metastasis by suppressing p300
activity and p300/HIF-1/CREB binding to the promoter region of the
PAI-1 gene. Free Radic Biol Med. 94:185–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lampelj M, Arko D, Cas-Sikosek N, Kavalar
R, Ravnik M, Jezersek-Novakovic B, Dobnik S, Dovnik NF and Takac I:
Urokinase plasminogen activator (uPA) and plasminogen activator
inhibitor type-1 (PAI-1) in breast cancer - correlation with
traditional prognostic factors. Radiol Oncol. 49:357–364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deepak V, Ramachandran S, Balahmar RM,
Pandian SR, Sivasubramaniam SD, Nellaiah H and Sundar K: In vitro
evaluation of anticancer properties of exopolysaccharides from
Lactobacillus acidophilus in colon cancer cell lines. In Vitro Cell
Dev Biol Anim. 52:163–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Langenskiöld M, Holmdahl L, Angenete E,
Falk P, Nordgren S and Ivarsson ML: Differential prognostic impact
of uPA and PAI-1 in colon and rectal cancer. Tumour Biol.
30:210–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zong W, Yu C, Wang P and Dong L:
Overexpression of SASH1 inhibits TGF-β1-induced EMT in gastric
cancer cells. Oncol Res. 24:17–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vayalil PK, Iles KE, Choi J, Yi AK,
Postlethwait EM and Liu RM: Glutathione suppresses TGF-beta-induced
PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of
AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell
Mol Physiol. 293:L1281–L1292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goto N, Hiyoshi H, Ito I, Iida K, Nakajima
Y, Nagasawa K and Yanagisawa J: Identification of a novel compound
that suppresses breast cancer invasiveness by inhibiting
transforming growth factor-β signaling via estrogen receptor α. J
Cancer. 5:336–343. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Argentou N, Germanidis G, Hytiroglou P,
Apostolou E, Vassiliadis T, Patsiaoura K, Sideras P, Germenis AE
and Speletas M: TGF-β signaling is activated in patients with
chronic HBV infection and repressed by SMAD7 overexpression after
successful antiviral treatment. Inflamm Res. 65:355–365. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Y, Wu C, Boye A, Wu J, Wang J, Yang
X and Yang Y: MAPK inhibitors modulate Smad2/3/4 complex
cyto-nuclear translocation in myofibroblasts via Imp7/8 mediation.
Mol Cell Biochem. 406:255–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Wang Y, Yan R, Li S, Shi M, Xiao Y
and Guo B: Oxymatrine inhibits renal tubular EMT induced by high
glucose via upregulation of SnoN and inhibition of TGF-β1/Smad
signaling pathway. PLoS One. 11:e01519862016. View Article : Google Scholar : PubMed/NCBI
|