Introduction
Osteosarcoma is the most common primary malignant
bone tumor in children, adolescents, and young adults. Osteosarcoma
has been treated using various chemotherapy regimens that have been
developed over more than 40 years (1). Approximately 20% of patients with
osteosarcoma develop metastases. More than 85% of metastatic
disease occurs in the lung, which is the most common site of
metastasis (2). Metastatic
osteosarcoma exhibits resistance to chemotherapeutic treatment
(3). There has been little
improvement in the survival rates of osteosarcoma patients since
the 1980s (1).
To improve the prognosis of osteosarcoma, many
researchers have investigated molecular targets with which to
inhibit osteosarcoma growth and metastasis. Casein kinase 2 (CK2)
is a highly conserved serine/threonine kinase that comprises two α
catalytic (α and α') and two β regulatory subunits (4). Hundreds of CK2 substrates have been
identified, and many substrates continue to be discovered (5). CK2 has important roles in cell growth
and cell fate. In addition, deregulated CK2 activation promotes
many types of human cancers (6–9).
CX-4945 is a potent and selective orally
bioavailable small molecule inhibitor of CK2 that has been
investigated in clinical trials (10–14).
CX-4945 promotes significant reductions in the proliferation and
survival of non-small cell lung carcinoma, squamous cell carcinoma,
breast cancer, pancreatic cancer, and B-cell lymphoma cells
(10,15,16).
In this study, we found that the expression of CK2 is upregulated
in human osteosarcoma cells. We evaluated the function of CK2 in
human osteosarcoma using siRNA and CX-4945.
Materials and methods
Cell lines and reagents
The human osteosarcoma cell lines 143B, SaOS-2,
U2OS, and MG63 were purchased from the American Type Culture
Collection (Manassas, VA, USA). The human osteosarcoma cell lines
NY and Hu09 were purchased from the Health Science Research
Resources Bank (Osaka, Japan). The human osteosarcoma cell lines
NOS1 and HSOS1 were purchased from Riken Cell Bank (Tsukuba,
Japan). Normal human osteoblast cells (NHost) were purchased from
Sanko Junyaku (Tokyo, Japan). The human mesenchymal stem cell line
UBE6T15 was purchased from Health Science Research Resources Bank.
The cell lines were cultured at 37°C in 5% CO2. CX-4945
was purchased from MedChem Express (Princeton, NJ, USA). Control
siRNA, CK2α siRNA, and CK2β siRNA were purchased from Dharmacon
(Pittsburgh, PA, USA).
Analysis of cell viability
Cells were treated with CX-4945 and a control
vehicle. Cell viability was evaluated by a WST-1 assay (Roche,
Basel, Switzerland) for mitochondrial dehydrogenase activity, as
described previously (17).
Western blot analysis
Cells were lysed using Mammalian Protein Extraction
Reagent (Thermo Scientific, Waltham, MA, USA), 3 mM pAPMSF (Wako
Chemicals, Kanagawa, Japan), 5 mg/ml aprotinin (Sigma-Aldrich, St.
Louis, MO, USA), and 2 mM sodium orthovanadate (Wako Chemicals).
SDS-PAGE and immunoblotting were performed, and the following
antibodies were used: CK2α (Merck Millipore, Billerica, MA, USA),
CK2β (Merck Millipore), cleaved-PARP (Cell Signaling, Danvers, MA,
USA), cleaved caspase-3 (Cell Signaling), Bcl-xL (Cell Signaling),
Bcl-2 (Cell Signaling), and tubulin (Sigma-Aldrich). ECL Western
Blotting Reagent (GE Healthcare, Amersham, UK).
Flow cytometry
143B and Saos-2 were cultured with CX4945 or vehicle
at 37°C in 5% CO2 for 24 and 48 h. Cells were treated
with the Annexin V-FITC/7-AAD kit (Beckman Coulter, Brea, CA, USA),
and fluorescence-activated cell sorting was performed with a CyAn
ADP analyzer (Beckman Coulter).
Animal studies
Mouse xenograft models were created as previously
described (17,18). For CX-4945 and control vehicle
treatment, 143B cells (1×106) were suspended in 100 µl
of Matrigel (BD Biosciences, Franklin Lakes, NJ, USA).
Five-week-old nude mice were subcutaneously inoculated with the
mixture of 143B cells and Matrigel. The tumor volume was evaluated
using the formula LW2/2, where L and W represent the
length and width of the tumor, respectively. Xenograft models were
randomly treated with either CX-4945 (150 mg/kg/day) or an equal
volume of vehicle as a control administered via oral tube every
day. All animal experiments were performed in compliance with the
guidelines of the Institute of Laboratory Animal Sciences, Graduate
School of Medical and Dental Sciences, Kagoshima University (permit
no. MD15045). Every effort was employed to minimize both the number
of animals used and animal pain.
Statistical analysis
The Kolmogorov-Smirnov test was performed to examine
the distribution of data. Statistical analyses were performed using
the Mann-Whitney U test. The survival rate was evaluated using the
Kaplan-Meier method and the log-rank test. All statistical analyses
were performed using BellCurve for Excel 2015 (SSRI, Osaka, Japan).
P<0.05 was considered statistically significant.
Results
Upregulated expression of CK2 in human
osteosarcoma cells
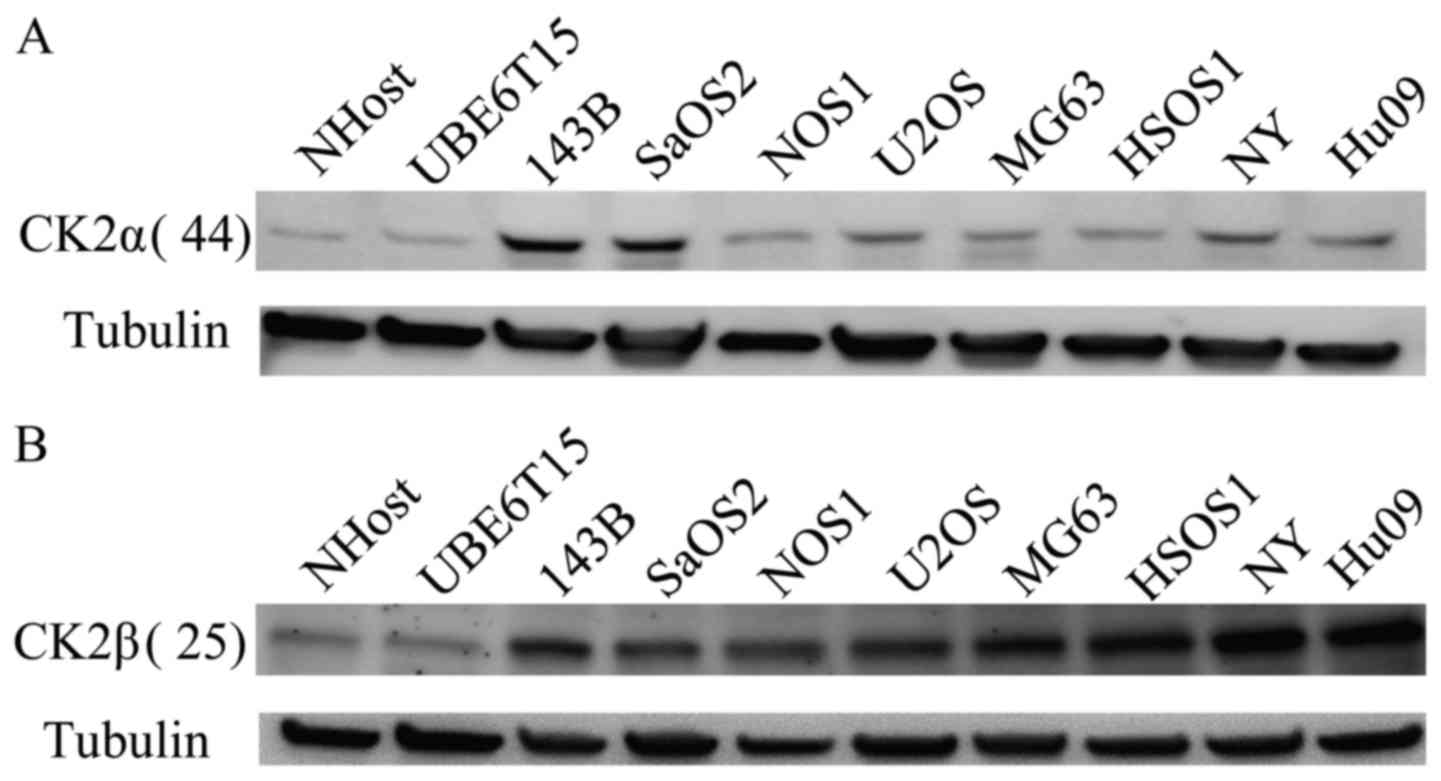
Western blot analysis showed that the expression of
both CK2α and CK2β protein was upregulated compared with NHost
normal human osteoblasts and UBE6T15 human mesenchymal stem cells
(Fig. 1).
Knockdown of CK2α or CK2β inhibited
the proliferation of human osteosarcoma cells
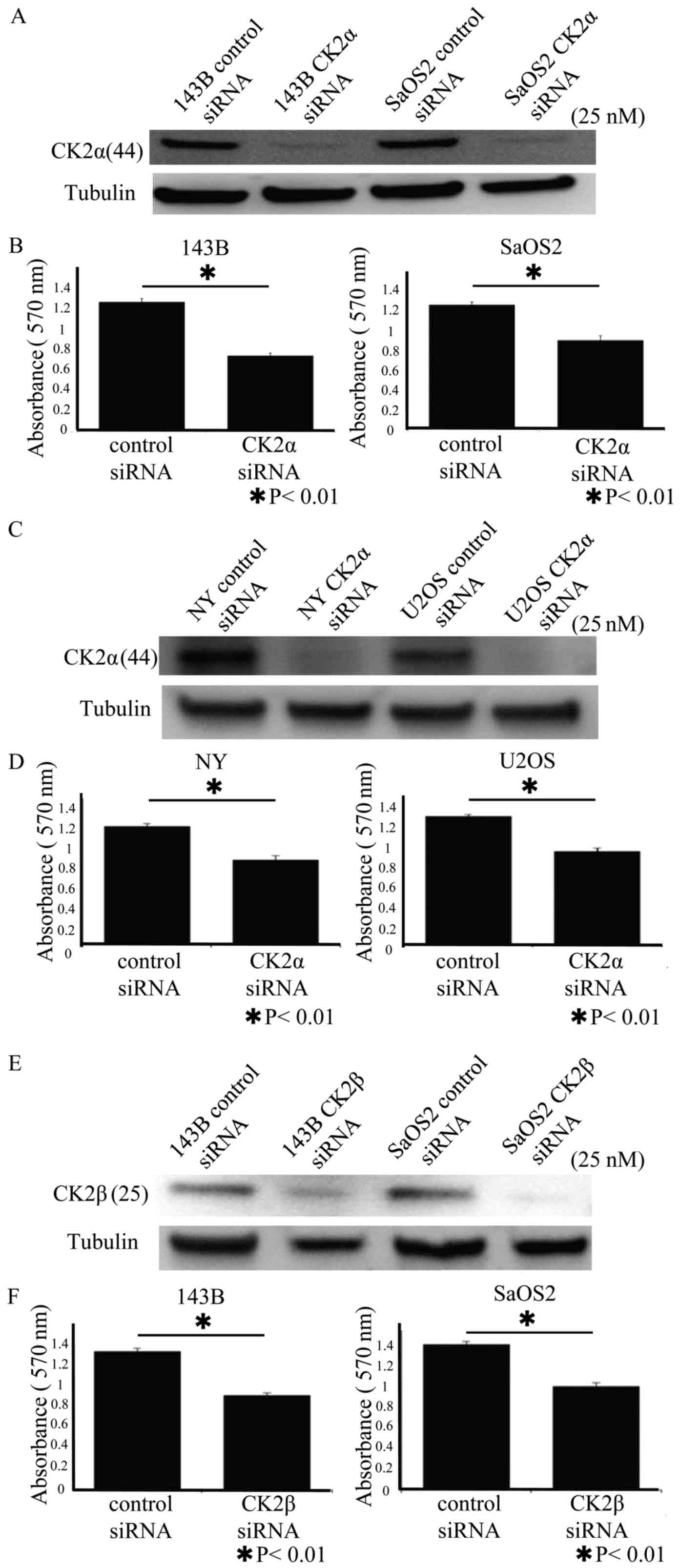
We used siRNA to determine whether CK2α promoted the
proliferation of osteosarcoma cells. Western blot analysis revealed
that CK2α siRNA decreased the expression level of CK2α protein
(Fig. 2A and C). WST assay revealed
that knockdown of CK2α inhibited the proliferation of the 143B,
SaOS-2, NY, and U2OS human osteosarcoma cell lines (Fig. 2B and D). In addition, we used siRNA
to determine whether CK2β promoted the proliferation of
osteosarcoma cells. Western blot analysis revealed that CK2β siRNA
decreased the expression of CK2β protein (Fig. 2E). WST assay revealed that knockdown
of CK2β inhibited the proliferation of the 143B and SaOS-2 human
osteosarcoma cell lines (Fig.
2F).
CX-4945 inhibited the proliferation of
human osteosarcoma cell lines, but not the proliferation of
mesenchymal stem cells
We used 4 human osteosarcoma cell lines, 143B,
SaOS2, NY, and U2OS which expressed high levels of CK2α for further
examinations. Treatment with 3 µM CX-4945 did not inhibit the
proliferation of UBE6T15 human mesenchymal stem cells (Fig. 3A); however, treatment with 3 µM
CX-4945 caused a significant reduction in osteosarcoma cell
proliferation. (Fig. 3B). WST assay
revealed that treatment with CX-4945 inhibited the proliferation of
the 143B, Saos-2, NY, and U2OS human osteosarcoma cell lines in a
dose-dependent manner (Fig.
3C).
CX-4945 promotes apoptotic cell death
of human osteosarcoma cell lines
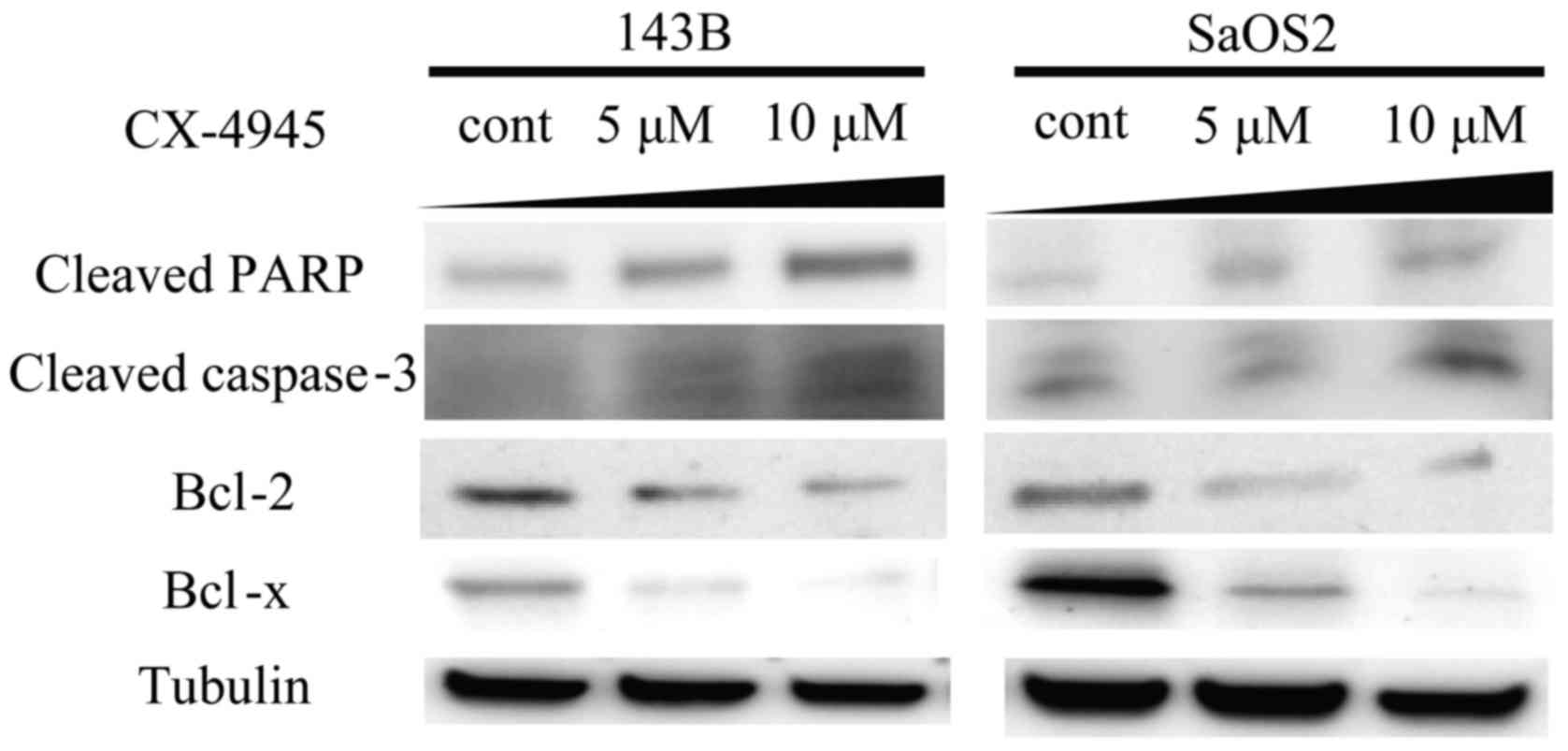
Western blot analysis revealed that treatment with
CX-4945 increased the expression of cleaved PARP and cleaved
caspase-3 (Fig. 4). Treatment with
CX-4945 also decreased the expression of Bcl-2 and Bcl-xL. Analysis
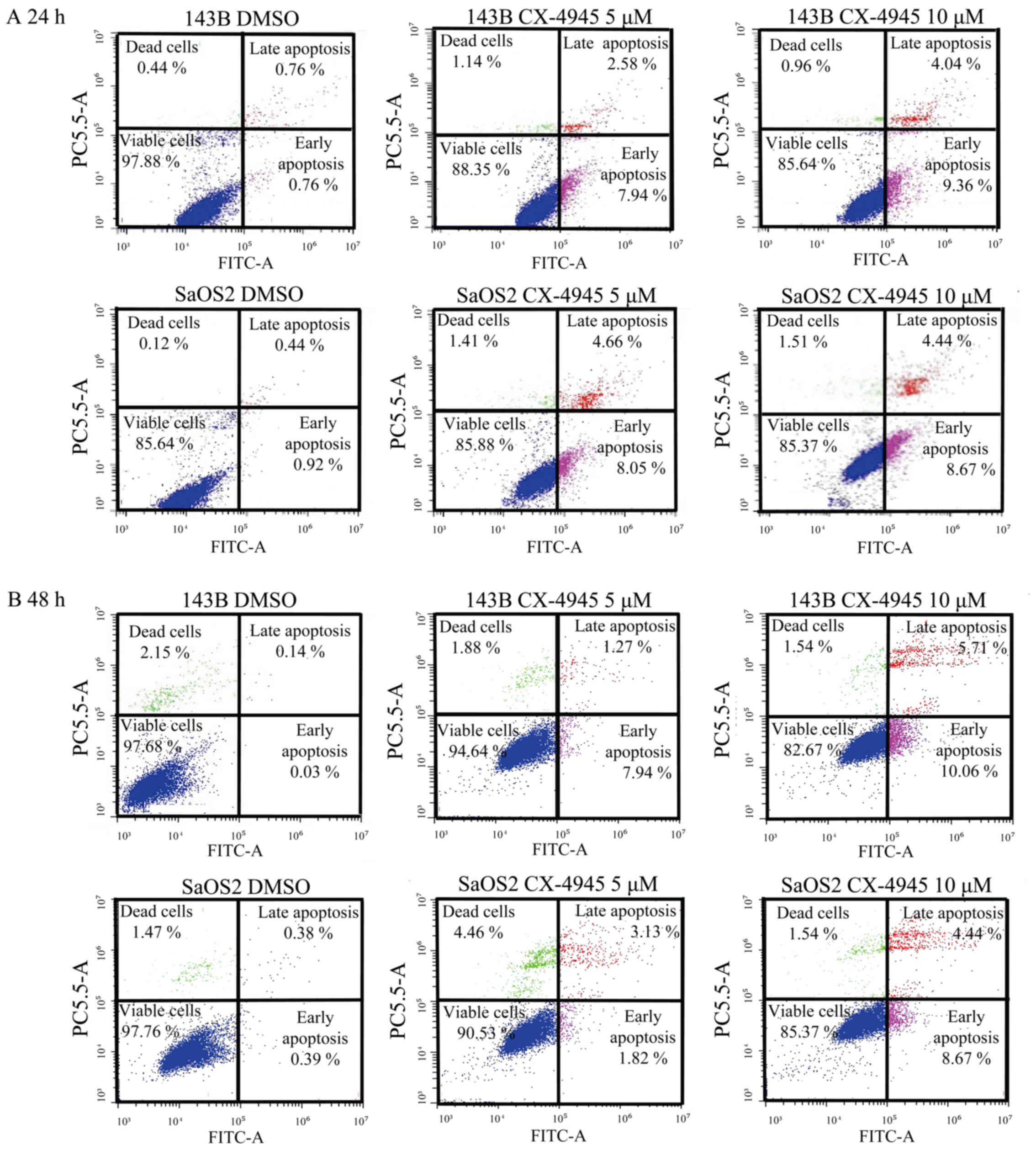
by flow cytometry showed that treatment with CX-4945 increased the
population of early and late apoptotic cells in 143B and SaOS-2
osteosarcoma cells at 24 and 48 h (Fig.
5). These findings indicate that CX-4945 treatment promoted
apoptotic death of human osteosarcoma cells.
CX-4945 prevents osteosarcoma growth
in vivo
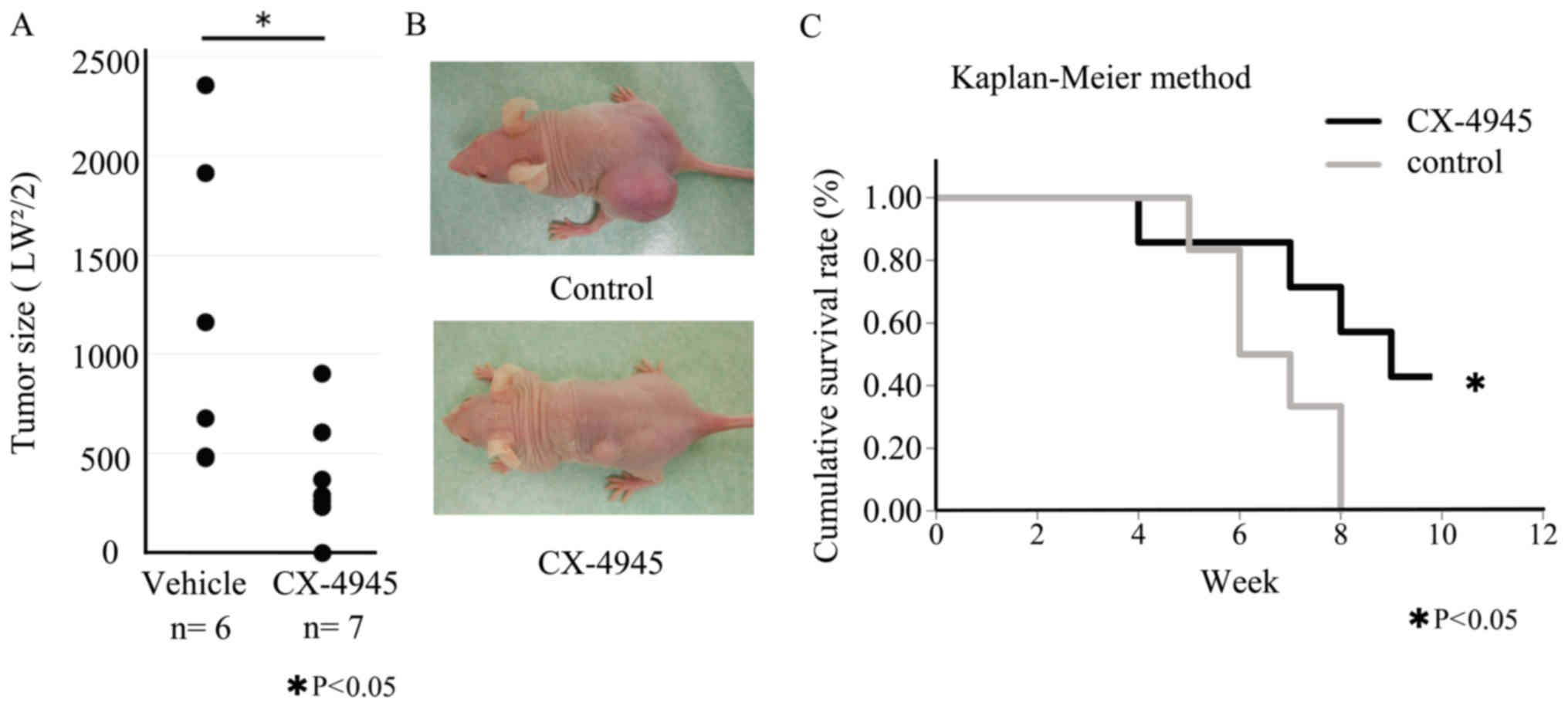
Palpable tumors were confirmed in nude mice 7 days
after inoculation with 143B osteosarcoma cells. Compared with
control vehicle treatment, treatment with CX-4945 significantly
inhibited the growth of the osteosarcoma xenografts (Fig. 6A). Kaplan-Meier analysis showed that
CX-4945 treatment provided a significant survival benefit (Fig. 6B).
Discussion
CK2 is upregulated in many types of malignant
tumors, and maintains the phenotype of malignancy (6,19). CK2
is a potential therapeutic target for human cancers, and has been
tested in clinical trials (10–14).
We found that human osteosarcoma cells upregulated the expression
of CK2α and CK2β. To the best of our knowledge, this is the first
report to show the upregulation of CK2 in human osteosarcoma. We
showed that treatment with 3 µM CX-4945 inhibited osteosarcoma
growth in vitro, but did not inhibit mesenchymal stem cell
proliferation. These findings suggest that 3 µM of CX-4945 might be
a safe and effective dose for osteosarcoma treatment. We also
showed that CX-4945 prevented the growth of osteosarcoma in
vivo.
CX-4945 reportedly has a long half-life, high oral
bioavailability, and non-cardiac toxicity (20). Furthermore, CX-4945 inhibits
osteoclast differentiation and enhances osteoblast differentiation
(21), indicating that CX-4945
inhibits osteosarcoma growth while promoting the regeneration of
affected bone. As CK2 reportedly drives the metastatic development
of lung cancer, prostate cancer, squamous cell carcinoma, and
breast carcinoma (9,22–25),
the CK2 inhibitor CX-4945 is a promising drug for the treatment of
metastatic bone tumors.
Human osteosarcoma specimens and cell lines
reportedly have an overexpression of Hedgehog pathway-related
genes, including SMO and GLI2. Furthermore, inhibition of the
Hedgehog pathway prevents osteosarcoma growth and metastasis
(17,18,26–30).
CK2 activates the Hedgehog pathway in many types of cells,
including mesothelioma, ovarian cancer, hepatocellular carcinoma,
and lung cancer cells (31–36). There is a possibility that CX-4945
inhibits the Hedgehog pathway via inhibition of CK2. Osteosarcoma
growth is inhibited in vitro by a combination of arsenic
trioxide, a Hedgehog signal inhibitor, with conventional
FDA-approved anticancer agents including cisplatin, ifosfamide, or
doxorubicin. In vivo tumors treated with a combination of
arsenic trioxide with either cisplatin or ifosfamide grew
significantly less than tumors treated with the vehicle alone
(17,27,29).
These findings suggest that combining CX-4945 with conventional
anticancer agents might more effectively prevent osteosarcoma
growth, and that this combination therapy might decrease the
required concentration of each drug. The lower levels of each agent
in these combinations might reduce the toxicities associated with
the use of each single drug. However, osteosarcoma is an extremely
heterogeneous tumor. Preselection of patients with osteosarcoma
exhibiting upregulated CK2 is required for CK2-targeted
treatment.
CX-4945 reportedly modulates not only CK2 activity,
but also PI3K-Akt-mTOR signaling, the Notch pathway, the
PI3K-Akt-mTOR pathway, the focal adhesion kinase-Src-paxillin
signaling cascade, ER stress signaling, NF-κB, and Bcl-xL, ERK,
AP-1, and IL-8 gene activities (37–42).
The pleotropic effect of CX-4945 and off-target effects of CK2
might potentially affect the inhibition of osteosarcoma growth.
Nonetheless, CX-4945 showed promising therapeutic efficacy for
osteosarcoma in this study.
Taken together, our findings indicate that CK2 might
be an attractive therapeutic target, and that CX-4945 might be a
promising new reagent for the treatment of osteosarcoma.
Acknowledgements
We are grateful to Hui Gao for her excellent
technical assistance. We wish to thank the joint research
laboratory of Kagoshima University Graduate School of Medical and
Dental Sciences. This study was supported by Grants-in-Aid for
Scientific Research (KAKENHI) (C) 19591725, (C) 20591786, (C)
21591919, (C) 21591920, (C) 22591663, (C) 23592195, and (C)
16K10868.
References
|
1
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He JP, Hao Y, Wang XL, Yang XJ, Shao JF,
Guo FJ and Feng JX: Review of the molecular pathogenesis of
osteosarcoma. Asian Pac J Cancer Prev. 15:5967–5976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanif IM, Hanif IM, Shazib MA, Ahmad KA
and Pervaiz S: Casein Kinase II: An attractive target for
anti-cancer drug design. Int J Biochem Cell Biol. 42:1602–1605.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meggio F and Pinna LA:
One-thousand-and-one substrates of protein kinase CK2? FASEB J.
17:349–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmad KA, Harris NH, Johnson AD, Lindvall
HC, Wang G and Ahmed K: Protein kinase CK2 modulates apoptosis
induced by resveratrol and epigallocatechin-3-gallate in prostate
cancer cells. Mol Cancer Ther. 6:1006–1012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faust RA, Gapany M, Tristani P, Davis A,
Adams GL and Ahmed K: Elevated protein kinase CK2 activity in
chromatin of head and neck tumors: Association with malignant
transformation. Cancer Lett. 101:31–35. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JS, Eom JI, Cheong JW, Choi AJ, Lee
JK, Yang WI and Min YH: Protein kinase CK2alpha as an unfavorable
prognostic marker and novel therapeutic target in acute myeloid
leukemia. Clin Cancer Res. 13:1019–1028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Amin EB, Mayo MW, Chudgar NP,
Bucciarelli PR, Kadota K, Adusumilli PS and Jones DR: CK2α drives
lung cancer metastasis by targeting BRMS1 nuclear export and
degradation. Cancer Res. 76:2675–2686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siddiqui-Jain A, Drygin D, Streiner N,
Chua P, Pierre F, O'Brien SE, Bliesath J, Omori M, Huser N, Ho C,
et al: CX-4945, an orally bioavailable selective inhibitor of
protein kinase CK2, inhibits prosurvival and angiogenic signaling
and exhibits antitumor efficacy. Cancer Res. 70:10288–10298. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferguson AD, Sheth PR, Basso AD, Paliwal
S, Gray K, Fischmann TO and Le HV: Structural basis of CX-4945
binding to human protein kinase CK2. FEBS Lett. 585:104–110. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cozza G, Pinna LA and Moro S: Protein
kinase CK2 inhibitors: A patent review. Expert Opin Ther Pat.
22:1081–1097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim J and Kim SH: Druggability of the CK2
inhibitor CX-4945 as an anticancer drug and beyond. Arch Pharm Res.
35:1293–1296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarno S, Papinutto E, Franchin C, Bain J,
Elliott M, Meggio F, Kazimierczuk Z, Orzeszko A, Zanotti G,
Battistutta R, et al: ATP site-directed inhibitors of protein
kinase CK2: An update. Curr Top Med Chem. 11:1340–1351. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bettendorff L, Wirtzfeld B, Makarchikov
AF, Mazzucchelli G, Frédérich M, Gigliobianco T, Gangolf M, De Pauw
E, Angenot L and Wins P: Discovery of a natural thiamine adenine
nucleotide. Nat Chem Biol. 3:211–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pizzi M, Piazza F, Agostinelli C, Fuligni
F, Benvenuti P, Mandato E, Casellato A, Rugge M, Semenzato G and
Pileri SA: Protein kinase CK2 is widely expressed in follicular,
Burkitt and diffuse large B-cell lymphomas and propels malignant
B-cell growth. Oncotarget. 6:6544–6552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura S, Nagano S, Nagao H, Ishidou Y,
Yokouchi M, Abematsu M, Yamamoto T, Komiya S and Setoguchi T:
Arsenic trioxide prevents osteosarcoma growth by inhibition of GLI
transcription via DNA damage accumulation. PLoS One. 8:e694662013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagao H, Ijiri K, Hirotsu M, Ishidou Y,
Yamamoto T, Nagano S, Takizawa T, Nakashima K, Komiya S and
Setoguchi T: Role of GLI2 in the growth of human osteosarcoma. J
Pathol. 224:169–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kulbe H, Iorio F, Chakravarty P, Milagre
CS, Moore R, Thompson RG, Everitt G, Canosa M, Montoya A, Drygin D,
et al: Integrated transcriptomic and proteomic analysis identifies
protein kinase CK2 as a key signaling node in an inflammatory
cytokine network in ovarian cancer cells. Oncotarget.
7:15648–15661. 2016.PubMed/NCBI
|
|
20
|
Son YH, Song JS, Kim SH and Kim J:
Pharmacokinetic characterization of CK2 inhibitor CX-4945. Arch
Pharm Res. 36:840–845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Son YH, Moon SH and Kim J: The protein
kinase 2 inhibitor CX-4945 regulates osteoclast and osteoblast
differentiation in vitro. Mol Cells. 36:417–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trembley JH, Unger GM, Korman VL, Abedin
MJ, Nacusi LP, Vogel RI, Slaton JW, Kren BT and Ahmed K: Tenfibgen
ligand nanoencapsulation delivers bi-functional anti-CK2 RNAi
oligomer to key sites for prostate cancer targeting using human
xenograft tumors in mice. PLoS One. 9:e1099702014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Unger GM, Kren BT, Korman VL, Kimbrough
TG, Vogel RI, Ondrey FG, Trembley JH and Ahmed K: Mechanism and
efficacy of sub-50-nm tenfibgen nanocapsules for cancer
cell-directed delivery of anti-CK2 RNAi to primary and metastatic
squamous cell carcinoma. Mol Cancer Ther. 13:2018–2029. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giusiano S, Cochet C, Filhol O,
Duchemin-Pelletier E, Secq V, Bonnier P, Carcopino X, Boubli L,
Birnbaum D, Garcia S, et al: Protein kinase CK2α subunit
over-expression correlates with metastatic risk in breast
carcinomas: Quantitative immunohistochemistry in tissue
microarrays. Eur J Cancer. 47:792–801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gapany M, Faust RA, Tawfic S, Davis A,
Adams GL and Ahmed K: Association of elevated protein kinase CK2
activity with aggressive behavior of squamous cell carcinoma of the
head and neck. Mol Med. 1:659–666. 1995.PubMed/NCBI
|
|
26
|
Yang W, Liu X, Choy E, Mankin H, Hornicek
FJ and Duan Z: Targeting hedgehog-GLI-2 pathway in osteosarcoma. J
Orthop Res. 31:502–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saitoh Y, Setoguchi T, Nagata M, Tsuru A,
Nakamura S, Nagano S, Ishidou Y, Nagao-Kitamoto H, Yokouchi M,
Maeda S, et al: Combination of Hedgehog inhibitors and standard
anticancer agents synergistically prevent osteosarcoma growth. Int
J Oncol. 48:235–242. 2016.PubMed/NCBI
|
|
28
|
Nagao-Kitamoto H, Setoguchi T, Kitamoto S,
Nakamura S, Tsuru A, Nagata M, Nagano S, Ishidou Y, Yokouchi M,
Kitajima S, et al: Ribosomal protein S3 regulates GLI2-mediated
osteosarcoma invasion. Cancer Lett 356B. 855–861. 2015. View Article : Google Scholar
|
|
29
|
Nagao-Kitamoto H, Nagata M, Nagano S,
Kitamoto S, Ishidou Y, Yamamoto T, Nakamura S, Tsuru A, Abematsu M,
Fujimoto Y, et al: GLI2 is a novel therapeutic target for
metastasis of osteosarcoma. Int J Cancer. 136:1276–1284. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirotsu M, Setoguchi T, Sasaki H,
Matsunoshita Y, Gao H, Nagao H, Kunigou O and Komiya S: Smoothened
as a new therapeutic target for human osteosarcoma. Mol Cancer.
9:52010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Yang YL, Wang Y, You B, Dai Y,
Chan G, Hsieh D, Kim IJ, Fang LT, Au A, et al: CK2α, over-expressed
in human malignant pleural mesothelioma, regulates the Hedgehog
signaling pathway in mesothelioma cells. J Exp Clin Cancer Res.
33:932014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang AQ, Cao XC, Tian L, He L and Liu F:
Apigenin inhibits the self-renewal capacity of human ovarian cancer
SKOV3-derived sphere-forming cells. Mol Med Rep. 11:2221–2226.
2015.PubMed/NCBI
|
|
33
|
Wu D, Sui C, Meng F, Tian X, Fu L, Li Y,
Qi X, Cui H, Liu Y and Jiang Y: Stable knockdown of protein kinase
CK2-alpha (CK2α) inhibits migration and invasion and induces
inactivation of hedgehog signaling pathway in hepatocellular
carcinoma Hep G2 cells. Acta Histochem. 116:1501–1508. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Wang Y, Mao JH, Hsieh D, Kim IJ,
Hu LM, Xu Z, Long H, Jablons DM and You L: Inhibition of CK2α
down-regulates Hedgehog/Gli signaling leading to a reduction of a
stem-like side population in human lung cancer cells. PLoS One.
7:e389962012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Z, Mei W, Strack S, Jia J and Yang J:
The antagonistic action of B56-containing protein phosphatase 2As
and casein kinase 2 controls the phosphorylation and Gli turnover
function of Daz interacting protein 1. J Biol Chem.
286:36171–36179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jia H, Liu Y, Xia R, Tong C, Yue T, Jiang
J and Jia J: Casein kinase 2 promotes Hedgehog signaling by
regulating both smoothened and Cubitus interruptus. J Biol Chem.
285:37218–37226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim J, Choi WJ, Moon SH, Jung J, Park JK,
Kim SH and Lee JO: Micropillar arrays as potential drug screens:
Inhibition of micropillar-mediated activation of the
FAK-Src-paxillin signaling pathway by the CK2 inhibitor CX-4945.
Acta Biomater. 27:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bian Y, Han J, Kannabiran V, Mohan S,
Cheng H, Friedman J, Zhang L, VanWaes C and Chen Z: MEK inhibitor
PD-0325901 overcomes resistance to CK2 inhibitor CX-4945 and
exhibits anti-tumor activity in head and neck cancer. Int J Biol
Sci. 11:411–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Intemann J, Saidu NE, Schwind L and
Montenarh M: ER stress signaling in ARPE-19 cells after inhibition
of protein kinase CK2 by CX-4945. Cell Signal. 26:1567–1575. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang S, Long H, Yang YL, Wang Y, Hsieh D,
Li W, Au A, Stoppler HJ, Xu Z, Jablons DM, et al: Inhibition of
CK2α down-regulates Notch1 signalling in lung cancer cells. J Cell
Mol Med. 17:854–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bliesath J, Huser N, Omori M, Bunag D,
Proffitt C, Streiner N, Ho C, Siddiqui-Jain A, O'Brien SE, Lim JK,
et al: Combined inhibition of EGFR and CK2 augments the attenuation
of PI3K-Akt-mTOR signaling and the killing of cancer cells. Cancer
Lett. 322:113–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martins LR, Lúcio P, Melão A, Antunes I,
Cardoso BA, Stansfield R, Bertilaccio MT, Ghia P, Drygin D, Silva
MG, et al: Activity of the clinical-stage CK2-specific inhibitor
CX-4945 against chronic lymphocytic leukemia. Leukemia. 28:179–182.
2014. View Article : Google Scholar : PubMed/NCBI
|