Introduction
Breast cancer is the second leading cause of
cancer-related deaths, and the most common cancer among women
worldwide (1). Although numerous
measures and drugs have been applied to the clinic, metastasis is
still one of the most critical issues in patients with breast
cancer (2). Therefore, the
identification of effective drugs and exploring the mechanisms
which suppress breast cancer metastasis may provide hope to
clinical therapies.
The metastasis of cancer cells are reminiscent of
epithelial-mesenchymal transition (EMT) usually occurring in
embryonic development, tissue repair and tumor progression
(3,4). During the EMT process, epithelial-like
cancer cells lose cell-cell contacts and acquire mesenchymal
properties, which are believed to help cancer cells to gain the
abilities of migration and invasion, resulting in the
disassociation of cancer cells from the primary tumor and migration
to distant organs. From a molecular viewpoint, EMT is characterized
by downregulation of epithelial cell markers such as E-cadherin and
claudin, and upregulation of mesenchymal molecules such as vimentin
and N-cadherin (5,6). Several transcriptional factors,
including Snail, Slug, ZEB and Twist, have been found to be
involved in the regulation of EMT (6,7). These
transcription factors directly or indirectly repress the
transcriptional expression of E-cadherin, resulting in the loss of
epithelial markers and the acquisition of mesenchymal features
(3). Recent studies have also
suggested that EMT is connected with the acquisition of cancer stem
cell properties, development of drug resistance, and induction of
angiogenesis, providing various distinct benefits to tumor
progression (8–12).
Supporting evidence from epidemiology indicates that
some natural dietaries may exhibit beneficial actions against the
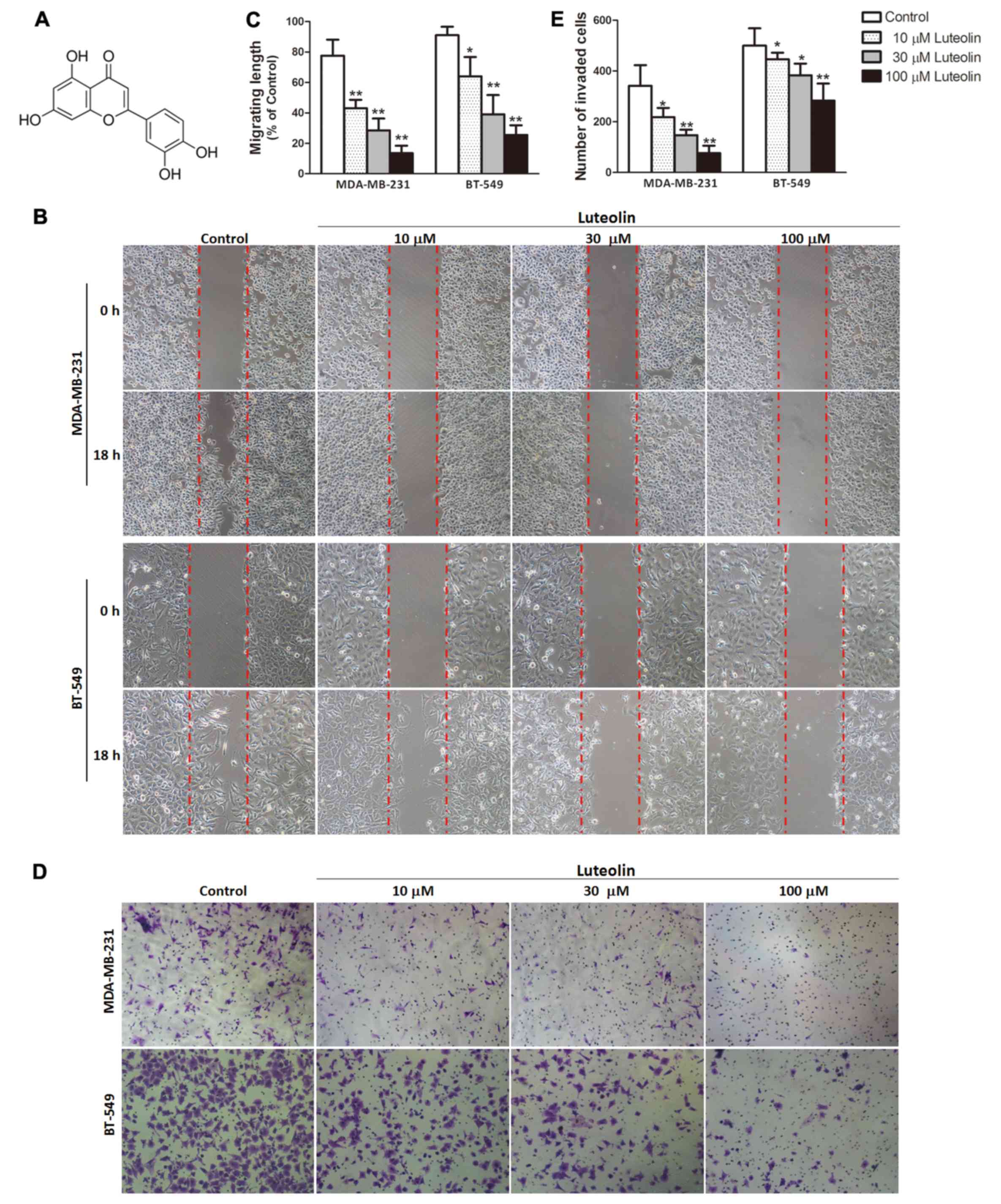
risk or progression of breast cancer (13). Luteolin (Fig. 1A), one of the natural flavonoid
compounds found in many plants such as carrots, celery, broccoli,
perilla leaf and seed, has been reported to possess many biological
properties such as anti-inflammation, anti-allergy, antioxidant,
anticancer and anti-microbial (14–16).
Previous studies indicate that luteolin exhibits a wide range of
antitumor activities in various types of cancers by inhibiting cell
proliferation and tumor growth, promoting cancer cell apoptosis and
cell cycle arrest, sensitizing drug resistance, and mitigating
invasiveness and metastasis of cancer cells (14,17–19).
Specifically, it has been found that luteolin enhanced
paclitaxel-induced apoptosis in human breast cancer (20), and sensitized drug-resistant human
breast cancer cells to tamoxifen (21). Furthermore, luteolin effectively
blocked progestin-dependent angiogenesis and the stem cell-like
phenotype in human breast cancer cells (22). Although these studies revealed its
protective roles in malignancy, the effects and underlying
mechanisms of luteolin on the metastasis of highly aggressive
triple-negative breast cancer (TNBC) remain largely unexplored.
In the present study, we chose two TNBC cell lines
MDA-MA-231 and BT5-49, which have been indicated to possess highly
malignant transfer traits (23,24),
to investigate the potential functions and mechanisms of luteolin
on the metastasis of TNBC in vitro and in vivo.
Materials and methods
Reagents and antibodies
Luteolin (purity >99%) was obtained from Pulus
Biology Technology (Chengdu, China). RNA isolation kit, PrimeScript
RT and PCR reagent kits were purchased from Takara (Dalin, China).
Matrigel and Transwell chambers were obtained from Corning
(Corning, NY, USA). Antibodies used in the present study were
purchased from the following sources: anti-E-cadherin,
anti-claudin, anti-vimentin, anti-N-cadherin, anti-Snail,
anti-Slug, anti-ZEB1 and anti-β-catenin antibodies were from Cell
Signaling Technology (Danvers, MA, USA); anti-GAPDH antibody was
from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-Ki67 was
from Abcam (Cambridge, UK); anti-F-actin-Red 555 was from
Invitrogen (Carlsbad, CA, USA).
Cell culture and treatment
Human breast cancer cell lines MDA-MB-231 and BT5-49
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured at 37°C in Dulbecco's modified
Eagles medium (DMEM) (HyClone, Los Angeles, CA, USA) with 10% fetal
bovine serum (FBS) (Gibco, Grand Island, NY, USA) and 1%
penicillin/streptomycin in a humidified atmosphere of 5%
CO2. The cells were treated with various concentrations
(10, 30 and 100 µM) of luteolin for the indicated time.
Nude mice and in vivo metastasis
assay
Female nude mice at 4–5 weeks old following approval
by the Animal Ethics Committee of Chongqing Medical University were
housed according to the National and Institutional Guidelines for
Humane Animal Care in specific pathogen-free (SPF) laboratory
animal environmental. For the xenograft metastasis experiment,
female nude mice were subcutaneously injected with 1×106
MDA-MB-231 cells with or without transduction of Ad-GFP or
Ad-β-catenin, and subjected to intraperitoneal injection of
luteolin (100 mg/kg) three times every week when the tumor reached
1 cm3 in volume. Eight weeks later, the mice were
sacrificed under anesthesia. The tumor nodules on the lung were
counted. The primary tumor tissues and lung were collected for
further analyses.
Scratch migration assay
MDA-MB-231 and BT5-49 cells were seeded into a
6-well plate in culture medium and allowed to grow to 100%
confluence. A sterile toothpick was used to scrap the monolayer
cells creating a wound. The scraped cells were washed out with
phosphate-buffered saline (PBS), and the remaining cells continued
to culture in the absence or presence of different concentration of
luteolin for 18 h. The breast cancer cells were observed and the
areas of the wound were measured.
Transwell invasion assay
The invasion was measured using the Matrigel-coated
24-well Transwell chamber. Briefly, MDA-MB-231 and BT5-49 cells
were trypsinized, washed and seeded into the upper chamber in a
serum-free medium at a density of 2.5×105 cells/well.
The lower chamber contained complete DMEM with 10% FBS. Following
incubation for 8 h, the cells on the Matrigel membrane were fixed
and stained using crystal violet. The invaded cells were
counted.
Immunofluorescent (IF) staining
For IF analysis, the cells or tissues on the slides
were fixed in 4% paraformaldehyde at room temperature and
permeabilized using 0.05% Triton X-100. Then, the slides were first
immersed in blocking solution containing 1% BSA in PBS, followed by
incubation with the primary antibody overnight at 4°C. The slides
were then incubated in PBS buffer with fluorescently labeled
secondary antibody for 1 h at room temperature in the dark. Nuclei
were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and
observed under a fluorescence microscope (Nikon, Tokyo, Japan).
Protein isolation and western
blotting
The cells were lysed in protein lysis buffer to
collect total proteins. The concentration of protein was determined
using the BCA kit. Total proteins (40 µg) were fractionated on 10%
SDS gel, and then transferred to polyvinylidene fluoride (PVDF)
membranes. The membranes were blocked with 5% BSA in Tris-buffered
saline (TBS) containing 0.1% Tween-20, followed by incubation with
the primary antibody at 4°C overnight. After washing with TBST for
three times, the membranes were incubated with secondary antibody
conjugated with horseradish peroxidase (HRP). The membranes were
visualized using the enhanced chemiluminescent system and short
exposure of the membrane to X-ray film.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA from the cancer cells was extracted using
the RNA isolation kit according to the manufacturer's instructions.
Then cDNA was synthesized using PrimeScript RT reagent kit with 1
µg total RNA. qPCR was performed using the PCR kit. The following
primer sequences were used: E-cadherin sense,
5′-TCCTGGGCAGAGTGAATTTTGAAGA-3′ and antisense,
5′-AAACGGAGGCCTGATGGGG-3′; claudin sense,
5′-CCTCCTGGGAGTGATAGCAAT-3′ and antisense,
5′-GGCAACTAAAATAGCCAGACCT-3′; vimentin sense,
5′-TACAGGAAGCTGCTGGAAGG-3′ and antisense,
5′-ACCAGAGGGAGTGAATCCAG-3′; N-cadherin sense,
5′-AGCCAACCTTAACTGAGGAGT-3′ and antisense,
5′-GGCAAGTTGATTGGAGGGATG-3′; Snail sense,
5′-TCGGAAGCCTAACTACAGCGA-3′ and antisense,
5′-AGATGAGCATTGGCAGCGAG-3′; Slug sense, 5′-GGGGAGAAGCCTTTTTCTTG-3′
and antisense, 5′-TCCTCATGTTTGTGCAGGAG-3′; GAPDH (control) sense,
5′-TGTTGCCATCAATGACCCCTT-3′ and antisense, 5′-CTCCACGACGTACTCAG
CG-3′. Relative quantification was achieved by normalization of
GAPDH.
Adenovirus transduction
Recombinant adenoviruses Ad-β-catenin or Ad-GFP were
transduced into MDA-MB-231 cells according to the manufacturer's
instructions. The expression of GFP was used as a marker for
monitoring transduction efficiency. After 48 h of transduction, the
cells were used for experiments and the target gene expression was
also determined by qRT-PCR and western blotting.
Immunohistochemistry (IHC)
staining
The expression of molecules which were association
with EMT was detected by IHC staining. Briefly, 5-µm thick tissue
sections were cut and deparaffinized, and an antigen retrieval
procedure was performed. Endogenous peroxidases were quenched by
incubating tissue with hydrogen peroxide, followed by incubating
with the primary antibody at 4°C overnight and the HRP-labeled
secondary antibody sequentially. Finally, the sections were
visualized with DAB staining and imaged.
Statistical analysis
All experiments were conducted for at least three
independent times. All data are expressed as mean ± SD and the
Student's t-test and one-way ANOVA analysis were used to determine
significance. P<0.05 was considered significant.
Results
Luteolin inhibits breast cancer cell
migration and invasion in vitro
To determine whether luteolin influences breast
cancer cell migration and invasion, wound-healing migration and
Transwell invasion assays were performed to evaluate the metastasis
of breast cancer cell lines MDA-MB-231 and BT5-49. Pretreatment
with luteolin resulted in a concentration-dependent slowing of the
wound healing ability of breast cancer cells compared with the
control group (Fig. 1B and C). The
Transwell invasion assay revealed a dose-dependent decrease in cell
invasion in the luteolin-treated breast cancer cell lines when
compared with the control group (Fig.
1D and E).
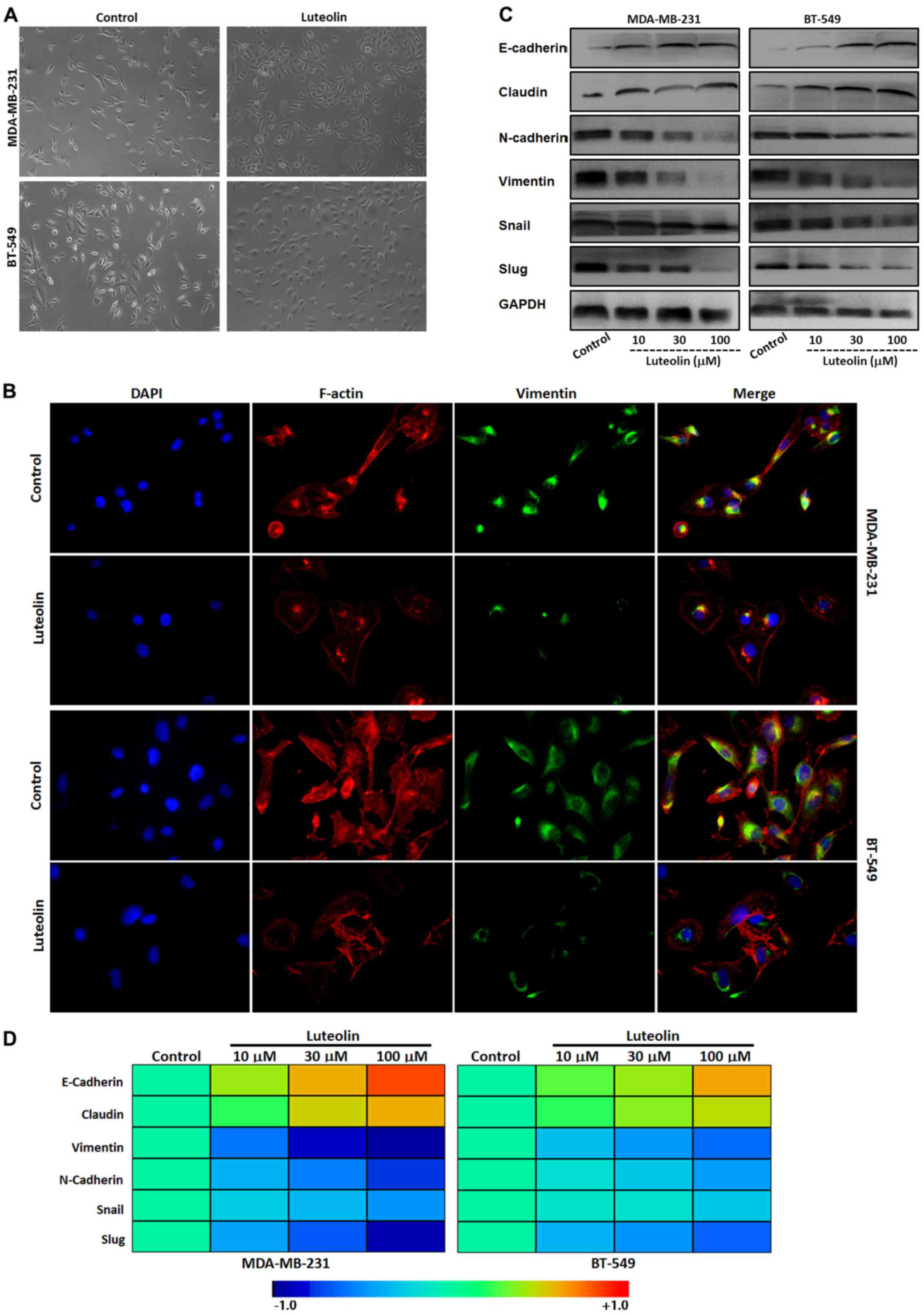
Luteolin reverses EMT in the
MDA-MB-231 and BT5-49 cells
As previously stated, the morphology of MDA-MB-231
and BT5-49 cells display a long spindle mesenchymal feature.
Following treatment with luteolin, the cancer cell morphology
shifted to an oval epithelial type (Fig. 2A). In agreement with this
observation, IF assay showed that luteolin downregulated the
mesenchymal marker vimentin and reorganized cytoskeletal protein
F-actin in the cytoplasm in the two breast cancer cell lines
(Fig. 2B). Western blot analysis
further showed that levels of epithelial markers E-cadherin and
claudin were upregulated, while mesenchymal markers N-cadherin and
vimentin with the two EMT-related transcription factors Snail and
Slug, particularly Slug, were downregulated in the luteolin-treated
breast cancer cells in a concentration-dependent manner (Fig. 2C). Consistently, mRNA expression
analysis by qRT-PCR showed similar results with the proteins of EMT
(Fig. 2D).
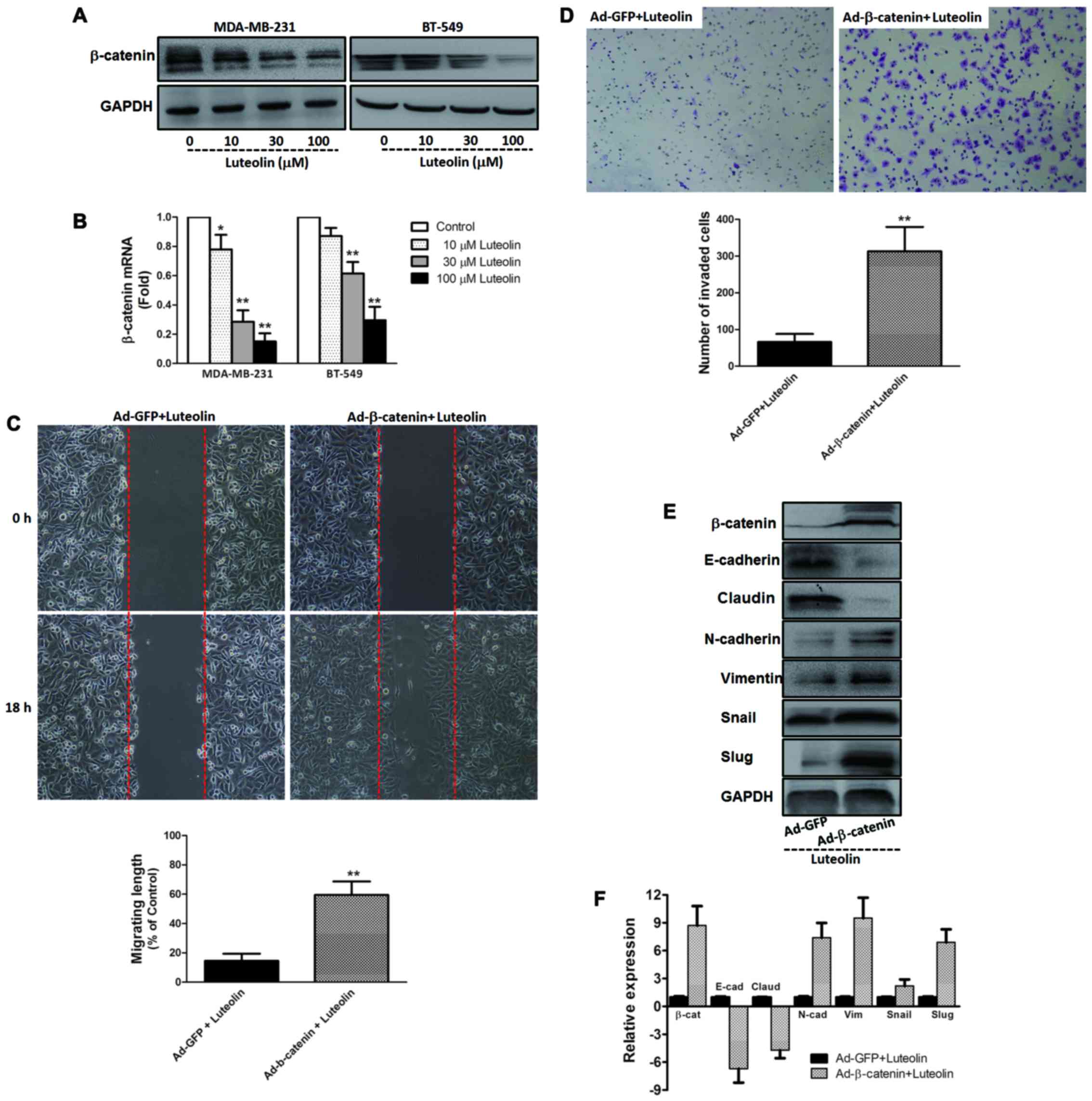
β-catenin is responsible for the
anti-migration and anti-invasion effect of luteolin in vitro
In light of the key role of β-catenin in metastasis
and EMT of cancer, we determined the expression of β-catenin by
western blotting and qRT-PCR. As shown in Fig. 3A and B, compared with the control
group, the two breast cancer cell lines expressed higher levels of
β-catenin protein and mRNA, which were inhibited by luteolin in a
dose-dependent manner. Furthermore, overexpression of β-catenin by
an adenovirus vector system was carried out in the breast cancer
cell line MDA-MB-231. As shown in Fig.
3C and D, overexpression of β-catenin abrogated the
anti-migration and anti-invasion effect of luteolin in the
MDA-MB-231 cells. Meantime, we found that these benefits of
luteolin on the expression of EMT markers and EMT-related
transcription factors were abrogated by overexpression of β-catenin
(Fig. 3E and F).
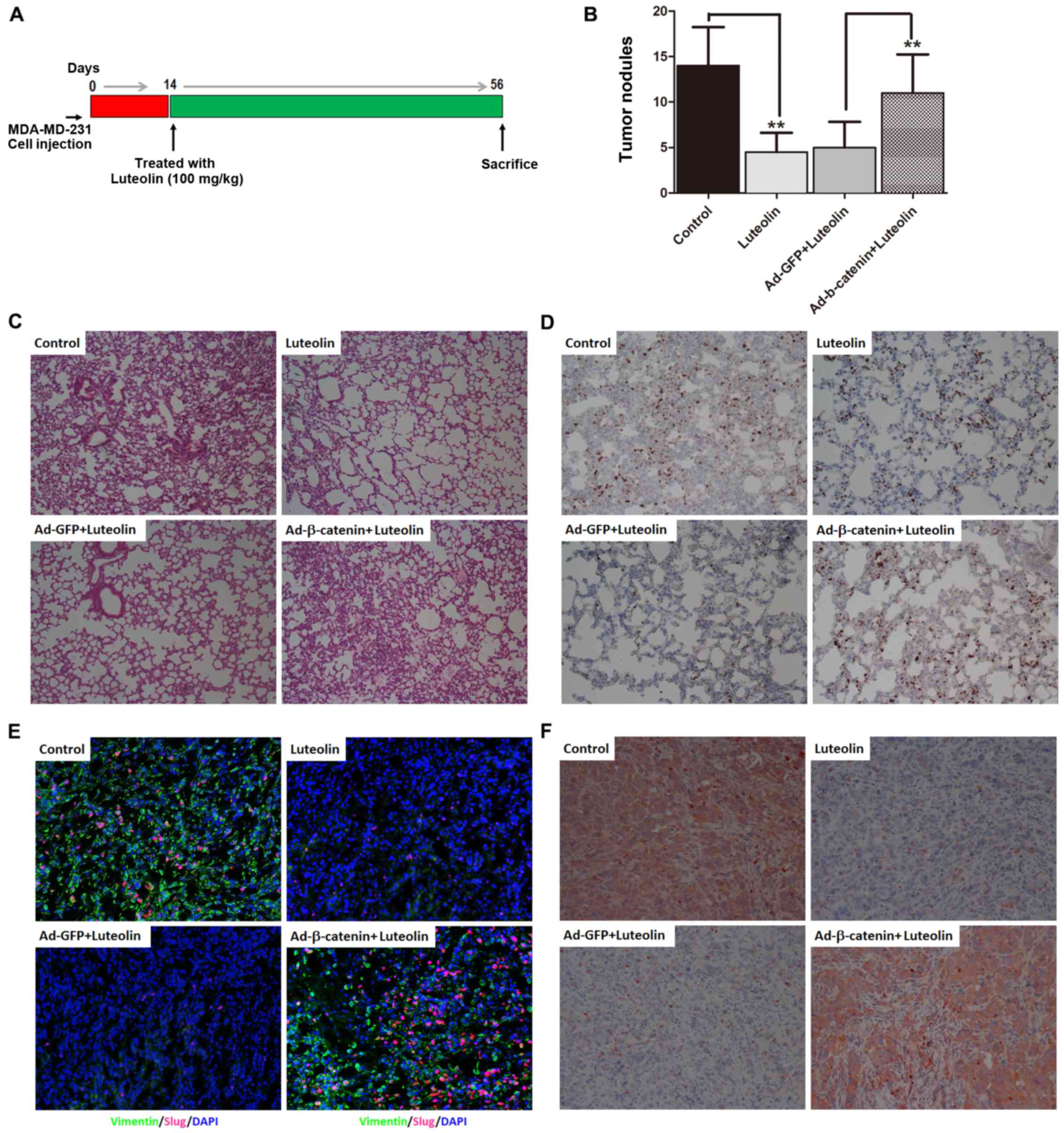
Luteolin suppresses metastasis of
breast cancer cells in vivo
Finally, we explore the in vivo effect of
luteolin on cancer metastasis. As illustrated in Fig. 4A, using a xenograft metastasis tumor
model, we found that the prominent metastatic nodules on the
surface of the lung were obviously observed in the mice bearing
TNBC cells. Luteolin markedly decreased the number of nodules in
the lung, which were abrogated by overexpression of β-catenin
(Fig. 4B). H&E and Ki67
staining of lungs isolated from the mice receiving orthotopic
transplantation displayed that luteolin markedly inhibited breast
cancer cell metastases to the lung, while overexpression of
β-catenin abolished the inhibitory effect of luteolin on lung
metastases of breast cancer (Fig. 4C
and D). Noteworthy, the levels of mesenchymal marker vimentin
and transcription repressor Slug in the primary tumor tissues were
decreased in the orthotopic tumors of the mice after luteolin
treatment, while overexpression of β-catenin upregulated vimentin
and Slug (Fig. 4E). In agreement
with these in vivo observations, β-catenin emerged as a high
expression protein in primary tumors of xenograft metastatic mice
but was obviously downregulated by luteolin treatment (Fig. 4F).
Discussion
Breast cancer is a heterogeneous disease, which is
classified into at least five distinct subtypes according to
molecular profiles of gene expression (25). Among these subtypes, triple-negative
breast cancer (TNBC), defined as estrogen receptor-negative,
progesterone receptor-negative, and lack of HER-2 expression, is an
aggressive metastatic and higher histological grade subtype of
breast cancer (23). Due to the
lack of effective targeted therapy and highly metastatic behavior,
patients with TNBC have a relatively poor outcome (26). In the present study, we confirmed
that luteolin efficiently suppressed the migration and invasion of
TNBC cell lines MDA-MB-231 and BT-549, and inhibited the lung
metastases of breast cancer xenograft tumors derived from
MDA-MD-231 cells, implying that luteolin may serve as a potential
drug for the treatment of cancer metastasis in TNBC.
Accumulating evidence reveals that EMT plays a
critical role in invasiveness and the metastasis of breast cancer.
It has been proposed that the EMT process occurs during metastatic
breast cancer in which cancer cells show a fibroblast-like and
spindle morphology with loss of epithelial markers E-cadherin and
claudin and gain of mesenchymal markers vimentin and N-cadherin
(27). Clinical data suggest that
in patients with TNBC, cancer cells display a particular
mesenchymal phenotypic shift, and the expression of mesenchymal
markers is associated with poor prognosis and metastatic potential
(23,28). Previous studies have shown that
mesenchymal molecules promote migration and invasion of breast
cancer cells in vitro and in vivo models, and
knockout of the vimentin or N-cadherin gene effectively attenuates
breast cancer cell motility and invasion (29,30).
Based on the antimetastatic capacity of luteolin, we aimed to
ascertain whether luteolin affects EMT of TNBC. Our present results
indicated that following treatment with luteolin, TNBC cells not
only acquired epithelial characteristics, but also lost mesenchymal
characteristics, implying that luteolin could effectively reverse
EMT in highly metastatic TNBC. This is consistent with a study
describing that luteolin inhibited EMT and invasiveness in
pancreatic cancer cell lines (31).
Furthermore, we also analyzed the expression of two transcription
factors Snail and Slug, which have been demonstrated to regulate
EMT and promote tumor metastasis in breast cancer (32). The results showed that luteolin
dose-dependently inhibited the expression of these transcription
repressors, particularly Slug. These data confirmed that the
inhibition of metastasis by luteolin involves the reversion of
EMT.
Many signaling mechanisms have been implicated to be
involved in the regulation of EMT and cancer metastasis, including
the PI3K/Akt, MAPK/ERK and Wnt/β-catenin pathways (3). Several studies have uncovered that
Wnt/β-catenin signaling is significantly activated in highly
aggressive metastatic breast cancer cell lines and patients with
TNBC, and hyperactivated Wnt/β-catenin signaling is positively
correlated with poor clinical prognosis (33,34).
In canonical Wnt/β-catenin signaling, β-catenin plays a central
role. Activation of Wnt signaling leads to the stabilization and
subsequent nuclear translocation of β-catenin, which induces
expression of transcriptional repressors such as Snail, triggering
EMT that is linked to cancer metastasis (34,35).
Inhibition or silencing of β-catenin markedly inhibits the
expression of EMT-related transcriptional factors Snail and Slug,
which lead to the expression of epithelial markers E-cadherin,
resulting in reversal of EMT and alleviation of the metastasis of
breast cancer (36,37). Thereby, β-catenin may become a key
target for treating metastatic cancer. Notably, a previous study
showed that luteolin inhibited cell proliferation and tumor growth
in experimental colon carcinogenesis by inhibiting the β-catenin
signaling pathway (38). In the
present study, using in vitro and in vivo metastasis
breast cancer models, we first found that β-catenin expression was
dose-dependently reduced by luteolin in two TNBC cell lines, and we
further found that luteolin markedly downregulated β-catenin in
primary tumor tissues of the xenografts, which coincided with the
abilities of luteolin on metastasis and EMT of breast cancer.
Hence, we deduced that the antimetastatic affect of luteolin may be
mediated by β-catenin signaling. In order to verify the hypothesis,
ectopic expression of β-catenin was determined before luteolin
treatment in vitro and in vivo metastasis
experiments. We found that these beneficial effects of luteolin
were abrogated by overexpression of β-catenin, indicating that
downregulation of β-catenin expression may mediate the inhibitory
effects of luteolin on metastasis and EMT of TNBC.
Taken together, our experimental data indicated that
luteolin exerted a potent therapeutic effect on invasion and
metastasis of TNBC, which may be involved in the reversal of EMT by
downregulation of β-catenin. These findings suggest that luteolin
may be applied as a potential candidate treatment for the
prevention and intervention of metastatic breast cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81472475). We
thank Dr Jianfei Guo, James Winkle College of Pharmacy, University
of Cincinnati, OH for the discussion and proofreading of the
manuscript.
References
|
1
|
DeSantis CE, Fedewa SA, Sauer A Goding,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnedos M, Vicier C, Loi S, Lefebvre C,
Michiels S, Bonnefoi H and Andre F: Precision medicine for
metastatic breast cancer - limitations and solutions. Nat Rev Clin
Oncol. 12:693–704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baranwal S and Alahari SK: Molecular
mechanisms controlling E-cadherin expression in breast cancer.
Biochem Biophys Res Commun. 384:6–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Zhang L, Hu C, Liang S, Fei X, Yan
N, Zhang Y and Zhang F: WNT pathway inhibitor pyrvinium pamoate
inhibits the self-renewal and metastasis of breast cancer stem
cells. Int J Oncol. 48:1175–1186. 2016.PubMed/NCBI
|
|
13
|
Ross JA and Kasum CM: Dietary flavonoids:
Bioavailability, metabolic effects, and safety. Annu Rev Nutr.
22:19–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CY, Peng WH, Tsai KD and Hsu SL:
Luteolin suppresses inflammation-associated gene expression by
blocking NF-kappaB and AP-1 activation pathway in mouse alveolar
macrophages. Life Sci. 81:1602–1614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung JG, Hsia TC, Kuo HM, Li YC, Lee YM,
Lin SS and Hung CF: Inhibitory actions of luteolin on the growth
and arylamine N-acetyltransferase activity in strains of
Helicobacter pylori from ulcer patients. Toxicol In Vitro.
15:191–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du Y, Feng J, Wang R, Zhang H and Liu J:
Effects of flavonoids from Potamogeton crispus L. on proliferation,
migration, and invasion of human ovarian cancer cells. PLoS One.
10:e01306852015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma L, Peng H, Li K, Zhao R, Li L, Yu Y,
Wang X and Han Z: Luteolin exerts an anticancer effect on NCI-H460
human non-small cell lung cancer cells through the induction of
Sirt1-mediated apoptosis. Mol Med Rep. 12:4196–4202.
2015.PubMed/NCBI
|
|
19
|
Shi R, Huang Q, Zhu X, Ong YB, Zhao B, Lu
J, Ong CN and Shen HM: Luteolin sensitizes the anticancer effect of
cisplatin via c-Jun NH2-terminal kinase-mediated p53
phosphorylation and stabilization. Mol Cancer Ther. 6:1338–1347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ
and Tseng TH: Luteolin enhances paclitaxel-induced apoptosis in
human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol
Interact. 213:60–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tu SH, Ho CT, Liu MF, Huang CS, Chang HW,
Chang CH, Wu CH and Ho YS: Luteolin sensitises drug-resistant human
breast cancer cells to tamoxifen via the inhibition of cyclin E2
expression. Food Chem. 141:1553–1561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cook MT, Liang Y, Besch-Williford C,
Goyette S, Mafuvadze B and Hyder SM: Luteolin inhibits
progestin-dependent angiogenesis, stem cell-like characteristics,
and growth of human breast cancer xenografts. Springerplus.
4:4442015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao C, Qiao Y, Jonsson P, Wang J, Xu L,
Rouhi P, Sinha I, Cao Y, Williams C and Dahlman-Wright K:
Genome-wide profiling of AP-1-regulated transcription provides
insights into the invasiveness of triple-negative breast cancer.
Cancer Res. 74:3983–3994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Voduc KD, Cheang MCU, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carey L, Winer E, Viale G, Cameron D and
Gianni L: Triple-negative breast cancer: Disease entity or title of
convenience? Nat Rev Clin Oncol. 7:683–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chung S, Yao J, Suyama K, Bajaj S, Qian X,
Loudig OD, Eugenin EA, Phillips GR and Hazan RB: N-cadherin
regulates mammary tumor cell migration through Akt3 suppression.
Oncogene. 32:422–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X, Dai S, Dai J, Xiao Y, Bai Y, Chen
B and Zhou M: Luteolin decreases invasiveness, deactivates STAT3
signaling, and reverses interleukin-6 induced
epithelial-mesenchymal transition and matrix metalloproteinase
secretion of pancreatic cancer cells. Onco Targets Ther.
8:2989–3001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Herreros AG, Peiró S, Nassour M and
Savagner P: Snail family regulation and epithelial mesenchymal
transitions in breast cancer progression. J Mammary Gland Biol
Neoplasia. 15:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dey N, Barwick BG, Moreno CS,
Ordanic-Kodani M, Chen Z, Oprea-Ilies G, Tang W, Catzavelos C,
Kerstann KF, Sledge GW Jr, et al: Wnt signaling in triple negative
breast cancer is associated with metastasis. BMC Cancer.
13:5372013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geyer FC, Lacroix-Triki M, Savage K,
Arnedos M, Lambros MB, MacKay A, Natrajan R and Reis-Filho JS:
β-Catenin pathway activation in breast cancer is associated with
triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol.
24:209–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sánchez-Tilló E, de Barrios O, Siles L,
Cuatrecasas M, Castells A and Postigo A: β-catenin/TCF4 complex
induces the epithelial-to-mesenchymal transition (EMT)-activator
ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA.
108:19204–19209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG and
Weiss SJ: Canonical Wnt signaling regulates Slug activity and links
epithelial-mesenchymal transition with epigenetic Breast Cancer 1,
Early Onset (BRCA1) repression. Proc Natl Acad Sci USA.
109:16654–16659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim
NH, Cha SY, Ryu JK, Choi YJ, Kim J, et al: A Wnt-Axin2-GSK3beta
cascade regulates Snail1 activity in breast cancer cells. Nat Cell
Biol. 8:1398–1406. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ashokkumar P and Sudhandiran G: Luteolin
inhibits cell proliferation during Azoxymethane-induced
experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest
New Drugs. 29:273–284. 2011. View Article : Google Scholar : PubMed/NCBI
|