Introduction
Cholangiocarcinoma arises from the epithelial cells
of the intrahepatic, perihilar and distal biliary tree (1). Despite significant improvements in
surgery combined with chemotherapy using gemcitabine and cisplatin,
the prognosis of cholangiocarcinoma patients still remains poor
with a median survival of less than one year (1). In the recent decade, deregulations of
various oncogenes or tumor suppressors have been implicated in
malignant progression of human cancers (2–4).
Therefore, revealing the underlying mechanism of cholangiocarcinoma
development and progression may help improve the development of
effective therapeutic strategies.
Chemokines, secreted by various cell types, play
central roles in chemotaxis (5,6).
According to the order of conserved cysteine residues, chemokines
are classified into C, CC, CXC and C(X)3C, and CXC chemokines are
further classified into ELR+ CXC and ELR-CXC, according
to the absence or presence of the amino-terminal ELR motif
(6,7). Many studies have shown that chemokines
are involved in the tumorigenesis and malignant progression of
human cancers, and therefore may become potential therapeutic
target for cancer treatment (8–10). For
instance, lung cancer cells were found to utilize the CXCL12/CXCR4
signaling to benefit growth and distant spread (4). CXCL5/CXCR2 axis can promote the
migration and invasion of bladder cancer cells by activating
PI3K/AKT-induced upregulation of MMP2/MMP9 (11).
CXCL7 is a platelet-derived growth factor that
belongs to the CXC chemokine family, functioning as a potent
chemoattractant and activator of neutrophils through binding to its
receptor CXCR2 (12). CXCL7 has
been demonstrated to participate in a variety of cellular
processes, such as DNA synthesis, glycolysis, mitosis,
intracellular cAMP accumulation, prostaglandin E2 secretion, as
well as the synthesis of hyaluronic acid and plasminogen activator
(12–17). Moreover, it is also an antimicrobial
protein with bactericidal and antifungal activity (18). Recently, CXCL7 has been found to be
deregulated in human cancers, and plays a role in tumor growth. For
instance, CXCL7 was found to promote the growth of clear cell renal
cell carcinoma (19). Desurmont
et al reported that overexpression of CXCL7 and CXCR2 in
liver metastases from colon cancer was correlated to shorter
disease-free and overall survival (20). However, the role of CXCL7 in
cholangiocarcinoma has not been previously reported.
The present study aimed to investigate the
expression of CXCL7 in cholangiocarcinoma tissues, as well as the
regulatory role of CXCL7 in the malignant phenotypes of
cholangiocarcinoma cells.
Materials and methods
Tissue sample collection
This study was approved by the legislation and
ethical boards of Guangdong General Hospital, Guangdong Academy of
Medical Sciences, Guangzhou, China. A total of 156
cholangiocarcinoma tissues and 35 adjacent non-tumor tissues were
collected from our hospital. Written informed consent was obtained
from all the patients. The patients involved in this study received
no preoperative chemotherapy or radiotherapy. The clinicopathologic
characteristics of cholangiocarcinoma samples were summarized in
Table I. All tissues were formalin
fixed and paraffin-embedded.
 | Table I.Association between CXCL7 expression
and clinicopathological characteristics in cholangiocarcinoma. |
Table I.
Association between CXCL7 expression
and clinicopathological characteristics in cholangiocarcinoma.
| Variables | No. | Low CXCL7 (n=79) | High CXCL7
(n=77) | P-value |
|---|
| Age |
|
|
|
|
|
<55 | 54 | 25 | 29 | 0.43 |
| ≥55 | 102 | 54 | 48 |
|
| Gender |
|
|
|
|
| Male | 94 | 50 | 44 | 0.433 |
|
Female | 62 | 29 | 33 |
|
| Tumor size |
|
|
|
|
| <4
cm | 105 | 66 | 49 | 0.143 |
| ≥4
cm | 51 | 23 | 28 |
|
| Differentiation |
|
|
|
|
|
Well-Moderate | 88 | 51 | 37 | 0.038 |
| Poor | 68 | 28 | 40 |
|
| Lymph node
metastasis |
|
|
|
|
| No | 68 | 46 | 22 | 0.0002 |
| Yes | 88 | 33 | 55 |
|
| Vascular
invasion |
|
|
|
|
| No | 81 | 58 | 23 | <0.0001 |
|
Yes | 75 | 21 | 54 |
|
| Clinical stage |
|
|
|
|
|
I–II | 61 | 44 | 17 | <0.0001 |
|
III–IV | 95 | 35 | 60 |
|
Immunohistochemical staining
assay
Sections (4 µm) were deparaffinized and subjected to
heat-induced antigen retrieval using citrate buffer for 22 min
using a microwave oven, which were then incubated with primary
antibodies, and then with secondary antibody for 1 h at room
temperature. The reaction was developed using substrate
diaminobenzidine and counterstained with hematoxylin. The protein
expression was scored by 3 pathologists independently. The
percentage of positively staining cells was graded as 0 (no
staining, negative), +: >0 and ≤25% of cells positive, ++:
>25 and ≤75% of cells positive, +++: >75% of cells
positive.
Cell cultures
Human cholangiocarcinoma cell lines (HuCCT1, HuH28,
QBC939, EGI-1, OZ and WITT) and human hepatic stellate cell line
LX-1 were purchased from Cell Bank of Chinese Academic Institute,
Shanghai, China. All cell lines were cultured in DMEM (Gibco,
Carlsbad, CA, USA) added with 10% FBS (Gibco) in a 37°C incubator
with 5% CO2.
Cell transfection
Lipofectamine 2000 was used to conduct cell
transfection, according to the manufacturer's instructions.
Briefly, cells were cultured to 70% confluence, and resuspended in
serum-free DMEM medium. The blank pcDNA3.1 vector, pcDNA3.1-CXCL7
plasmid, non-specific siRNA, CXCL7 siRNA, and Lipofectamine 2000
were diluted with serum-free medium, respectively. The diluted
Lipofectamine 2000 was then added into the diluted plasmid or
siRNA, and incubated for 20 min at room temperature, and then added
into the cell suspension. After incubation at 37°C for 6 h, the
medium was replaced by the normal serum-containing medium. Then,
cells were cultured for 48 h before the following assays.
RT-PCR analysis
Total RNA was extracted by using TRIzol Reagent
(Life Technologies), according to the manufacturer's instructions.
A total of 800 ng RNA was converted into cDNA using Reverse
Transcription kit (Life Technologies), according to the
manufacturer's instructions. Real-time PCR was then performed by
using Q-PCR Detection kit (Life Technologies) on ABI 7500
thermocycler. The PCR steps were 95°C for 10 min, and 40 cycles of
denaturation at 95°C for 15 sec and annealing/elongation step at
60°C for 60 sec. GAPDH was used as an internal control. The
relative expression was analyzed by the 2−∆∆Ct
method.
Western blotting
Cells were lysed with ice-cold lysis buffer (50 mM
Tris-HCl, pH 6.8, 100 mM 2-ME, 2% w/v SDS, 10% glycerol). Protein
was separated with 10% SDS-PAGE and then transferred onto a
polyvinylidene difluoride (PVDF) membrane (Life Technologies). The
PVDF membrane was incubated with PBS containing 5% milk overnight
at 4°C. After washing with PBS 3 times, the PVDF membrane was
incubated with primary antibodies (Abcam, Cambridge, MA, USA) at
room temperature for 3 h. After washing with PBS 3 times, the PVDF
membrane was incubated with secondary antibody (Abcam) at room
temperature for 1 h. Super Signal West Pico Chemiluminescent
Substrate kit (Pierce, Rockford, IL, USA) was then used to detect
signals, according to the manufacturer's instructions. The relative
protein expression was analyzed by Image-Pro plus software 6.0,
represented as the density ratio versus GAPDH.
Enzyme-linked immunosorbent assay
(ELISA)
Cells in DMEM containing 10% FBS were seeded in a
6-well plate (1.5×105 cells/well) and cultured for 48 h.
Then, the supernatant was collected, and centrifuged at 12000 × g
for 10 min. The secretion level of CXCL7 was detected using a human
CXCL7 ELISA kit (Thermo Fisher Scientific, Inc., Bethesda, MA,
USA), according to the manufacturer's instructions.
MTT assay
MTT assay was used to examine cell proliferation.
Briefly, cells were plated at a density of 10,000 cells per well in
96-well plates. After cultured for 0, 24, 48 and 72 h, the cells
were incubated with MTT at a final concentration of 0.5 mg/ml for 4
h at 37°C. After the removal of the medium, 150 mM of DMSO solution
was added. The absorbance was read at 570 nm using a Bio-Tek™
ELX-800™ Absorbance Microplate reader.
Transwell assay
Transwell assay was performed to examine the cell
invasion using Transwell chambers (BD Biosciences, Franklin Lakes,
NJ, USA). Cell suspension containing 5×105 cells/ml was
prepared in serum-free media, and 300 µl of cell suspension was
added into the upper chamber. Then, 500 µl of DMEM with 10% FBS was
added into the lower chamber. Cells were incubated for 24 h. Then,
a cotton-tipped swab was used to carefully wipe out the cells that
did not migrate through the pores. The filters were fixed in 90%
alcohol and stained by crystal violet, and observed under an
inverted microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analysis was performed using SPSS 17.0 (SPSS, Armonk, NY, USA). The
differences between two groups were analyzed using Student's
t-test. The differences among more than two groups were analyzed
using ANOVA. P<0.05 indicated significant differences.
Results
Upregulation of CXCL7 is associated
with cholangiocarcinoma progression
In the present study, our data indicated that the
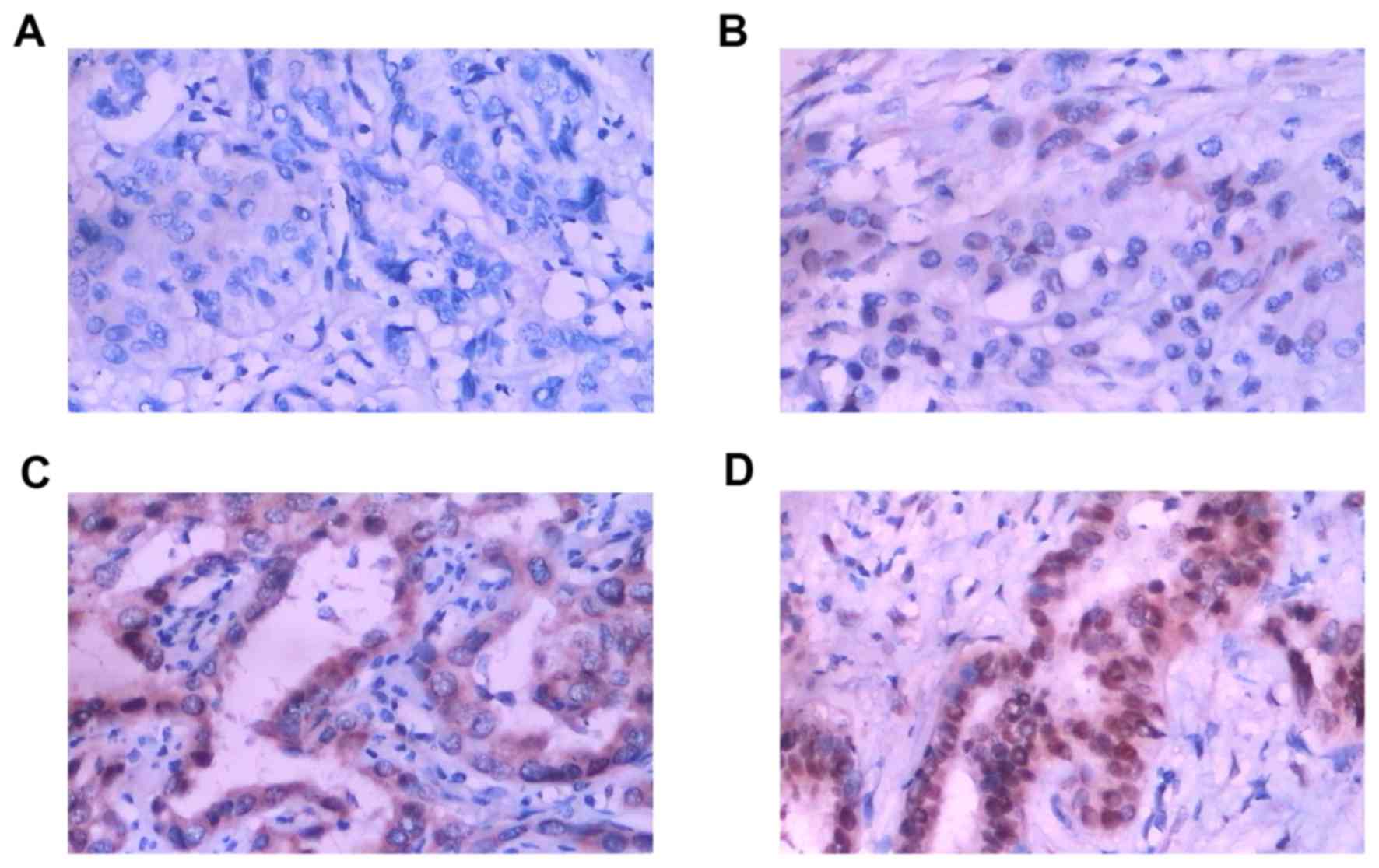
CXCL7 protein was mainly in the cytoplasm (Fig. 1). Fig.
1A represents negative expression (−), Fig. 1B weak expression (+), Fig.1C moderate expression (++), and
Fig. 1D strong expression (+++),
respectively. The positive expression of CXCL7 was found in 66%
(103/156) of cholangiocarcinoma cases, while only 23% (8/35) was
detected in adjacent non-tumor tissues. We further investigated the
clinical significance of CXCL7 expression in cholangiocarcinoma.
The cholangiocarcinoma patients were divided into two groups, low
CXCL7 expression group (negative and weak expression) and high
CXCL7 expression group (moderate and strong expression). As
indicated in Table I, high CXCL7
expression was significantly associated with poor differentiation,
lymph node metastasis, vascular invasion, and advanced clinical
stage of cholangiocarcinoma. However, the expression of CXCL7 was
not associated with age, gender, and tumor size (Table I). We further investigated the
relationship between CXCL7 protein expression and the expression of
Ki67, CA199, AFP, and P53 in cholangiocarcinoma. As indicated in
Table II, the expression of CXCL7
was significantly associated with the Ki67 expression. However, the
CXCL7 expression was not associated with CA199, AFP, or P53
expression in cholangiocarcinoma.
 | Table II.Association between the expression
levels of CXCL7 and other markers in cholangiocarcinoma. |
Table II.
Association between the expression
levels of CXCL7 and other markers in cholangiocarcinoma.
| Variables | No. | Low CXCL7
(n=79) | High CXCL7
(n=77) | P-value |
|---|
| Ki67 |
|
|
|
|
|
Negative | 50 | 38 | 12 | <0.0001 |
|
Positive | 106 | 41 | 65 |
|
| CA199 |
|
|
|
|
|
Negative | 58 | 34 | 24 | 0.125 |
|
Positive | 98 | 45 | 53 |
|
| AFP |
|
|
|
|
|
Negative | 52 | 24 | 28 | 0.428 |
|
Positive | 104 | 55 | 49 |
|
| P53 |
|
|
|
|
|
Negative | 69 | 32 | 37 | 0.343 |
|
Positive | 87 | 47 | 40 |
|
Increased CXCL7 expression is
associated with poor prognosis of cholangiocarcinoma patients
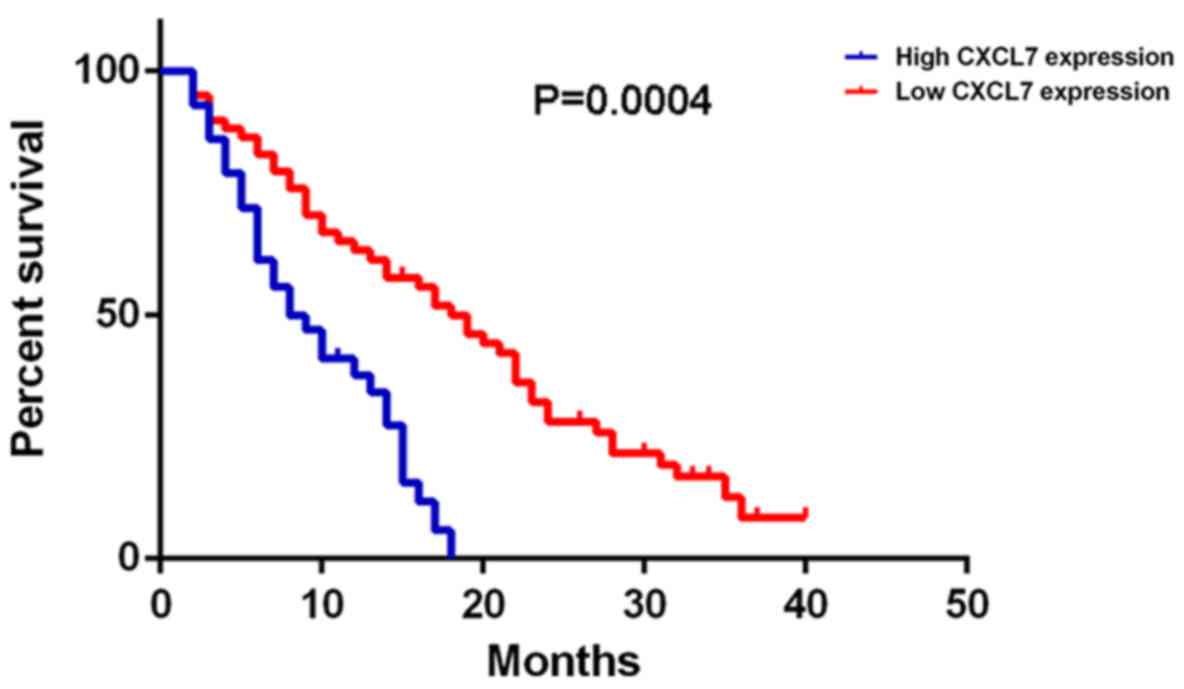
Further investigation indicated that the
cholangiocarcinoma patients with high CXCL7 expression had shorter
overall survival time, when compared with those with low CXCL7
expression (Fig. 2). Therefore, the
increased expression of CXCL7 is associated with the advanced
progression and poor prognosis of patients with
cholangiocarcinoma.
Knockdown of CXCL7 reduces the
proliferation and invasion of cholangiocarcinoma cells
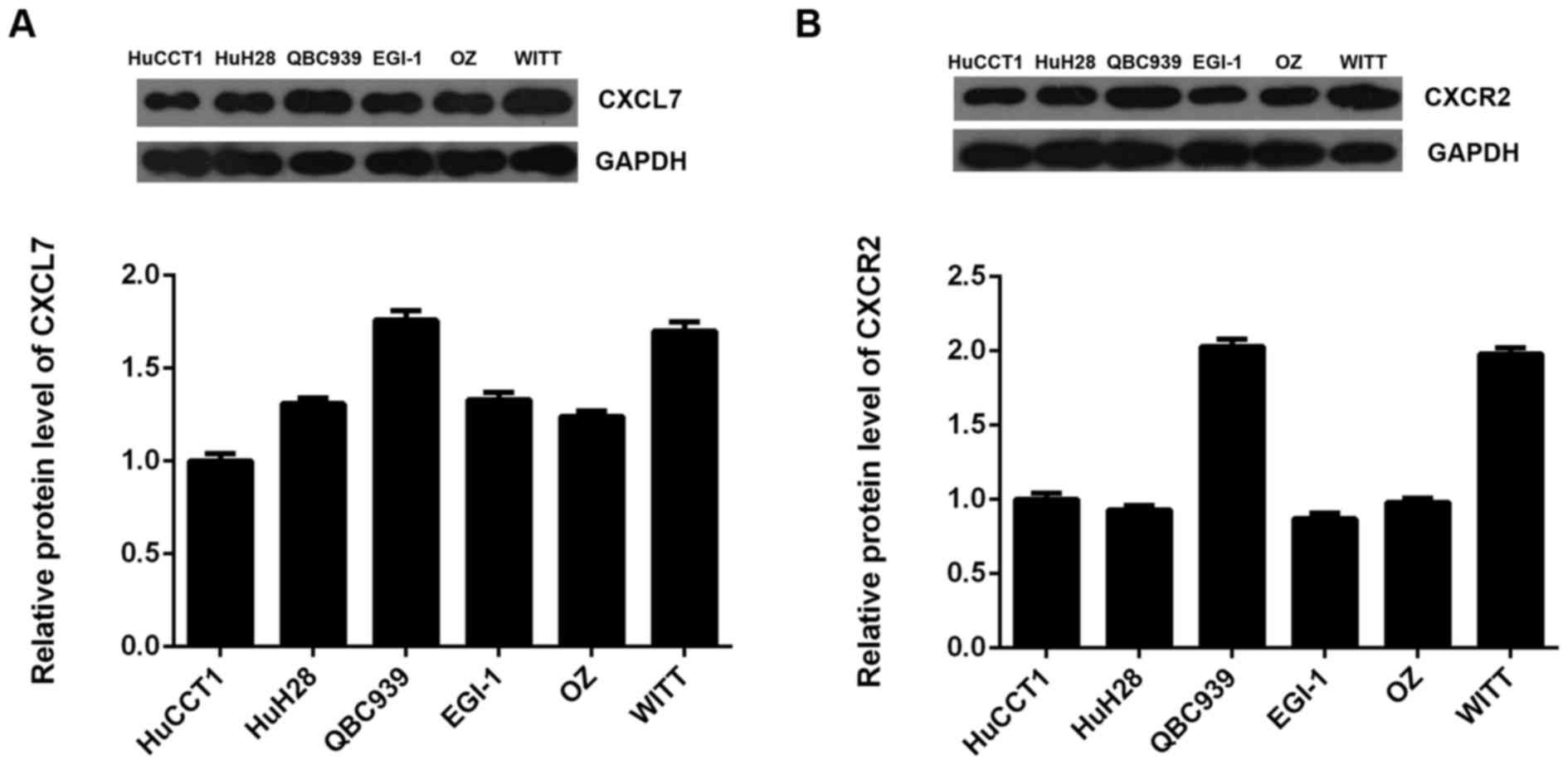
We further examined the protein expression of CXCL7
and CXCR2 in several common cholangiocarcinoma cell lines including
HuCCT1, HuH28, QBC939, EGI-1, OZ and WITT. As indicated in Fig. 3A and B, the protein expression of
CXCL7 and CXCR2 was positively expressed in these
cholangiocarcinoma cell lines. As QBC939 cells showed the highest
expression of CXCL7, we used this cell line in the following
experiments.
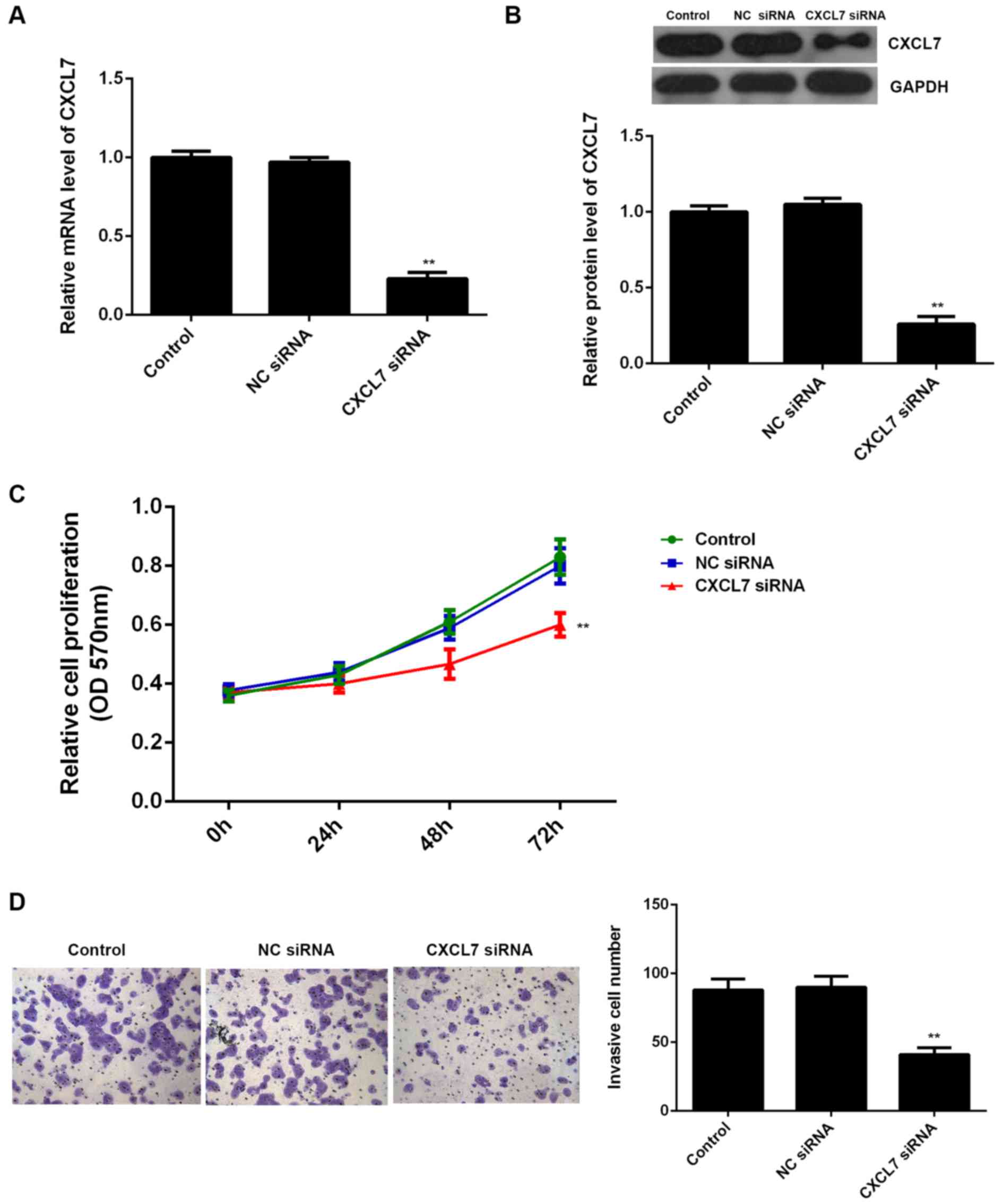
The effects of CXCL7 on the proliferation and
invasion of QBC939 cells were further studied. To knock down the
expression of CXCL7, QBC939 cells were transfected with
CXCL7-specific siRNA, or non-specific siRNA (NC siRNA),
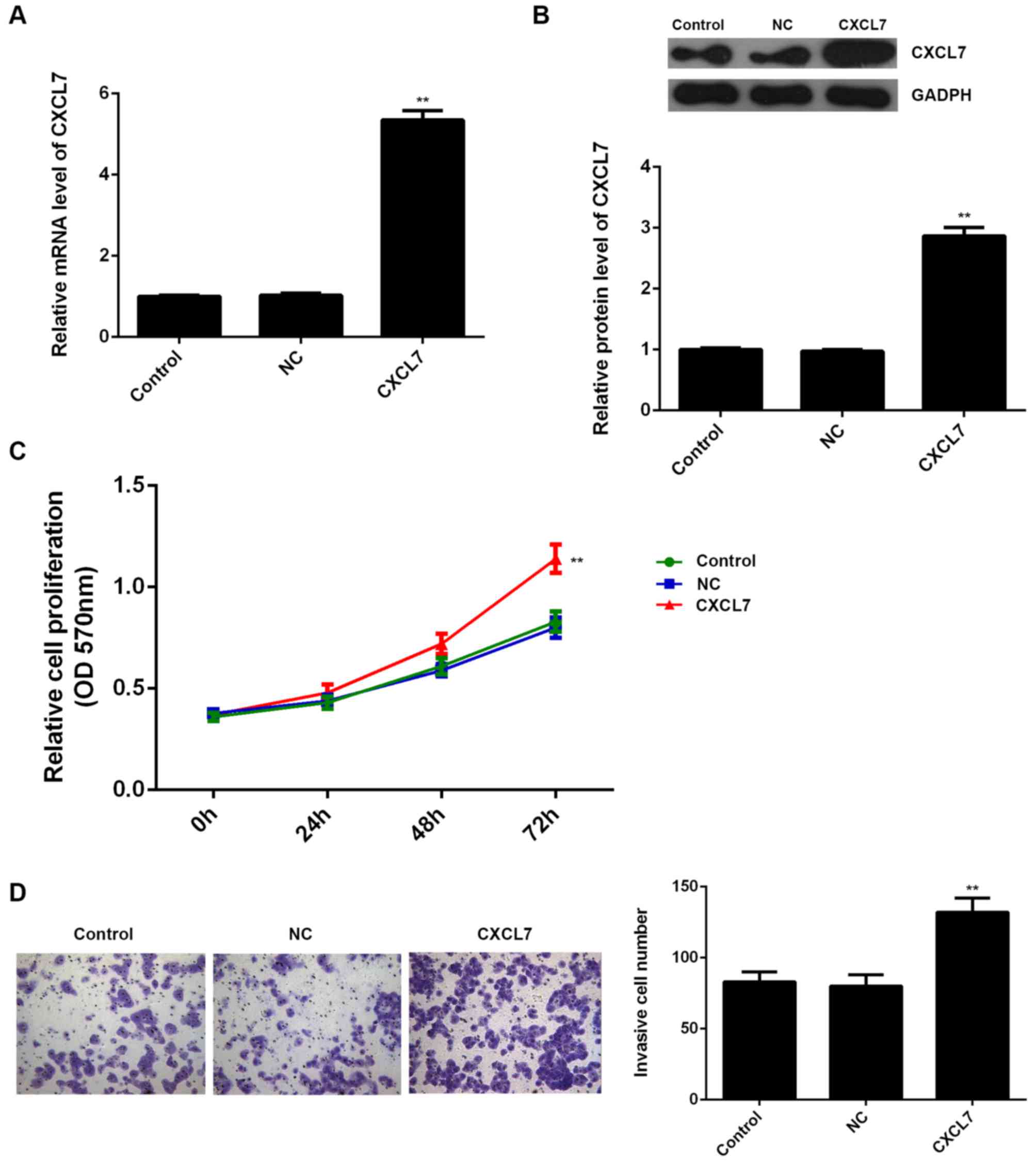
respectively. Our data showed that transfection with CXCL7-specific
siRNA significantly decreased the mRNA and protein expression of
CXCL7 compared to the control group (Fig. 4A and B). MTT assay and Transwell
assay further showed that knockdown of CXCL7 caused a significant
decrease in the proliferation and invasion of QBC939 cells
(Fig. 4C and D). These data suggest
that CXCL7 plays a promoting role in the regulation of the
malignant phenotypes of QBC939 cells. To further confirm these
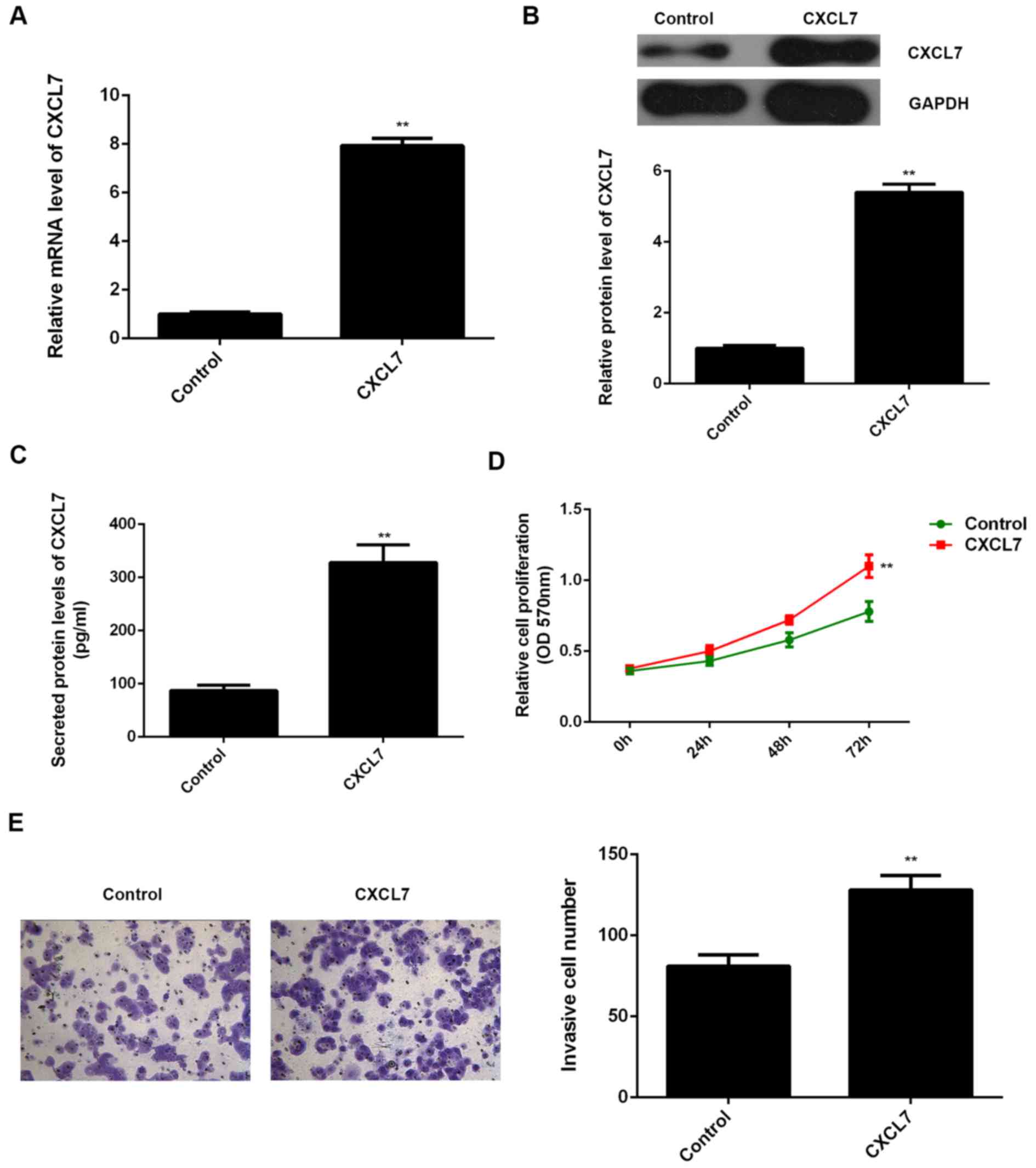
findings, QBC939 cells were transfected with pcDNA3.1-CXCL7 ORF
plasmid, or blank vector as NC group, respectively. After
transfection with pcDNA3.1-CXCL7 ORF plasmid, the mRNA and protein
levels of CXCL7 were significantly increased, when compared to the
control group, respectively (Fig. 5A
and B). Moreover, overexpression of CXCL7 remarkably enhanced
the proliferation and invasion of QBC939 cells (Fig. 5C and D). Taken together, we
demonstrate that CXCL7 can promote the proliferation and invasion
of cholangiocarcinoma cells.
CXCL7 promotes the malignant
phenotypes of cholangiocarcinoma cells in a paracrine-dependent
manner
As non-tumor cells in the tumor microenvironment can
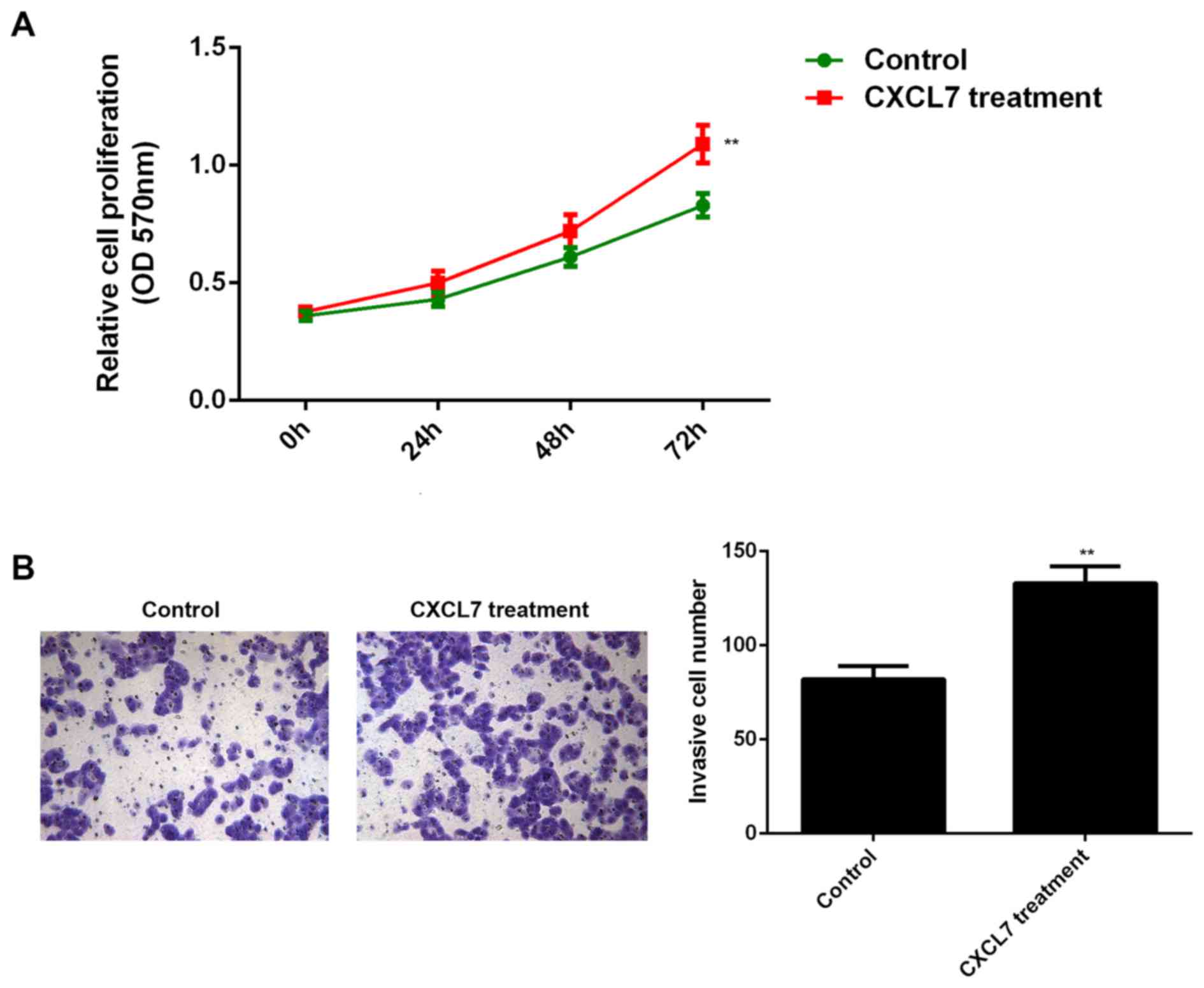
also secret CXCL7, we used 50 ng/ml of recombinant human CXCL7 to
treat QBC939 cells for 24 h. After treatment, the cell
proliferation and invasion of QBC939 cells were examined. As shown
in Fig. 6A and B, treatment with
CXCL7 significantly increased the proliferation and invasion of
QBC939 cells, when compared to the control group, respectively.
We further studied the effect of normal cell-derived
CXCL7 on the malignant phenotypes of cholangiocarcinoma cells.
Human hepatic stellate cell line LX-1 was transfected with CXCL7
ORF plasmid or blank vector, respectively. After transfection with
CXCL7 ORF plasmid, the mRNA and protein expression of CXCL7 in LX-1
cells were significantly increased compared to the control group
(Fig. 7A and B). ELISA data further
indicated that the CXCL7 levels in the CM of CXCL7-overexpressing
LX-1 cells were higher than those in the control group (Fig. 7C). The CM of LX-1 cells were further
used to culture QBC939 cells. As shown in Fig. 7D and E, the proliferation and
invasion of QBC939 cells cultured with the CM of
CXCL7-overexpressing LX-1 cells were significantly increased, when
compared to control group, respectively. These findings confirmed
that CXCL7 may also play a promoting role in cholangiocarcinoma in
a paracrine-dependent manner.
The AKT signaling is activated by
CXCL7 in cholangiocarcinoma QBC939 cells
AKT signaling has been demonstrated to be involved
in the development and malignant progression of human cancers. In
the present study, we studied whether the activity of AKT signaling
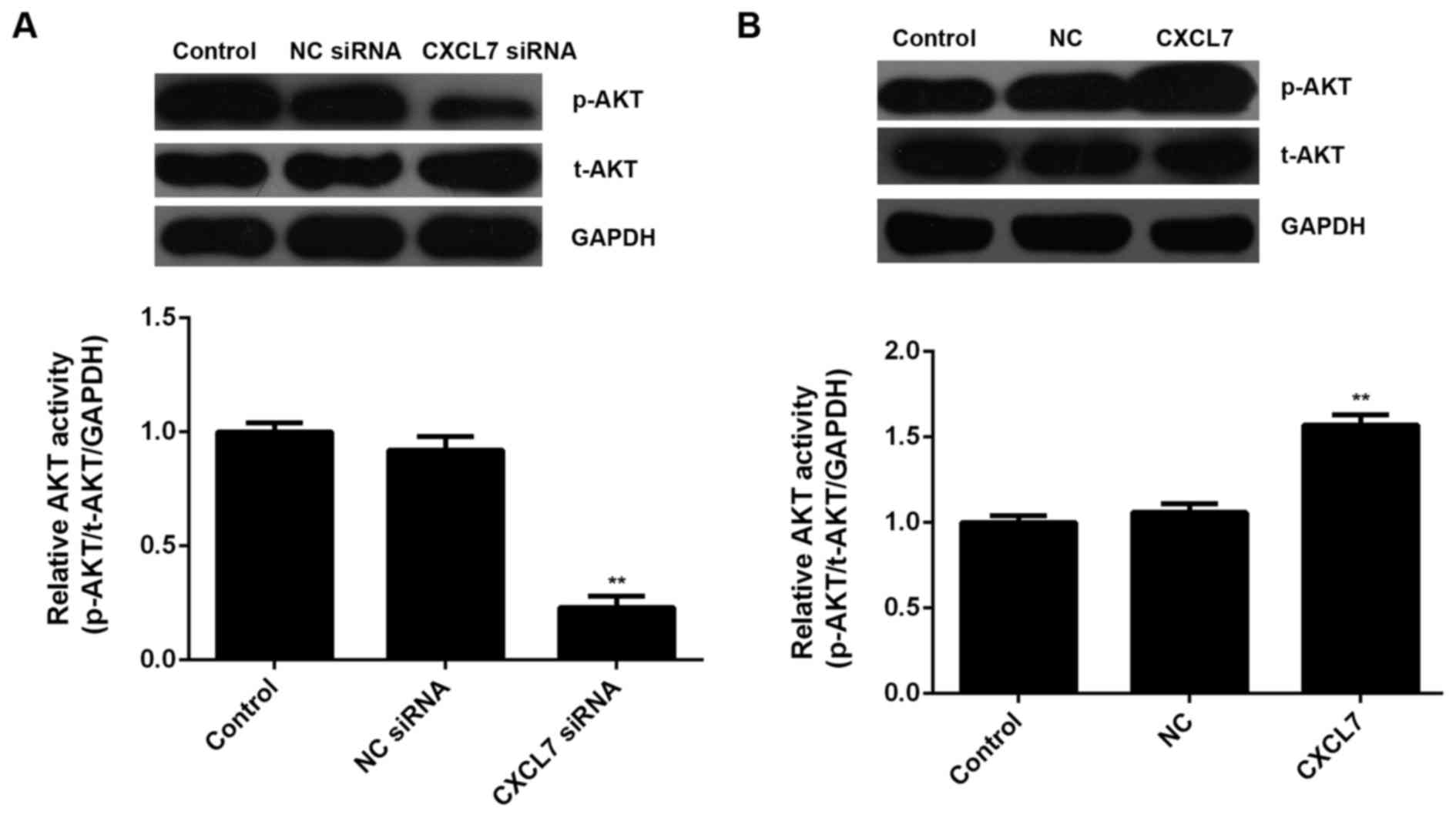
was affected by CXCL7 in cholangiocarcinoma cells. Western blotting
data showed that knockdown of CXCL7 significantly decreased the
activity of AKT signaling, when compared to the control group
(Fig. 8A). On the contrary,
overexpression of CXCL7 enhanced its activity, when compared to the
control group in cholangiocarcinoma QBC939 cells (Fig. 8B). According to these data, we
suggest that the activation of AKT signaling is involved in the
CXCL7-induced proliferation and invasion of cholangiocarcinoma
cells.
Discussion
The clinical outcomes of cholangiocarcinoma patients
remain poor. Therefore, understanding the molecular mechanism
underlying cholangiocarcinoma development and progression is
urgently needed, which may promote the potential benefit of
targeted therapy. In the present study, we investigated the
expression of CXCL7 in cholangiocarcinoma, as well as the role and
molecular mechanism of CXCL7 in the regulation of the malignant
phenotypes of cholangiocarcinoma cells. We found that the increased
expression of CXCL7 was significantly associated with advanced
progression and poor prognosis of cholangiocarcinoma patients.
In vitro study showed that CXCL7 plays a promoting role in
cholangiocarcinoma QBC939 cell proliferation and invasion.
Moreover, treatment with both exogenous CXCL7 and the CM of
CXCL7-overexpressing LX-1 cells could also promote the malignant
phenotypes of QBC939 cells. In addition, the activity of AKT
signaling was found to be upregulated after CXCL7
overexpression.
Deregulations of CXCL7 have been observed in several
human cancers, and it generally plays an oncogenic role. For
instance, CXCL7 is an independent prognostic factor for overall
survival in clear cell renal cell carcinoma (ccRCC). Moreover, it
was also reported that SB225002, an inhibitor of CXCR1 and CXCR2,
could inhibit endothelial cell proliferation, tumor angiogenesis
and ccRCC growth. On the contrary, overexpression of CXCL7 enhanced
ccRCC cell proliferation in vitro and tumor growth in
vivo (19). Here we for the
first time reported that CXCL7 was mainly expressed in
cholangiocarcinoma tissues compared to adjacent non-tumor tissues,
and the increased expression of CXCL7 was significantly associated
with poor differentiation, vascular invasion, lymph node
metastasis, advanced clinical stage, as well as shorter overall
survival time, but was not associated with age, gender and tumor
size. We further showed that CXCL7 and its receptor CXCR2 were
positively expressed in several common cholangiocarcinoma cell
lines. Therefore, CXCL7 may play a promoting role in the malignant
progression of cholangiocarcinoma.
We further studied the regulatory role of CXCL7 in
cholangiocarcinoma cells. Cholangiocarcinoma QBC939 cells were
transfected with CXCL7-specific siRNA or ORF plasmid to
downregulate or upregulate its expression, respectively. We found
that knockdown of CXCL7 significantly decreased QBC939 cell
proliferation and invasion. On the contrary, overexpression of
CXCL7 remarkably promoted these cellular events of QBC939 cells.
These findings suggest that CXCL7 may play a promoting role in
cholangiocarcinoma growth and metastasis. Similar findings were
also reported in other cancer types. For instance, tumors
established from Lewis lung carcinoma (LLC) cells overexpressing
CXCL7 increased the infiltration of M2 macrophages at the early
stages of tumorigenesis, and these CXCL7-overexpressing LLC tumors
developed faster than control tumors, suggesting that CXCL7
attracts macrophages especially at the tumor site and may
accelerate lung tumor development in the early stages (21). Therefore, CXCL7 may become a
potential therapeutic target for human cancers including
cholangiocarcinoma.
As CXCL7 could also be secreted by other normal cell
types in tumor microenvironment, we studied whether CXCL7 could
also promote the malignant phenotypes of cholangiocarcinoma cells
in a paracrine-dependent manner. Recombinant human CXCL7 was used
to treat QBC939 cells, and the exogenous CXCL7 was able to promote
proliferation and invasion of QBC939 cells. Moreover, we used the
CM of CXCL7-overexpressing LX-1 cells to culture the QBC939 cells,
and found that the CM of CXCL7-overexpressing LX-1 cells
significantly enhanced the malignant phenotypes of QBC939 cells.
These findings indicate that CXCL7 also plays a promoting role in
cholangiocarcinoma in a paracrine-dependent manner.
Moreover, we studied the alteration of the AKT
signaling in cholangiocarcinoma cells after CXCL7 overexpression.
AKT signaling pathway is present in all cells of higher eukaryotes
and is highly conserved. Activated Akt participates in the
regulation of such cellular processes as cell growth,
proliferation, survival, migration, and angiogenesis, by
phosphorylating a range of intracellular proteins (22,23).
Moreover, it has been found to play a key role in tumor growth and
metastasis, and thus can become an important target for cancer
treatment (24,25). Wilson et al reported that
inhibition of the AKT signaling pathway suppressed cell viability
via induction of apoptosis in cholangiocarcinoma (26). Huang et al also showed that
FHIT suppresses proliferation and promotes apoptosis in
cholangiocarcinoma cells by blockage of Akt signaling pathway
(27). Besides, dual inhibition of
AKT and ERK signaling is synergistic in cholangiocarcinoma and
reverses acquired resistance to MEK-inhibitors (28). In the present study, we found that
overexpression of CXCL7 enhanced the activity of AKT signaling in
cholangiocarcinoma cells, suggesting that this signaling pathway is
probably involved in the CXCL7-mediated malignant phenotypes of
cholangiocarcinoma cells.
In conclusion, the present study suggests that CXCL7
plays promoting role in the proliferation and invasion of
cholangiocarcinoma cells through activation of AKT signaling
pathways, and blockage of the connection between cholangiocarcinoma
and adjacent tissue may be an effective strategy for the treatment
of cholangiocarcinoma.
References
|
1
|
Chong DQ and Zhu AX: The landscape of
targeted therapies for cholangiocarcinoma: Current status and
emerging targets. Oncotarget. Apr 18–2016.(Epub ahead of
print).
|
|
2
|
Andersen JB: Molecular pathogenesis of
intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci.
22:101–113. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ching CB and Hansel DE: Expanding
therapeutic targets in bladder cancer: The PI3K/Akt/mTOR pathway.
Lab Invest. 90:1406–1414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Sun J, Feng Y, Tian X, Wang B and
Zhou Y: Oncogenic roles and drug target of CXCR4/CXCL12 axis in
lung cancer and cancer stem cell. Tumour Biol. 37:8515–8528. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo N, Liu F, Yang L, Huang J, Ding X and
Sun C: Chemokine receptor 7 enhances cell chemotaxis and migration
of metastatic squamous cell carcinoma of head and neck through
activation of matrix metalloproteinase-9. Oncol Rep. 32:794–800.
2014.PubMed/NCBI
|
|
6
|
van der Vorst EP, Döring Y and Weber C:
Chemokines. Arterioscler Thromb Vasc Biol. 35:e52–e56. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding J and Tredget EE: The role of
chemokines in fibrotic wound healing. Adv Wound Care (New
Rochelle). 4:673–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duda DG, Kozin SV, Kirkpatrick ND, Xu L,
Fukumura D and Jain RK: CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway
inhibition: An emerging sensitizer for anticancer therapies? Clin
Cancer Res. 17:2074–2080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kowalczuk O, Burzykowski T, Niklinska WE,
Kozlowski M, Chyczewski L and Niklinski J: CXCL5 as a potential
novel prognostic factor in early stage non-small cell lung cancer:
Results of a study of expression levels of 23 genes. Tumour Biol.
35:4619–4628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rehman AO and Wang CY: CXCL12/SDF-1 alpha
activates NF-kappaB and promotes oral cancer invasion through the
Carma3/Bcl10/Malt1 complex. Int J Oral Sci. 1:105–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan
J, Wang X and Li L: CXCL5/CXCR2 axis promotes bladder cancer cell
migration and invasion by activating PI3K/AKT-induced upregulation
of MMP2/MMP9. Int J Oncol. 47:690–700. 2015.PubMed/NCBI
|
|
12
|
von Hundelshausen P, Petersen F and Brandt
E: Platelet-derived chemokines in vascular biology. Thromb Haemost.
97:704–713. 2007.PubMed/NCBI
|
|
13
|
Kalwitz G, Neumann K, Ringe J, Sezer O,
Sittinger M, Endres M and Kaps C: Chondrogenic differentiation of
human mesenchymal stem cells in micro-masses is impaired by high
doses of the chemokine CXCL7. J Tissue Eng Regen Med. 5:50–59.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blunk JA, Sauerstein K and Schmelz M:
Experimental thermal lesions induce beta-thromboglobulin release
from activated platelets. Eur J Pain. 15:23–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuang P, Wo D, Xu ZG, Wei W and Mao HM:
Dynamic changes in plasma tissue plasminogen activator, plasminogen
activator inhibitor-1 and beta-thromboglobulin content in ischemic
stroke. J Clin Neurosci. 22:1123–1127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tai PK, Liao JF, Hossler PA, Castor CW and
Carter-Su C: Regulation of glucose transporters by connective
tissue activating peptide-III isoforms. J Biol Chem.
267:19579–19586. 1992.PubMed/NCBI
|
|
17
|
Kwiatkowski S, Czajka R, Dołegowska B,
Chlubek D and Torbé A: Evaluation of neutrophile elastase and
isoprostane 8epiPGF2alpha concentrations in maternal and umbilical
cord blood serum and in amniotic fluid in pregnancies complicated
by premature rupture of membranes. Ginekol Pol. 79:281–286.
2008.(In Polish). PubMed/NCBI
|
|
18
|
González-Cortés C, Diez-Tascón C,
Guerra-Laso JM, González-Cocaño MC and Rivero-Lezcano OM:
Non-chemotactic influence of CXCL7 on human phagocytes. Modulation
of antimicrobial activity against L. pneumophila. Immunobiology.
217:394–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grépin R, Guyot M, Giuliano S, Boncompagni
M, Ambrosetti D, Chamorey E, Scoazec JY, Negrier S, Simonnet H and
Pagès G: The CXCL7/CXCR1/2 axis is a key driver in the growth of
clear cell renal cell carcinoma. Cancer Res. 74:873–883. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desurmont T, Skrypek N, Duhamel A,
Jonckheere N, Millet G, Leteurtre E, Gosset P, Duchene B, Ramdane
N, Hebbar M, et al: Overexpression of chemokine receptor CXCR2 and
ligand CXCL7 in liver metastases from colon cancer is correlated to
shorter disease-free and overall survival. Cancer Sci. 106:262–269.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Unver N, Esendagli G, Yilmaz G and Guc D:
CXCL7-induced macrophage infiltration in lung tumor is independent
of CXCR2 expression: CXCL7-induced macrophage chemotaxis in LLC
tumors. Cytokine. 75:330–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Qiu T, Zhang P, Wang X, Yin Y and Li
S: IKVAV regulates ERK1/2 and Akt signalling pathways in BMMSC
population growth and proliferation. Cell Prolif. 47:133–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin Y, Cui W, Yang X and Tong B:
Kaempferol inhibits the growth and metastasis of cholangiocarcinoma
in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai).
48:238–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilson JM, Kunnimalaiyaan S,
Kunnimalaiyaan M and Gamblin TC: Inhibition of the AKT pathway in
cholangiocarcinoma by MK2206 reduces cellular viability via
induction of apoptosis. Cancer Cell Int. 15:132015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Q, Liu Z, Xie F, Liu C, Shao F, Zhu
CL and Hu S: Fragile histidine triad (FHIT) suppresses
proliferation and promotes apoptosis in cholangiocarcinoma cells by
blocking PI3K-Akt pathway. Sci World J. 2014:1796982014. View Article : Google Scholar
|
|
28
|
Ewald F, Nörz D, Grottke A, Hofmann BT,
Nashan B and Jücker M: Dual inhibition of PI3K-AKT-mTOR- and
RAF-MEK-ERK-signaling is synergistic in cholangiocarcinoma and
reverses acquired resistance to MEK-inhibitors. Invest New Drugs.
32:1144–1154. 2014. View Article : Google Scholar : PubMed/NCBI
|