Introduction
Cervical cancer is a common gynecological tumor with
a high incidence rate especially among younger population groups.
Early detection and treatment are critical for cervical cancer
prognosis. Effective treatment is challenging when cervical cancer
is detected after metastasis has occurred, which diminishes the
efficacy of surgical treatment. Hence, it is of great importance to
identify the features of advanced cervical carcinoma and establish
new therapeutic strategy for clinical treatments. However, the
mechanism of cervical cancer metastasis has not been fully
clarified. The epithelial-to-mesenchymal transition (EMT) of
cervical cancer cells is closely related to the occurrence and
development of epithelial malignancies, thus this is a research
hotspot. The process of EMT occurs in multiple steps, with
corresponding changes in cell polarity and mobility, which allows
tumor cells to infiltrate surrounding tissue and metastasize to
distant sites (1,2). Important indicators of EMT include a
reduction in E-cadherin expression levels and an increase in
N-cadherin expression levels (1,2).
Numerous in vitro and in vivo studies have suggested
that EMT has a key role in the occurrence, development, and
metastasis of many malignancies, such as colonic, breast, lung,
cervical, pancreatic, and renal cancer. There are also reports that
high-mobility group box 1 protein (HMGB1) induces tumor
development, and HMGB1 is associated with invasion and metastasis
of several types of tumors (3).
HMGB1 activates mitogen-activated protein kinase (MAPK) and nuclear
factor-κB (NF-κB) pathways. During cell stimulation, cytoplasmic
inhibitor κB (IκB) is phosphorylated and degraded causing NF-κB
release which is correlated with EMT in many tumor types (4,5).
However, whether HMGB1 promotes invasion and metastasis of cervical
carcinoma and the related mechanisms remain to be investigated.
Combining international research findings and preliminary study
results, we hypothesized that HMGB1 facilitates the invasion and
migration of cervical cancer by activating the NF-κB signaling
pathway. This study was designed as an experiment to explore the
expression of HMGB1 in cervical tissues and the effects of HMGB1 on
invasion and migration in cervical cancer cells and the relevant
mechanism, which will provide a new strategy for the prevention and
treatment of cervical cancer and a potential oncogenic biomarker
and therapeutic target for late-stage and metastatic cervical
cancer patients.
Materials and methods
Patients and samples
All 123 cervical samples were collected from
patients who had undergone surgery at Shengjing Hospital (Shenyang,
Liaoning, China) between 2011 and 2013. The specimens included 48
locally advanced invasive cervical cancers (ICC), 51 cervical
intraepithelial neoplasia (CIN), and 24 normal squamous epithelial
specimens (NSCES). The median age of all patients was 44 years
(range, 19–74 years). Normal squamous epithelial specimens were
collected from uteri of patients who had undergone hysterectomy
without malignancy. This study was approved by the Ethics Committee
of China Medical University, and informed written consent was
obtained from all subjects prior to the study.
A histopathological diagnosis of cancer was based on
World Health Organization classifications, and the clinical staging
was defined according to the International Federation of Gynecology
and Obstetrics (FIGO) system. Complete clinical and pathological
data were available for all patients, and none had received
pre-operative radiotherapy, chemotherapy, or biological
therapy.
Immunohistochemistry
The tissues were embedded in paraffin and fixed in
4% formaldehyde, and serial sections were used. We used mouse
anti-HMGB1 at 1:40 (R&D Systems, Inc., Minneapolis, MN, USA);
rabbit anti-NF-κB at 1:100, rabbit anti-N-cadherin at 1:100 and
rabbit anti-E-cadherin at 1:200 (all from ProteinTech Group, Inc.,
Chicago, IL, USA). Phosphate-buffered saline (PBS) was substituted
for the primary antibody in the negative control. Serial sections
were used for all single staining to show that HMGB1, NF-κB,
E-cadherin and N-cadherin were related. After overnight incubation
at 4°C, 3 washes in PBS were performed. The procedure was based on
the SP kit system (Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China). Two researchers who were blinded to the patient
materials examined the immunostained slides with microscopy in a
bright-field. To assess immunostaining data for HMGB1, NF-κB,
E-cadherin and N-cadherin, an immunostaining scoring system
corresponding to total staining intensity was as follows: strong
staining, 3; moderate staining, 2; weak staining, 1; no staining,
0. Scores for the relative numbers of positive cells were as
follows: >75% of cells were positive, 4; 51–75% of cells were
positive, 3; 25–50% of cells were positive, 2; <25% of cells
were stained positive, 1; no positive cells, 0. The scores of
percentage and intensity reflect the sums of scores, with total
scores of 0 indicated as (−); total scores of 1–2 as (+); total
scores of 3–5 as (++); total scores of 6–7 as (+++).
Cell culture
HeLa cells (Institute of Biochemistry and Cell
Biology, Shanghai, China) were maintained in Dulbecco's modified
Eagle's medium (DMEM)/high glucose (HyClone, Logan, UT, USA). Media
were supplemented with 10% fetal bovine serum (FBS) (ExCell Bio,
Shanghai, China), and cells were cultured at 37°C in a humidified
chamber with 5% CO2. Cells were stimulated with HMGB1
(Sigma, St. Louis, MO, USA) for different intervals of 0, 24, 48
and 72 h at a dose of 1,000 ng/ml which were named group A, B, C
and D, respectively; or at different doses of 0, 10, 100 and 1,000
ng/ml for 48 h which were named group A’, B’, C’ and D’,
respectively. In addition, we used BAY11-7082 (Cayman Chemical Co.,
Ann Arbor, MI, USA) to stimulate cells for 6 h and then stimulated
the cells with HMGB1 for 48 h: a (control), b (only HMGB1), c
(HMGB1+DMSO), d (BAY11-7082+HMGB1); we used anti-receptor for
advanced glycation end products (RAGE) (BIOSS, Beijing, China) to
stimulate the cells for 6 h and then stimulated cells with HMGB1
for 48 h: a’ (control), b’ (only HMGB1), c’ (HMGB1+DMSO), and d’
(anti-RAGE+HMGB1).
MTT assay
Analysis was performed using a 96-well plate;
1×105 cells in 200 µl of DMEM/high glucose supplemented
with 10% FBS were added to each well, and the cells were cultured
for 24, 48 and 72 h at 37°C. After treating the cells with HMGB1,
20 µl of MTT (Sigma) was added. The cells were then incubated for 4
h and optical densities were measured at 490 nm.
Matrigel invasion analysis
Analysis was performed using a 24-well invasion
chamber system which contained polycarbonate filters with a pore
size of 8-µm (Corning Costar, Inc.) with a Matrigel (Sigma)
membrane. Each 500 µl of DMEM/high glucose supplemented with 10%
FBS was placed in the lower compartment of the chamber. In the
pre-warmed and rehydrated upper compartment, 1×105 cells
in 500 µl of DMEM/high glucose supplemented without FBS were added,
and the cells were allowed to migrate through the intermediate
membrane for 24, 48 and 72 h at 37°C. The membranes were then fixed
with neutral-buffered formalin and stained in hematoxylin and eosin
staining. The cells that had attached to the lower side of the
membrane were counted in ten high-powered fields (x400) under a
microscope. Each experiment was repeated three times.
Quantitative real-time PCR
Quantitative real-time PCR was performed using the
real-time PCR system 7300 (Applied Biosystems, Foster City, CA,
USA). In brief, the PCR amplification reaction mixtures (20 µl)
contained cDNA, primer pairs, the dual-labeled fuorogenic probe,
and TaqMan Universal PCR Master Mix (Takara Bio, Dalian,
China).
The primers were: E-cadherin forward,
5′-AGAACGCATTGCCACATACA-3′ and reverse, 5′-TAAGCGATGGCGGCATTGTA-3′;
N-cadherin forward, 5′-CAACACACTCGCAGACGCTCA-3′ and reverse,
5′-AAGACGGCTCCAGGCAGTTT-3′; β-actin forward,
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′.
PCRs were performed in triplicate. The relative fold
changes were calculated with the following formula:
2−δδCt, δCt = Ct (target) - Ct (β-actin), which
reflected the target gene expression normalized to β-actin levels.
The fold increase or decrease in HMGB1 expression was determined
for different groups and is expressed as mean ± standard deviation
(SD).
Western blot analysis
Proteins from the cell samples were extracted with
RIPA buffer. Proteins were resolved via sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and blotted onto a
nitrocellulose membrane. The membrane was incubated with the
indicated primary anti-E-cadherin (1:500); anti-N-cadherin (1:500);
anti-NF-κB (1:500); anti-IκB (1:500) and anti-β-actin (1:500),
followed by incubation with anti-mouse and anti-rabbit
immunoglobulin G. Protein expression was visualized using enhanced
chemiluminescence. Comparison between different treatment groups
was made by determining the specific protein/β-actin ratio of the
immunoreactive area with densitometry. Each experiment was repeated
three times.
Statistical analysis
We analyzed all the statistics using SPSS 17.0
software (2009; SPSS, Inc., Chicago, IL, USA). Fisher's exact
probability and Student's t-test were used for comparison between
groups. Data are expressed as mean ± SD and were analyzed with
one-way and two-way ANOVA. We also used the Kaplan-Meier method to
conduct the univariate overall survival analysis. Survival rate
differences were performed with the log-rank test. Statistical
significance was defined as P<0.05.
Results
HMGB1 expression in cervical tissues
and its clinical significance
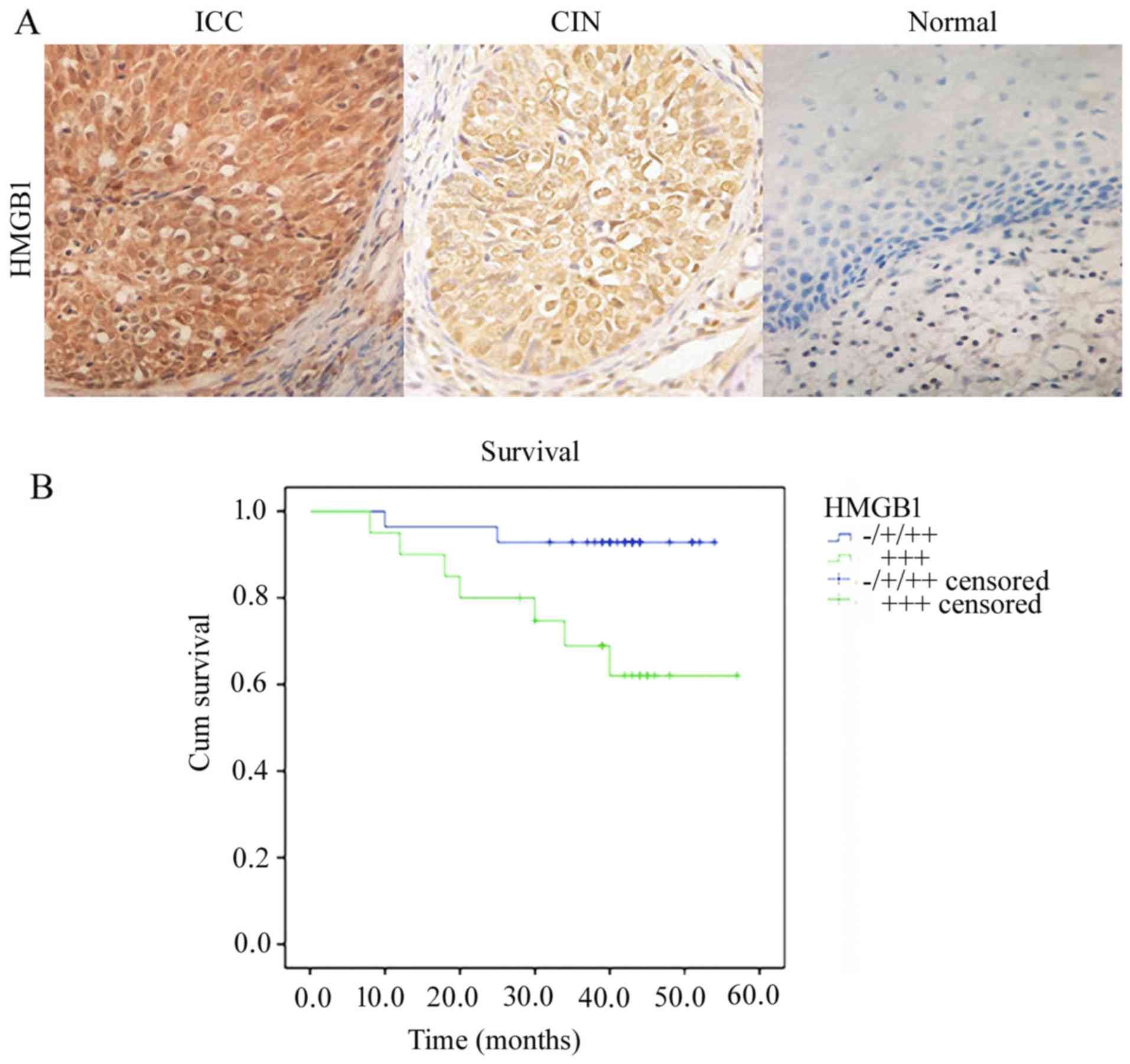
HMGB1 was observed in carcinoma cell cytoplasm and
nuclei, but was predominantly localized in the nuclei (Fig. 1A). The expression in the nuclei and
cytoplasm was increased in the cancer tissues compared to that in
the control tissues. Positive HMGB1 immunoreactivity was detected
in 89.58% (43/48) of the cervical cancer cases, in 54.90% (28/51)
of the CIN cases and 4.17% (1/24) of the control cases. Among the
cancer cases, positive HMGB1 immunoreactivity was detected in
33.33% (16/48) of the FIGO stage I cases and 56.25% (27/48) of the
FIGO stage II–III cases (P<0.05). In addition, HMGB1 protein was
positively associated with lymph node metastasis and cell
differentiation (P<0.05). However, there was no significant
correlation between HMGB1 and age or histological type (P>0.05)
(Table I). Kaplan-Meier analysis
suggested that the mean survival time of cervical cancer cases with
robust expression of HMGB1 was significantly lower (44.57±3.95
months) compared to the cervical cancer cases with weak or negative
expression of HMGB1 (51.39±1.82 months) (P<0.05). In our
results, patients strongly expressing HMGB1 had significantly
greater rates of death than patients with weak expression
(P<0.05, log-rank test) (Fig.
1B). These results suggest that HMGB1 may be a useful biomarker
with which to evaluate clinical significance and outcome of
cervical cancer.
 | Table I.Expression of HMGB1 protein in
cervical tissues and its relationship with clinicopathological
factors. |
Table I.
Expression of HMGB1 protein in
cervical tissues and its relationship with clinicopathological
factors.
|
|
| HMGB1 protein |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | n | − | + | ++ | +++ | P-value |
|---|
| NSCES | 24 | 18 | 5 | 1 | 0 |
|
| CIN | 51 | 2 | 21 | 28 | 0 | <0.05 |
| ICC | 48 | 2 | 3 | 23 | 20 |
|
| Age (years) |
|
|
|
|
| 0.93 |
|
<45 | 19 | 1 | 1 | 10 | 7 |
|
| ≥45 | 29 | 1 | 2 | 13 | 13 |
|
| FIGO |
|
|
|
|
| <0.05 |
| I | 21 | 2 | 3 | 15 | 1 |
|
|
II–III | 27 | 0 | 0 | 8 | 19 |
|
| Histological
type |
|
|
|
|
| 0.55 |
| SCC | 41 | 2 | 3 | 18 | 18 |
|
|
ADC | 7 | 0 | 0 | 5 | 2 |
|
|
Differentiation |
|
|
|
|
| 0.03 |
|
Well | 8 | 2 | 1 | 3 | 2 |
|
|
Moderate | 32 | 0 | 2 | 14 | 16 |
|
|
Poor | 8 | 0 | 0 | 6 | 2 |
|
| LN metastasis |
|
|
|
|
| 0.01 |
|
Positive | 14 | 0 | 0 | 3 | 11 |
|
|
Negative | 34 | 2 | 3 | 20 | 9 |
|
Relationship between HMGB1, NF-κB,
E-cadherin or N-cadherin
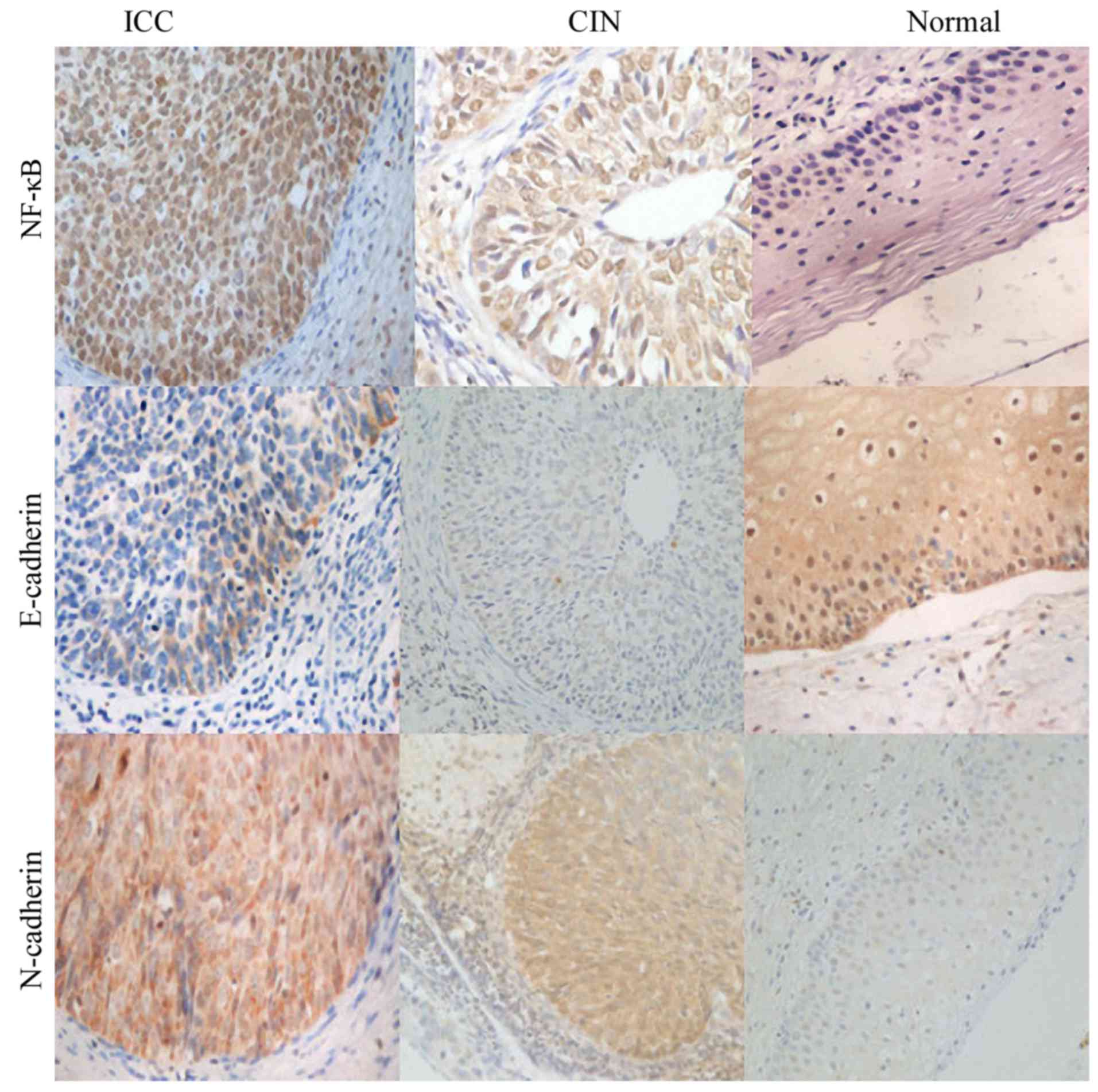
NF-κB staining was observed both in the cell
cytoplasm and nuclei in the cervical cancer cases and control
cases. However, in the cervical cancer cases, strong staining was
mainly localized in the nuclei. NF-κB was significantly increased
in cervical cancer samples compared to normal control samples
(Fig. 2). We found that E-cadherin
and N-cadherin were both located in the cytoplasm. E-cadherin
expression was downregulated in cervical cancer tissues compared to
that in the normal tissues (Fig.
2), on the contrary, N-cadherin expression was upregulated in
cervical cancer tissues compared to normal tissues (Fig. 2). According to the
immunohistochemistry results, we found that both HMGB1 and NF-κB
showed strong staining in the nuclei in cervical cancer cases, and
we found that HMGB1 expression was positively associated with NF-κB
and N-cadherin (r=0.76; r=0.69, both P<0.05); we also found that
E-cadherin expression was negatively associated with HMGB1
(r=−0.68, P<0.05) (Table
II).
 | Table II.Association between HMGB1 and NF-κB,
E-cadherin and N-cadherin expression in the cervical samples. |
Table II.
Association between HMGB1 and NF-κB,
E-cadherin and N-cadherin expression in the cervical samples.
|
| NF-κB |
| N-cadherin |
| E-cadherin |
|
|---|
|
|
|
|
|
|
|
|
|---|
|
| − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value |
|---|
| HMGB1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| − | 17 | 2 | 3 | 0 | <0.05 | 14 | 7 | 1 | 0 | <0.05 | 1 | 3 | 4 | 14 | <0.05 |
| + | 5 | 15 | 9 | 0 |
| 6 | 9 | 13 | 1 |
| 2 | 3 | 14 | 10 |
| ++ | 0 | 13 | 30 | 9 |
| 1 | 9 | 30 | 12 |
| 21 | 22 | 9 | 0 |
| +++ | 0 | 0 | 4 | 16 |
| 0 | 2 | 4 | 14 |
| 15 | 3 | 1 | 1 |
|
|
| r=0.76 |
|
|
| r=0.69 |
|
|
| r=−0.68 |
|
|
HMGB1 promotes morphological changes
in HeLa cells
Morphological observations of a normal control group
of HeLa cells indicated that cells were epithelial, polygonal, with
close cell-cell junctions, and growing in clusters. After
stimulation with 1,000 ng/ml HMGB1 for 72 h, the HeLa cells had
sparse cell-cell junctions, became spindle-shaped, and exhibited a
disappearance of polarity. After treatment with HMGB1, the HeLa
cells transitioned from an epithelial morphology into a mesenchymal
morphology. This was similar to the morphological changes that
occur after EMT (Fig. 3A).
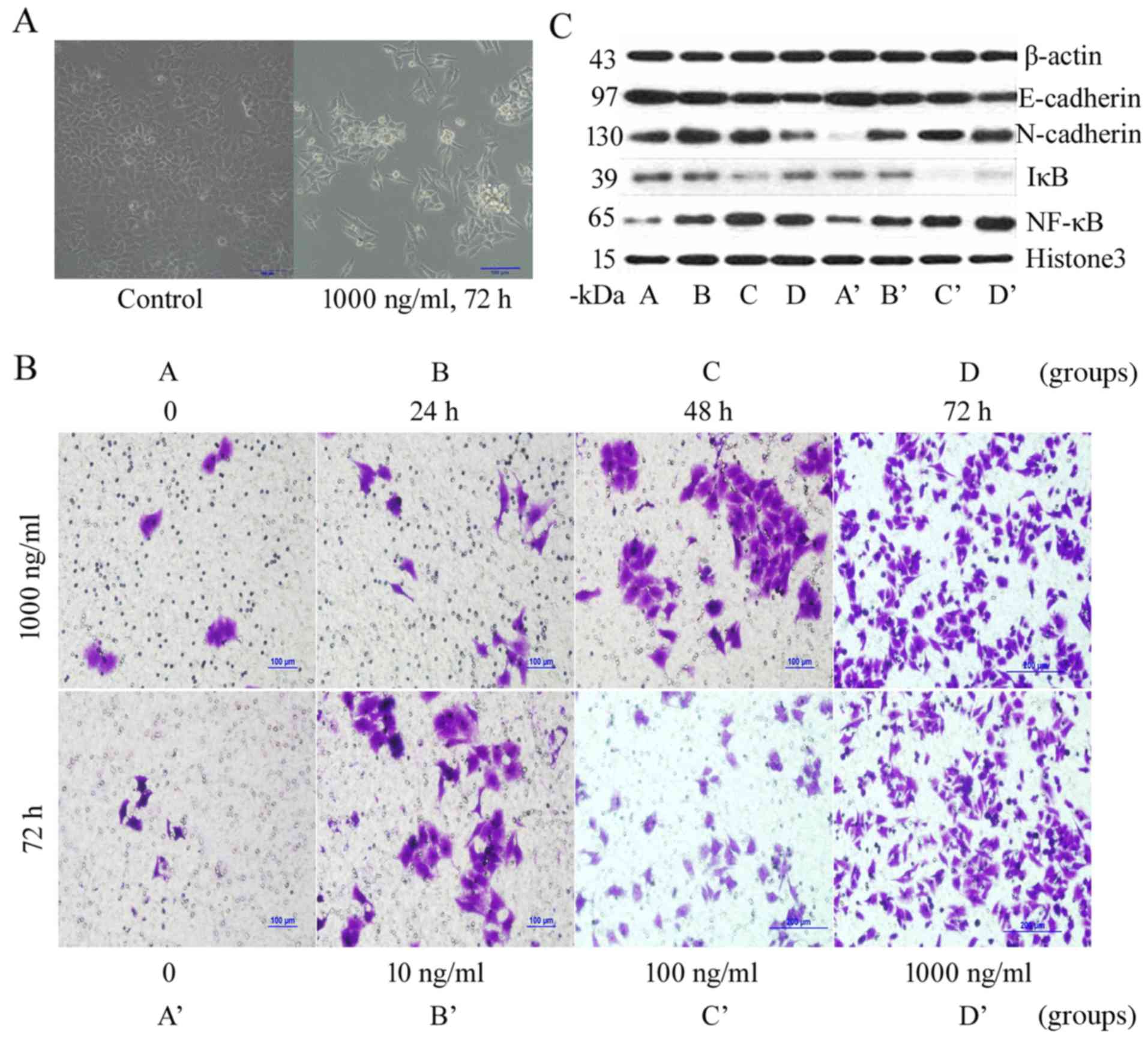 | Figure 3.Biological behavior of HeLa cells
after stimulation by high-mobility group box 1 protein (HMGB1). (A)
HeLa cell morphological changes. (B) Transwell assay following
HMGB1 stimulation. (C) Expression of E-cadherin, N-cadherin,
nuclear factor-κB (NF-κB) and inhibitor κB (IκB) protein in HeLa
cells by western blot analysis. Groups: A, cells were stimulated
with HMGB1 for 0 h at a dose of 1,000 ng/ml; B, cells were
stimulated with HMGB1 for 24 h at a dose of 1,000 ng/ml; C, cells
were stimulated with HMGB1 for 48 h at a dose of 1,000 ng/ml; D,
cells were stimulated with HMGB1 for 72 h at a dose of 1,000 ng/ml.
Groups: A’, at a dose of 0 ng/ml for 48 h; B’, at a dose of 10
ng/ml for 48 h; C’, at a dose of 100 ng/ml for 48 h; and D’, at a
dose of 1,000 ng/ml for 48 h. |
HMGB1 promotes proliferation and
invasion of HeLa cells
The MTT cell proliferation assay indicated that
treatment with 1,000 ng/ml HMGB1 for 48 h significantly increased
the culture optical density (OD) compared to that of the control
group (2.82±0.03 vs. 1.60±0.06). This treatment had the greatest
positive effect on cell proliferation of all tested treatments
(Table III). In the Transwell
assay, we observed that the number of penetrated cells in group D
(1,000 ng/ml HMGB1 for 72 h) was significantly higher than that in
group A (1,000 ng/ml HMGB1 for 0 h) (345.20±28.90 vs. 6.20±2.28),
and the number of penetrated cells in group D’ (1,000 ng/ml HMGB1
for 72 h) was evidently higher than that in group A’ (0 ng/ml HMGB1
for 72 h) (341.20±22.43 vs. 10.40±4.93). These results suggested
that stimulation with 1,000 ng/ml HMGB1 for 72 h had the greatest
positive effect in enhancing cell invasiveness (Table IV and Fig. 3B). These results demonstrated that
HMGB1 overexpression enhanced tumor migration and invasion in
vitro.
 | Table III.Proliferation ability of HeLa cells
after HMGB1 stimulation. |
Table III.
Proliferation ability of HeLa cells
after HMGB1 stimulation.
|
| OD value |
|---|
|
|
|
|---|
| HMGB1
stimulation | 24 h | 48 h | 72 h |
|---|
| 0 | 0.81±0.02 | 1.60±0.06 | 1.43±0.03 |
| 10 ng/ml | 1.02±0.11 | 2.06±0.07 | 1.50±0.02 |
| 100 ng/ml | 1.73±0.04 | 2.45±0.07 | 1.99±0.10 |
| 1,000 ng/ml | 1.91±0.02 | 2.82±0.03 | 2.21±0.03 |
 | Table IV.Invasion ability of HeLa cells after
HMGB1 stimulation. |
Table IV.
Invasion ability of HeLa cells after
HMGB1 stimulation.
|
| No. of invasive
HeLa cells |
|---|
|
|
|
|---|
|
| HMGB1 at 1,000
ng/ml (different intervals) | HMGB1 for 72 h
(different doses) |
|---|
|
|
|
|
|---|
| Group | A | B | C | D | A’ | B’ | C’ | D’ |
|---|
|
| 0 | 24 h | 48 h | 72 h | 0 | 10 ng/ml | 100 ng/ml | 1,000 ng/ml |
|---|
| No. | 6.20±2.28 | 23.20±3.70 | 72.60±4.83 | 345.20±28.90 | 10.40±4.93 | 57.00±6.89 | 74.60±6.88 | 341.20±22.43 |
HMGB1 promotes mesenchymal marker
expression and reduces epithelial marker expression in cervical
cancer cells
Western blot analysis showed that treatment with
1,000 ng/ml HMGB1 for 48 h significantly reduced cytoplasmic
E-cadherin and IκB expression levels, markedly increased
cytoplasmic N-cadherin expression levels, and significantly
increased nuclear NF-κB expression levels (Fig. 3C). These results indicated that
HMGB1 stimulation reduced the expression levels of epithelial
markers on the surface of HeLa cells, whereas that of mesenchymal
markers was increased, which indicates EMT. Reduced IκB expression
in the cytoplasm and increased NF-κB expression in the nucleus
suggested that NF-κB may be activated. Similar results were
obtained by performing real-time PCR. The relative expression level
of N-cadherin mRNA in group C (1,000 ng/ml HMGB1 for 48 h) was
1.00±0.05, which was higher than that in group A (1,000 ng/ml HMGB1
for 0 h) (0.27±0.02). The relative expression level of E-cadherin
mRNA in group C was 0.80±0.04, which was lower than that in group A
(1,000 ng/ml HMGB1 for 0 h) (1.00±0.04). The relative expression
level of N-cadherin mRNA in group D’ (1,000 ng/ml HMGB1 for 48 h)
was 0.93±0.05, which was higher than that in group A’ (0 ng/ml
HMGB1 for 48 h) (0.46±0.06). The relative expression level of
E-cadherin mRNA was 0.64±0.02, which was lower than that of group
A’ (0 ng/ml HMGB1 for 48 h) (0.98±0.06) (Table V).
 | Table V.mRNA expression of E-cadherin and
N-cadherin in the HeLa cells after HMGB1 stimulation. |
Table V.
mRNA expression of E-cadherin and
N-cadherin in the HeLa cells after HMGB1 stimulation.
|
| mRNA (mean ±
SD) |
|---|
|
|
|
|---|
|
| HMGB1 (1,000
ng/ml) | HMGB1 (48 h) |
|---|
|
|
|
|
|---|
| Groups | A | B | C | D | A’ | B’ | C’ | D’ |
|---|
|
| 0 | 24 h | 48 h | 72 h | 0 | 10 ng/ml | 100 ng/ml | 1,000 ng/ml |
|---|
| E-cad | 1.00±0.04 | 0.85±0.06 | 0.80±0.04 | 1.05±0.03 | 0.98±0.06 | 1.09±0.03 | 0.90±0.04 | 0.64±0.02 |
| N-cad | 0.27±0.02 | 0.49±0.03 | 1.00±0.05 | 0.59±0.02 | 0.46±0.06 | 0.59±0.01 | 1.05±0.09 | 0.93±0.05 |
Treatment with NF-κB inhibitor and
RAGE antagonist reduces HMGB1-mediated HeLa cell proliferation and
metastasis
For this study, HeLa cells were treated with NF-κB
inhibitor BAY11-7082 and anti-RAGE, and then stimulated with 1,000
ng/ml HMGB1 for 48 or 72 h. The results of MTT assays showed that
cell proliferation (OD) in group d was significantly lower than
that in group b (2.04±0.07 vs. 2.46±0.09), and the OD in group d’
was evidently lower than that in group b’ (1.70±0.07 vs.
2.67±0.05). These observations indicated that inhibition of the
NF-κB pathway significantly attenuated HMGB1-mediated stimulation
of HeLa cells (Table VI). After 72
h of HMGB1 stimulation, the Transwell assay indicated that the
number of penetrated cells in group d was significantly lower than
that in group b (102.40±8.20 vs. 290.40±11.33), and the number of
penetrated cells in group d’ was evidently lower than that in group
b’ (86.80±6.14 vs. 293.00±15.60) (Table VII and Fig. 4A). These results suggested that
HMGB1 may cause the morphological and biological changes observed
in HeLa cells by activating the NF-κB signaling pathway.
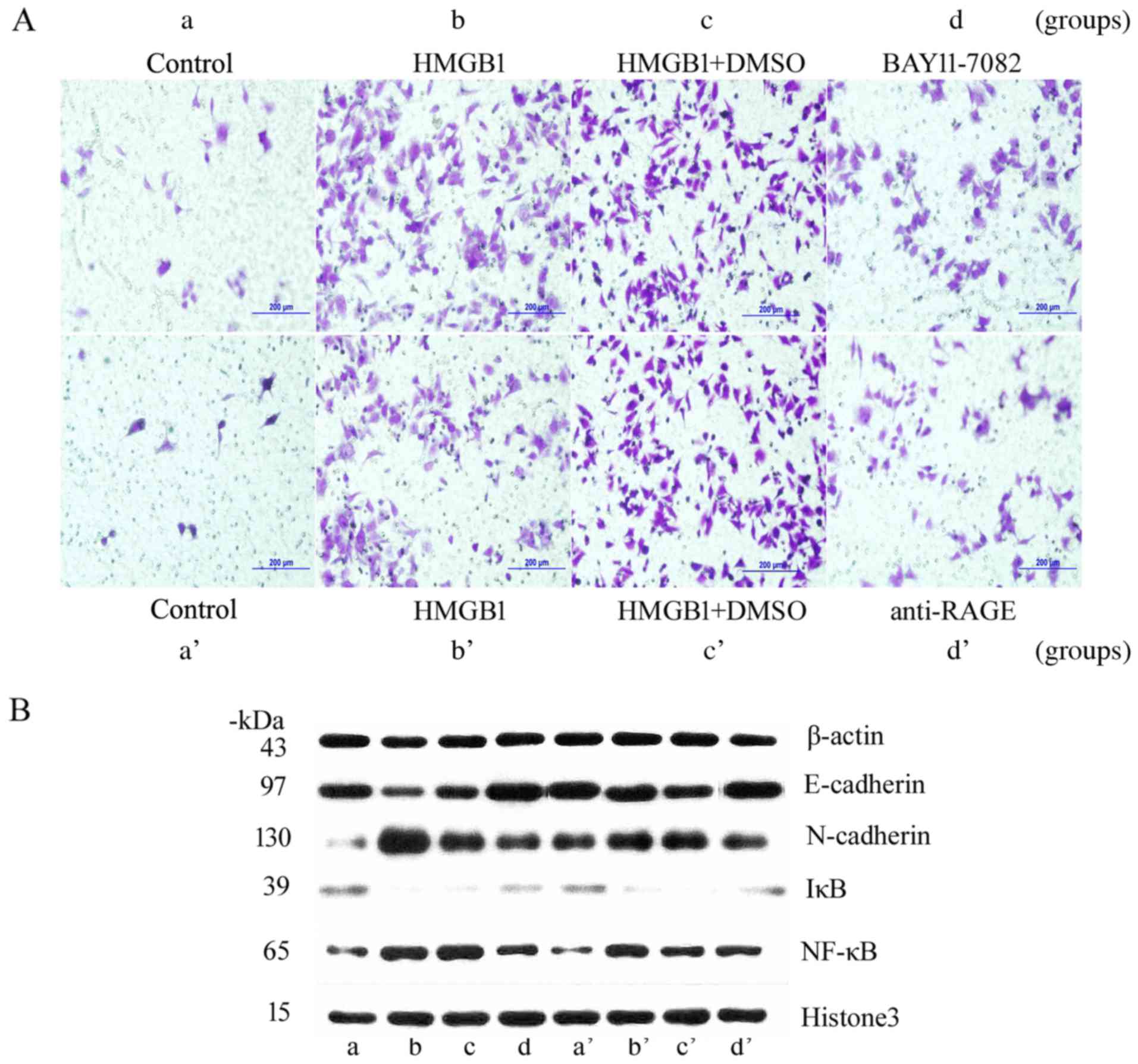 | Figure 4.Biological behavior of HeLa cells
after stimulation with high-mobility group box 1 protein (HMGB1)
when using BAY11-7082 or anti-receptor for advanced glycation end
products (RAGE). (A) Transwell assay. (B) Expression of E-cadherin,
N-cadherin, nuclear factor-κB (NF-κB) and inhibitor κB (IκB)
protein in HeLa cells treated with BAY11-7082 or anti-RAGE by
western blot analysis. Groups: a, control; b, cells cultured with
HMGB1 at a dose of 1,000 ng/ml for 48 h; c, cells cultured with
DMSO for 6 h and then stimulated the cells with HMGB1 for 48 h; and
d, cells cultured with BAY11-7082 for 6 h and then stimulated the
cells with HMGB1 for 48 h. Groups: a’, control; b’, cells cultured
with HMGB1 at a dose of 1,000 ng/ml for 48 h; c’, cells cultured
with DMSO for 6 h and then stimulated the cells with HMGB1 for 48
h; and d’, cells cultured with advanced glycation end products
(RAGE) for 6 h and then stimulated the cells with HMGB1 for 48
h. |
 | Table VI.Proliferation ability of HeLa cells
after suppression of HMGB1 expression. |
Table VI.
Proliferation ability of HeLa cells
after suppression of HMGB1 expression.
|
| BAY11-7082 (48
h) | Anti-RAGE (48
h) |
|---|
|
|
|
|
|---|
| Groups | a | b | c | d | a’ | b’ | c’ | d’ |
|---|
|
| Control | HMGB1 | HMGB1+DMSO |
HMGB1+BAY11-7082 | Control | HMGB1 | HMGB1+DMSO |
HMGB1+anti-RAGE |
|---|
| OD | 1.73±0.08 | 2.46±0.09 | 2.45±0.21 | 2.04±0.07 | 1.57±0.01 | 2.67±0.05 | 2.55±0.08 | 1.70±0.07 |
 | Table VII.Invasion ability of HeLa cells after
suppression of HMGB1 expression. |
Table VII.
Invasion ability of HeLa cells after
suppression of HMGB1 expression.
|
| No. of invasive
HeLa cells |
|---|
|
|
|
|---|
|
| BAY11-7082 (72
h) | Anti-RAGE (72
h) |
|---|
|
|
|
|
|---|
| Groups | a | b | c | d | a’ | b’ | c’ | d’ |
|---|
|
| Control | HMGB1 | HMGB1+DMSO |
HMGB1+BAY11-7082 | Control | HMGB1 | HMGB1+DMSO |
HMGB1+anti-RAGE |
|---|
| No. | 24.80±3.27 | 290.40±11.33 | 301.20±13.83 | 102.40±8.20 | 18.60±4.36 | 293.00±15.60 | 280.40±6.54 | 86.80±6.14 |
Treatment with NF-κB inhibitor and
RAGE antagonist reduces HMGB1-mediated EMT in HeLa cells
For this study, HeLa cells were treated with NF-κB
inhibitor BAY11-7082 and anti-RAGE, and then stimulated with 1,000
ng/ml HMGB1 for 48 h. Western blot analysis showed that the level
of E-cadherin expression in the cytoplasm in group d with the
addition of BAY11-7082 was higher than that in group b, N-cadherin
expression levels in the cytoplasm were markedly reduced, and IκB
expression levels in the cytoplasm were increased. The level of
NF-κB expression in the nucleus was decreased. Similar changes also
were observed in group d’ with the addition of anti-RAGE (Fig. 4B). These results were consistent
with those of real-time PCR assays. The relative expression level
of N-cadherin mRNA in group d was 2.05±0.16, which was lower than
that in group b (3.72±0.08), and the relative expression level of
E-cadherin mRNA was 1.37±0.05, which was higher than that in group
b (0.89±0.02). The relative expression level of N-cadherin mRNA in
group d’ was 2.18±0.07, which was lower than that in group b’
(2.51±0.14); and the relative expression level of E-cadherin mRNA
was 1.78±0.04, which was higher than that in group b’ (0.62±0.03)
(Table VIII). HMGB1 stimulation
caused nuclear entry and activation of NF-κB, whereas NF-κB
inhibition and HMGB1 receptor blockade evidently suppressed NF-κB
activation. These results suggest that HMGB1 may regulate EMT in
HeLa cells via activation of the NF-κB signaling pathway. This
mechanism could be a crucial determinant of cervical cancer
metastasis.
 | Table VIII.mRNA expression of E-cadherin and
N-cadherin in the HeLa cells after suppression of HMGB1
expression. |
Table VIII.
mRNA expression of E-cadherin and
N-cadherin in the HeLa cells after suppression of HMGB1
expression.
|
| mRNA (mean ±
SD) |
|---|
|
|
|
|---|
|
| BAY11-7082 (48
h) | Anti-RAGE (48
h) |
|---|
|
|
|
|
|---|
| Groups | a | b | c | d | a’ | b’ | c’ | d’ |
|---|
|
| Control | HMGB1 | HMGB1+DMSO |
HMGB1+BAY11-7082 | Control | HMGB1 | HMGB1+DMSO |
HMGB1+anti-RAGE |
|---|
| E-cad | 1.00±0.02 | 0.89±0.02 | 1.02±0.02 | 1.37±0.05 | 1.42±0.04 | 0.62±0.03 | 0.85±0.01 | 1.78±0.04 |
| N-cad | 1.00±0.06 | 3.72±0.08 | 2.86±0.04 | 2.05±0.16 | 2.06±0.08 | 2.51±0.14 | 3.14±0.07 | 2.18±0.07 |
Discussion
Cervical cancer is the most common malignancy of the
female reproductive system. In recent years, cervical cancer
morbidity and mortality are increasing especially among younger
populations and in developing countries. Thus cervical cancer is a
major threat to the health and life of women (6). The current treatment for cervical
cancer is the comprehensive therapy of surgery (dominant) +
radiochemotherapy (adjuvant) (7).
Surgical treatment has a critical role in patient prognosis, and
cervical cancer metastasis has adverse consequences for patient
treatment and prognosis. Therefore, early diagnosis and treatment
are crucial. However, the primary mechanism of cervical cancer
metastasis is unknown.
There are many causes and mechanisms for the
invasion and metastasis of cervical cancer, and EMT is believed to
play an important role. EMT is a physiological phenomenon in
mammalian fetal development, and loss of epithelial cell polarity
and acquisition of mesenchymal properties has important roles in
tumor invasion and metastasis (8,9). The
significant features of EMT are the decrease or loss of E-cadherin
and the upregulation of transcription factors such as N-cadherin,
Snail, Twist, and the zinc finger E-box-binding protein 1/2
(ZEB1/2) (10–12). Normal epithelial cells have typical
apical-basal polarity, close cell-cell junctions, and are adherent
to basal cells, all of which limit cell migration capacity. During
tumor proliferation and metastasis, normal epithelial cells undergo
a series of changes, including the loss of epithelial cell
polarity, acquisition of mesenchymal properties, loss of close
cell-cell junctions and adhesion, and acquisition of free migration
capability. These changes enable tumor cells to metastasize locally
at the primary lesion, or metastasize via blood vessels, lymphatic
vessels, and other pathways; these changes have important roles in
tumor invasion and metastasis (13,14).
In clinical specimens of cervical cancer, Lee et al observed
that expression of the EMT marker E-cadherin was reduced, whereas
that of vimentin was increased. After treatment with endothelial
growth factor (EGF), cervical cancer cells demonstrated
morphological changes including a fusiform, spindle-shaped
fibroblastic morphology, an enlarged intercellular space, and
increased migration and invasion capabilities (15).
In a resting state, NF-κB binds with its inhibitory
protein IκB and is present in the cytoplasm in an inactivated form.
During cell stimulation, cytoplasmic IκB is phosphorylated and
degraded, which causes NF-κB release. Free NF-κB is then able to
enter into the nucleus, where it regulates the transcription and
expression of several genes that are involved in the EMT process of
tumors (16). NF-κB is a key factor
that stimulates transforming growth factor β (TGF-β)-induced EMT.
NF-κB inhibition can block TGF-β-induced EMT of breast epithelial
cells, whereas NF-κB activation can promote mesenchymal cell
morphology (17). A recent study
reported that NF-κB could suppress E-cadherin protein expression by
promoting Snail expression (18).
These results indicate that NF-κB activation has a crucial role in
EMT of tumor cells, and contributes to the development of tumor
metastasis.
It is widely reported that HMGB1 can act as a
cytokine or a growth factor that promotes extracellular signal
transmission by binding with the corresponding cell surface
receptors, and thereby participates in tumor invasion and
metastasis (19). Several roles of
HMGB1 have been reported. HMGB1 plays an important role in various
diseases, from autoimmune diseases and tumors to
ischemia-reperfusion injury and shock; HMGB1 is also closely
related to the development of prostatic, breast, pancreatic, and
intestinal cancer (20,21). Our results showed that HMGB1
expression in cervical carcinoma tissues was significantly
increased, was positively correlated with the grade of the lesion,
and was closely associated with invasion, lymph node metastasis,
clinical staging and outcomes. Therefore, HMGB1 expression could be
used as an important indicator for invasion, metastasis, and
prognosis of cervical carcinoma. In order to further explore the
mechanism of HMGB1 in invasive cancer, we analyzed the expression
of NF-κB and E-cadherin that were involved in the pathway of NF-κB
signaling. Studies have shown that nuclear expression of NF-κB is
increased in carcinoma groups. HMGB1 was positively correlated with
NF-κB expression and co-expressed in the cell nuclei, which we
observed in serial sections of cervical tissues indicating it may
activate NF-κB signaling through some mechanism mediated by HMGB1.
HMGB1 causes phosphorylation, ubiquitylation and degradation of IκB
so that IκB combining with NF-κB was decreased. HMGB1 may initiate
the process of NF-κB translocation to the nuclei. We also found
that HMGB1 and E-cadherin were negatively related; HMGB1 and
N-cadherin were positively related, which indicates that HMGB1 may
activate NF-κB via binding the receptor leading to a change in the
relative downstream target genes, which result in EMT of cervical
cancer. The specific mechanism needs to be further studied using
cervical cancer cell lines in vitro. Our results showed that
HMGB1-mediated stimulation of HeLa cells caused morphological
changes (polygonal, epithelial-to-fusiform, and spindle-shaped
transitions), significantly increased the intercellular space,
reduced cell adhesion, and promoted free cell migration. HMGB1
stimulation of HeLa cells increased cell proliferation and invasion
compared to that of the control group. Western blot analysis and
real-time PCR analyses showed a significant increase in protein and
mRNA levels of the mesenchymal marker N-cadherin on the HeLa cell
surface, but a significant reduction in protein and mRNA levels of
the epithelial marker E-cadherin. These effects were maximized by
treatment with 1,000 ng/ml HMGB1 for 48 h.
HMGB1 can bind RAGE and stimulate the MAPK and NF-κB
signaling pathways (22). HMGB1 has
a high affinity for RAGE, which is 7 times greater than the binding
affinity of other RAGE ligands. The binding of HMGB1 and RAGE can
activate NF-κB nuclear translocation and MAPK pathway signaling,
thereby inducing macrophages to express inflammatory and
chemotactic factors, and stimulating vascular endothelial cells to
express adhesion molecules (23–25).
During the transition of renal tubular epithelial cells to renal
fibrotic cells, HMGB1 has an important role in regulating the
occurrence of EMT as an intermediate (26). A study of a mouse pulmonary fibrosis
model reported that HMGB1 bound RAGE, caused an increase in TGF-β,
and promoted the transition from alveolar type II epithelial cells
to mesenchymal cells (i.e., EMT) (27). In the present study, HMGB1-mediated
cell stimulation was attenuated by the addition of anti-RAGE; the
expression of N-cadherin was decreased compared to that without
anti-RAGE treatment. This suggested that addition of anti-RAGE
blocked the EMT phenomenon which was caused by HMGB1. These results
indicate that anti-RAGE competitively bound with RAGE, which
suppressed extracellular HMGB1 binding with RAGE and its
tumor-promoting effect. We propose that RAGE antagonism therapy may
become a new target for cervical cancer treatment.
NF-κB is a nuclear factor that is present in many
cells. It participates in the regulation of several genes involved
in inflammation, immune reactions, cell proliferation and
apoptosis. RAGE-bound HMGB1 can activate extracellular
signal-regulated kinase (ERK) 1/2 and p38MAPK. The primary
mechanism is that HMGB1 binding to RAGE facilitates IκB
phosphorylation and degradation and suppresses NF-κB inhibition,
which activates NF-κB (28),
thereby promoting tumor occurrence, development, and metastasis.
HMGB1 and Toll-like receptor (TLR) binding can upregulate NF-κB
expression and promote NF-κB activation. Ligand-binding TLR4 and
TLR2 contribute to their own dimerization and their binding with
the connexin MyD88, and activate NF-κB and MAPK signaling pathways
by activating downstream interleukin receptor-associated kinase
(IRAK) and TNF receptor-associated factor 6 (TRAF6) (29,30).
In the resting state, NF-κB binds with its inhibitory protein IκB
and is present in the cytoplasm in an inactivated form. During cell
stimulation, cytoplasmic IκB is phosphorylated and degraded, which
relieves NF-κB inhibition and enables NF-κB entry into the nucleus.
Therefore, NF-κB activation has a crucial role in EMT of tumor
cells and contributes to tumor development and metastasis.
We hypothesized that HMGB1 could activate NF-κB and
MAPK signaling pathways, cause EMT in cervical cells, and thereby
promote cervical cancer metastasis. This study showed that
HMGB1-mediated stimulation of HeLa cells significantly increased
NF-κB protein expression levels in the nucleus, whereas the
expression level of IκB in the cytoplasm declined, indicating that
HMGB1 promoted NF-κB activation after stimulation of the HeLa
cells. Suppression of NF-κB pathway activation also suppressed
HMGB1-mediated EMT in HeLa cells, reduced HeLa cell proliferation
and invasion capabilities, reduced N-cadherin protein levels on the
HeLa cell surface, and increased E-cadherin protein and mRNA
levels. These results suggest that HMGB1 may contribute to the
invasion and migration of cervical cancer cells by activating the
NF-κB/IκB signaling pathway, and thus cause local invasion and
distant metastasis.
These combined results suggest that HMGB1 may
promote the invasion and migration of cervical cancer cells by
activating NF-κB signaling. However, tumor invasion and metastasis
is a complex process that requires many factors and multiple steps.
EMT is one of the initiating factors that cause tumor metastasis,
and many signaling pathways are involved in EMT regulation. Further
studies are required to investigate the role of HMGB1 in EMT
regulation during cervical cancer metastasis, which will clarify
the mechanism of cervical cancer metastasis, improve the diagnosis
of cervical cancer, and discover new therapeutic treatments.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81372776), the Youth Project
of National Natural Science Foundation (grant no. 81202048), the
Higher Specialized Research Fund for the Doctoral Program (grant
no. 20122104110014), and the Free Researcher Project of Shengjing
Hospital (grant no. 201302).
References
|
1
|
Lee MY and Shen MR: Epithelial-mesenchymal
transition in cervical carcinoma. Am J Transl Res. 4:1–13.
2012.PubMed/NCBI
|
|
2
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen RC, Yi PP, Zhou RR, Xiao MF, Huang
ZB, Tang DL, Huang Y and Fan XG: The role of HMGB1-RAGE axis in
migration and invasion of hepatocellular carcinoma cell lines. Mol
Cell Biochem. 390:271–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans A, Lennard TW and Davies BR:
High-mobility group protein 1(Y): Metastasis-associated or
metastasis-inducing? J Surg Oncol. 88:86–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XY and Yuan R: Expression and
significance of HMGB1 and E-cadherin in ovarian carcinoma. J
Chongqing Med Univ. 37:614–616. 2012.(In Chinese).
|
|
6
|
Zhao FH, Tiggelaar SM, Hu SY, Xu LN, Hong
Y, Niyazi M, Gao XH, Ju LR, Zhang LQ, Feng XX, et al: A
multi-center survey of age of sexual debut and sexual behavior in
Chinese women: Suggestions for optimal age of human papillomavirus
vaccination in China. Cancer Epidemiol. 36:384–390. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López-Novoa JM and Nieto MA: Inflammation
and EMT: An alliance towards organ fibrosis and cancer progression.
EMBO Mol Med. 1:303–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21:(Suppl 7). vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu ST, Sun GH, Hsu CY, Huang CS, Wu YH,
Wang HH and Sun KH: Tumor necrosis factor-α induces
epithelial-mesenchymal transition of renal cell carcinoma cells via
a nuclear factor kappa B-independent mechanism. Exp Biol Med
(Maywood). 236:1022–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL,
Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang SC, Lin XL, Wang HY, Qin YJ, Chen L,
Li J, Jia JS, Shen HF, Yang S, Xie RY, et al: Hes1 triggers
epithelial-mesenchymal transition (EMT)-like cellular marker
alterations and promotes invasion and metastasis of nasopharyngeal
carcinoma by activating the PTEN/AKT pathway. Oncotarget.
6:36713–36730. 2015.PubMed/NCBI
|
|
14
|
Lin Z, Li W, Zhang H, Wu W, Peng Y, Zeng
Y, Wan Y, Wang J and Ouyang N: CCL18/PITPNM3 enhances migration,
invasion, and EMT through the NF-κB signaling pathway in
hepatocellular carcinoma. Tumour Biol. 37:3461–3468. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strippoli R, Benedicto I, Pérez Lozano ML,
Cerezo A, López-Cabrera M and del Pozo MA:
Epithelial-to-mesenchymal transition of peritoneal mesothelial
cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis Model
Mech. 1:264–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huber MA, Beug H and Wirth T:
Epithelial-mesenchymal transition: NF-kappaB takes center stage.
Cell Cycle. 3:1477–1480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong XE, Ito N, Lotze MT, Demarco RA,
Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL,
et al: High mobility group box I (HMGB1) release from tumor cells
after treatment: Implications for development of targeted
chemoimmunotherapy. J Immunother. 30:596–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et
al: HMG-1 as a late mediator of endotoxin lethality in mice.
Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klune JR, Dhupar R, Cardinal J, Billiar TR
and Tsung A: HMGB1: Endogenous danger signaling. Mol Med.
14:476–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Olkhanud PB, Damdinsuren B, Bodogai M,
Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP and Biragyn A:
Tumor-evoked regulatory B cells promote breast cancer metastasis by
converting resting CD4+ T cells to T-regulatory cells.
Cancer Res. 71:3505–3515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian J, Avalos AM, Mao SY, Chen B, Senthil
K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al:
Toll-like receptor 9-dependent activation by DNA-containing immune
complexes is mediated by HMGB1 and RAGE. Nat Immunol. 8:487–496.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sha Y, Zmijewski J, Xu Z and Abraham E:
HMGB1 develops enhanced proinflammatory activity by binding to
cytokines. J Immunol. 180:2531–2537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang D, Chen Q, Yang H, Tracey KJ, Bustin
M and Oppenheim JJ: High mobility group box-1 protein induces the
migration and activation of human dendritic cells and acts as an
alarmin. J Leukoc Biol. 81:59–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lynch J, Nolan S, Slattery C, Feighery R,
Ryan MP and McMorrow T: High-mobility group box protein 1: A novel
mediator of inflammatory-induced renal epithelial-mesenchymal
transition. Am J Nephrol. 32:590–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He M, Kubo H, Ishizawa K, Hegab AE,
Yamamoto Y, Yamamoto H and Yamaya M: The role of the receptor for
advanced glycation end-products in lung fibrosis. Am J Physiol Lung
Cell Mol Physiol. 293:L1427–L1436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Beijnum JR, Buurman WA and Griffioen
AW: Convergence and amplification of toll-like receptor (TLR) and
receptor for advanced glycation end products (RAGE) signaling
pathways via high mobility group B1 (HMGB1). Angiogenesis.
11:91–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palumbo R, Galvez BG, Pusterla T, De
Marchis F, Cossu G, Marcu KB and Bianchi ME: Cells migrating to
sites of tissue damage in response to the danger signal HMGB1
require NF-kappaB activation. J Cell Biol. 179:33–40. 2007.
View Article : Google Scholar : PubMed/NCBI
|