Introduction
Nasopharyngeal carcinoma (NPC) is a type of
malignant tumor that originates in the nasopharyngeal epithelium.
The primary treatment method for NPC is radiation therapy (1,2). NPC
is sensitive to radiation; however, according to the cancer stem
cell (CSC) theory, NPC stem cells (NPCSCs) are resistant to
radiation (3–5). Many factors, including nuclear factor
(NF)-κB (6) and hypoxia-inducible
factor-1 (HIF-1) (7), which are
overexpressed in cells under hypoxic conditions, contribute to the
radioresistance of CSCs (8,9).
NF-κB, a nuclear transcription factor, is
overexpressed in various cancers containing NPCs, and p65 is a key
subunit required for functional NF-κB activity (10,11).
Previous studies have found that NF-κB regulates
epithelial-mesenchymal transition (EMT), thus contributing to
cancer occurrence and progression (12). Decreased protein expression of the
epithelium marker E-cadherin (E-cad) and increased protein
expression of N-cadherin (N-cad) and vimentin are direct evidence
of the occurrence of EMT (13).
2-Methoxyestradiol (2-ME2), an endogenous estradiol metabolite, is
being investigated in clinical trials as an anticancer agent
(14). 2-ME2 regulates NF-κB
activation to influence apoptosis and chemoresistance in various
tumor cell lines, including leukemia K562 cells (15), medulloblastoma cells (16), prostate cancer cells (17) and pancreatic cancer cells (18). Thus, we speculated that 2-ME2 may
play a vital role in the proliferation, migration and
radioresistance of NPCSCs via the regulation of NF-κB p65
activity.
Solid tumors generally have a hypoxic
microenvironment (19). HIF-1α, a
unique subunit of HIF-1, which functions in the cellular response
to oxygen levels and which is overexpressed in solid tumors, is
closely associated with CSC resistance to treatment (20–22). A
previous study found that 2-ME2 decreased the HIF-1α-binding
activity, affected the expression of downstream genes, inhibited
tumor growth and angiogenesis and augments paclitaxel efficacy in
head and neck squamous cell carcinoma (23). However, whether 2-ME2 affects NPCSC
proliferation, migration and treatment resistance through the
regulation of HIF-1α remains unclear.
The objective of this study was to determine the
antitumor effects of 2-ME2 on NPCSCs and to demonstrate the
underlying mechanism involved in the antitumor effects of 2-ME2
through the suppression of the NF-κB/HIF-1 signaling pathway. 2-ME2
could potentially become a novel radiotherapy sensitization agent
for clinical use.
Materials and methods
Reagents
The human NPC cell line CNE-2 was obtained from the
Tumor Laboratory of Chongqing Medical University (Chongqing,
China). Penicillin-streptomycin (1%), fetal bovine serum (FBS),
RPMI-1640 culture medium and Dulbecco's modified Eagle's medium/F12
(DMEM/F12; 1:1 volume) were purchased from Gibco (Grand Island, NY,
USA). B27 supplement (2%) was purchased from Invitrogen (Carlsbad,
CA, USA). Accutase was purchased from Innovative Cell Technologies,
Inc. (San Diego, CA, USA). Human recombinant basic fibroblast
growth factor (bFGF) and epidermal growth factor (EGF) were
purchased from Peprotech, Inc. (Rocky Hill, NJ, USA). 2-ME2 was
purchased from Selleck Chemicals (Shanghai, China). Real-time
polymerase chain reaction (RT-PCR) kits were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Transwell chambers with an
8-µm aperture were purchased from Beijing Mingyangkehua
Biotechnology Co., Ltd. (Beijing, China). Anti-CD133-PE antibody
for flow cytometry (FCM) was purchased from Miltenyi Biotec
(Beijing, China). The lentiviral vector encoding NF-κB p65-shRNA
(pMAGic 7.1) was obtained from Shanghai Sunbio Co. (Shanghai,
China). Mouse anti-human primary antibodies directed against NF-κB
p65, lamin B1, vimentin, E-cad, and N-cad were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). The mouse anti-human
primary antibodies directed against HIF-1α and GAPDH and secondary
goat anti-mouse IgG antibody were purchased from Wuhan Boster
Biological Engineering Co., Ltd. (Wuhan, China). A modified BCA
protein assay kit, an allergic ECL chemiluminescence reagent kit
and an SDS-PAGE gel preparation kit were purchased from Beyotime
Biotech (Jiangsu, China). Cell proliferation-toxicity testing kits
[Cell Counting Kit-8 (CCK-8)] were purchased from Dojindo
Laboratories Co., Ltd. (Kumamoto, Japan). An inverted fluorescence
microscope was obtained from Nikon Corporation (Tokyo, Japan), an
upright fluorescence microscope was obtained from Olympus Corp.
(Tokyo, Japan), a flow cytometer was obtained from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA), and a linear accelerator
for radiotherapy was obtained from Varian Medical Systems, Inc.
(Palo Alto, CA, USA).
Cell culture
To culture the parental CNE-2 cell lines, CNE-2
cells were cultured in RPMI-1640 supplemented with 1%
penicillin-streptomycin and 10% FBS, placed in an incubator with 5%
CO2 at 37°C, and passaged every other day.
To enrich stem-like cells, a non-adhesive culture
system of serum-free medium (SFM) consisting of DMEM/F12
supplemented with 20 ng/ml bFGF, 20 ng/ml EGF, 2% B27 supplement
and 1% penicillin-streptomycin was used to collect NPCSCs. The
parental CNE-2 cells were re-suspended in T25, placed vertically in
an incubator, shaken several times a day, and passaged every 4–6
days until the cells formed microspheres.
To culture the NPCSCs, the microsphere suspension
was cultured in SFM and passaged every 4–6 days. When the cells
were passaged, the tumor spheroids were dissociated with Accutase
into single cells, transferred to new T25 containing new SFM, and
placed in an incubator with 5% CO2 at 37°C.
NPCSC re-differentiation assay
NPCSCs were collected, dissociated into single cells
with Accutase, seeded in a 6-well plate with RPMI-1640 containing
10% FBS, and placed in an incubator with 5% CO2 at 37°C.
After 3–5 days, the changes in cellular morphology were observed
using an inverted microscope.
Transwell migration assay
The parental CNE-2 cells and the NPCSCs were
dissociated into single cells that were then re-suspended
(2×105 cells/ml) in SFM. The upper chamber was loaded
with a 100-µl cell suspension, and the lower chamber was loaded
with a 500 µl 10% serum-containing medium. The cells were fixed
after 24 h with methanol and stained with crystal violet. Five
random fields were analyzed, and the number of cells invading
through the membrane was counted under a microscope (Olympus
Corp.). Three independent experiments were performed.
Soft agar colony assay
The parental cells and the NPCSCs were dissociated
into single cells and then re-suspended (103 cells/ml)
with SFM in 0.3% agar overlaid in 6-well plates (2 ml/well)
containing a 0.6% agar base. After the cells were incubated for 3
weeks, the spheroids >0.2 mm in diameter were evaluated using
fluorescence microscopy. Three independent experiments were
performed.
RT-PCR
To quantify gene expression, RT-PCR was performed
with SYBR-Green. In brief, an RNAiso reagent was used to extract
total RNA, and a reverse transcriptase kit was used to synthesize
the cDNA according to the manufacturer's instructions. Then, PCR
was performed. The expression levels of target genes relative to
GAPDH were determined using a SYBR-Green-based comparative CT
method (relative fold-change, 2−ΔΔCt). The primers were
designed by Sangon Biotech Co., Ltd. (Shanghai, China), and are as
follows: NF-κB p65 forward, 5′-GGAGCACAGATACCACCAAGA-3′ and
reverse, 5′-CGGCAGTCCTTTCCTACAAG-3′; HIF-1α forward,
5′-CTGCCAACCCCGAAATGACAT-3′ and reverse,
5′-CGCCGCTTAATAGCCCTCTG-3′; Bmi-1 forward,
5′-CCTGATGTGTGTGCTTTGTG-3′ and reverse,
5′-GGTCTGGTCTTGTGAACTTGG-3′; Twist1 forward,
5′-GAGCAAGATTCAGACCCTCAA-3′ and reverse, 5′-CATCCTCCAGACCGAGAAG-3′;
Oct4 forward, 5′-AGCCCTCATTTCACCAGGCC-3′ and reverse,
5′-TGGGACTCCTCCGGGTTTTG-3′; GAPDH forward,
5′-ACGGGAAGCTCACTGGCATGG-3′ and reverse,
5′-GGTCCACCACCCTGTTGCTGTA-3′.
The NPCSCs are treated with 2-ME2 and
grouped
The NPCSCs were dispersed into single cells,
suspended in pre-made 4, 8 and 0 µM (control) 2-ME2 solution and
then placed in an incubator with 5% CO2 at 37°C for 24
h.
Colony formation assay
Cells that had been treated with 2-ME2 for 24 h or
that were in the early log phase were dispersed, plated in 6-well
plates at 200, 400, 800 and 2,000 cells/well and then treated with
0, 2, 4 and 8 Gy irradiation. The cells were incubated for 12 days
to allow colony formation. Subsequently, the cells were fixed and
stained with 0.5% crystal violet, and the number of colonies
containing >50 cells was counted. Survival curves were fitted
using the multi-target click model in GraphPad Prism 5.0 software.
Each point on the survival curve represents the mean surviving
fraction (SF) from at least three independent experiments.
Flow cytometric assay
The expression of CD133+, a surface
marker of CSCs, was analyzed using FCM. The cells were dissociated
into single cells, washed and re-suspended in PBS. Then, the cells
were counted, adjusted to 1×106 cells/group, and
incubated with the appropriate concentration of anti-CD133-PE
(Miltenyi Biotec). The proportion of CD133+ cells in
each sample was detected by FCM.
Western blot analysis
Cytoplasmic, nuclear or total protein extraction and
sample preparation for western blot analysis were performed as
previously described (24). The
membranes were then incubated with one or more of the following
primary antibodies: anti-NF-κB p65, anti-lamin B1, anti-E-cad,
anti-N-cad, and anti-vimentin (all 1:1,000), as well as anti-GAPDH
and anti-HIF-1α (each 1:200). The proteins of interest were then
detected with goat anti-mouse secondary antibody (1:3,000). The
band intensities were detected using a western blot analysis
system. The proteins were normalized to GAPDH or lamin B1 and
quantified using the ChemiDoc™XRS. Three independent experiments
were performed.
Cell viability test
Cells that had been treated with 2-ME2 (0–8 µM) for
24 h were dispersed into single cells, counted, and then seeded
into 96-well plates at 2×104 cells/well. Each group was
set in 8 repeated wells and each well was loaded with 20 µl of
CCK-8. After the plates were placed in an incubator for 3 h, cell
viability was detected using ELISA.
Immunofluorescence staining
The cells that had been treated with 2-ME2 (0–8 µM)
for 24 h were plated onto poly-L-lysine-coated glass coverslips,
fixed in 4% paraformaldehyde for 20 min at room temperature,
permeabilized with 0.1% Triton X-100 and blocked with 10% BSA. The
slides were incubated overnight at 4°C with mouse anti-NF-κB p65
followed by incubation with secondary fluorescein-conjugated
antibodies [goat anti-mouse-IgG, tetraethyl rhodamine
isothiocyanate (TRITC)] at room temperature for 1 h. The slides
were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) and
analyzed under a fluorescence microscope. Three independent
experiments were performed.
p65-shRNA-infected cells
Gene knockdown targeting NF-κB p65 was performed as
previously described (24). Two
oligonucleotides were used to target NF-κB p65. The cells were
grown to the log phase in T25, dispersed into single cells and
transfected with the virus shRNA or virus non-carrier in SFM. The
levels of NF-κB p65 gene silencing and protein expression were
evaluated by RT-PCR and western blot analysis, respectively.
Statistical analysis
The values are expressed as the means ± standard
deviation (SD). Differences between groups were analyzed by
Student's t-tests. Differences among three groups were analyzed
using one-way ANOVA, and differences between any two groups were
analyzed using least significant difference (LSD) tests. All
statistical analyses were performed using SPSS version 16.0 for
Windows. Values of *p<0.05 were considered significant, and
values of **p<0.01 were considered very significant.
Results
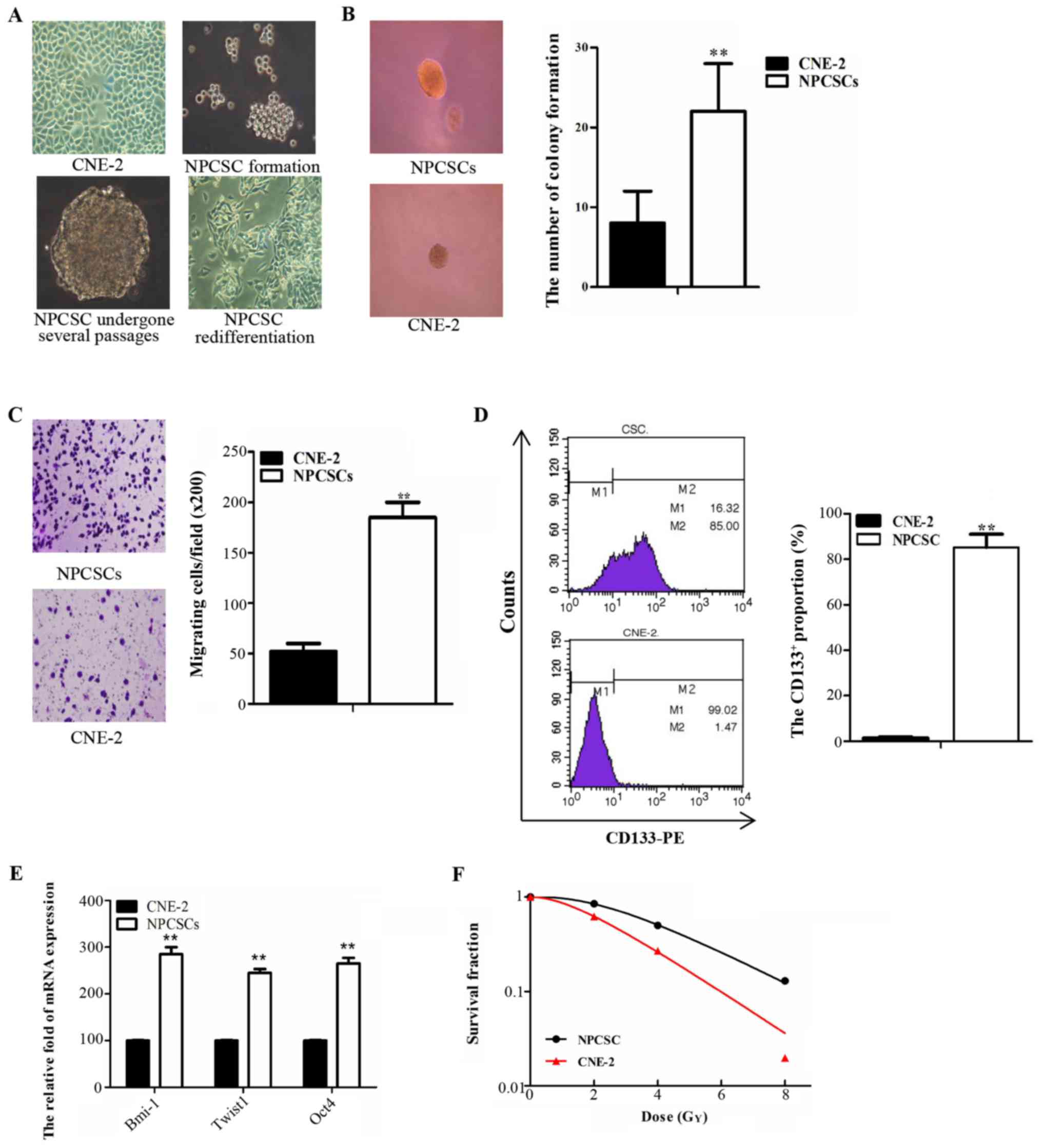
NPCSCs which were derived from CNE-2
cells acquired stem-like characteristics and radioresistance
The parental CNE-2 cells were cultured and routinely
passaged in RPMI-1640 medium containing 10% FBS (Fig. 1A left, upper panel). Cells formed
microspheres in the SFM approximately 8–10 days after being
suspended (Fig. 1A right, upper
panel). The microspheres maintained a strong ability to grow after
repeated passages (Fig. 1A left,
panel below) and differentiated into spindle adherent cells when
re-cultured in medium containing 10% FBS (Fig. 1A right, panel below). These data
demonstrated that the spherical cells have strong self-renewal and
differentiation abilities. These abilities were subsequently
identified as stem cell characteristics of NPCSCs. To identify the
stem cell characteristics of the spherical cells, soft agar cloning
and Transwell assays were used to detect the self-renewal and
migration abilities, respectively, of these cells in vitro.
The number of CNE-2 colonies was significantly reduced compared
with the number of spherical cells (Fig. 1B; p<0.01). The number of CNE-2
cells that migrated to the other side of the membrane was
significantly decreased compared with the number of spherical cells
that migrated (Fig. 1C; p<0.01).
The cell surface antigen CD133+ was used as a
CSC-specific marker, and the proportion of CD133+ cells
was detected by FCM. The results revealed that the proportion of
CD133+ cells was 85.2±5.88% for spherical cells and
1.47±0.45% for CNE-2 cells (p<0.01) (Fig. 1D). Several genes, including Bmi-1,
Twist1, and Oct4, which are associated with CSC properties, were
quantitatively analyzed by RT-PCR. We found that the expression
levels of these genes were significantly increased in spherical
cells compared with those in the CNE-2 cells (Fig. 1E). Colony formation assay was used
to investigate the radiosensitivity of the spherical cells. The
survival curve of the SF was generated using GraphPad software with
a multi-target single-hit model. This result demonstrated that the
spherical cells were more resistant to radiation therapy compared
with the CNE-2 cells (Fig. 1F). We
concluded that the spherical cells presented strong stemness, and
we used these cells in subsequent experiments as NPCSCs.
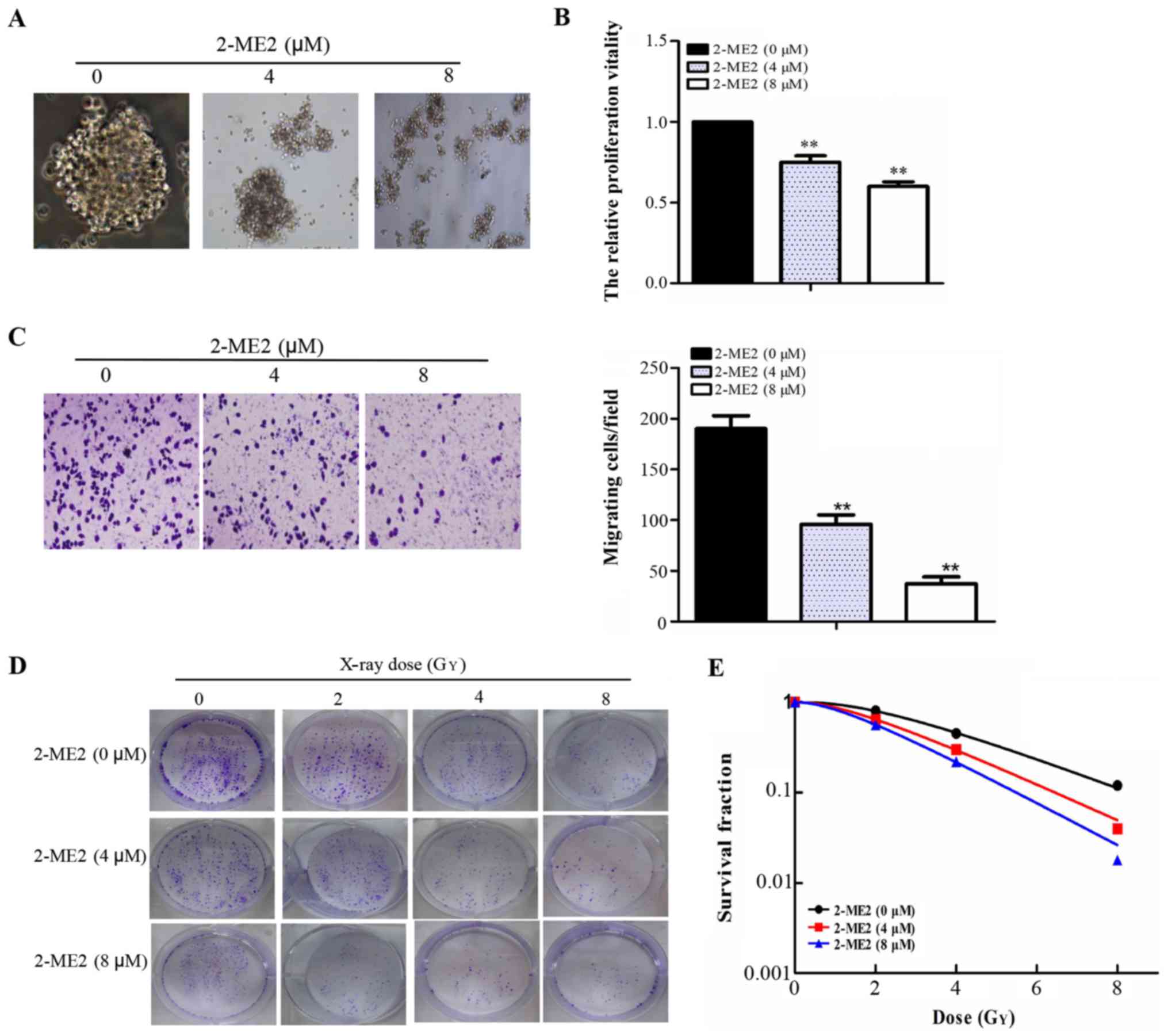
2-ME2 inhibits NPCSC proliferation and
migration and reduces NPCSC radioresistance
The compact and spherical growth of NPCSCs that had
been treated with 2-ME2 for 24 h dispersed gradually with
increasing concentrations of 2-ME2 as observed under an inverted
microscope (Fig. 2A). A CCK-8 assay
was used to detect cell proliferation. Cell proliferation clearly
decreased with increasing concentrations of 2-ME2 (Fig. 2B). A Transwell assay was used to
detect the migratory abilities of these cells. The numbers of cells
crossing the membrane were 190±12, 96±7, and 37±5 (Fig. 2C) for the groups treated with 2-ME2
(0, 4 and 8 µM, respectively), which indicated that 2-ME2 inhibited
NPCSC migration. To determine the radiosensitivity effect of 2-ME2
on the NPCSCs, a colony formation assay was performed (Fig. 2D). As depicted in Fig. 2E, the shoulder area of the survival
curves significantly narrowed, and the SFs at each dose (2, 4 and 8
Gy) decreased in a 2-ME2 concentration-dependent manner. The SER of
the 4 and 8 µM groups was 1.20 (p<0.05) and 1.39 (p<0.05),
respectively, indicating that 2-ME2 reduced NPCSC
radioresistance.
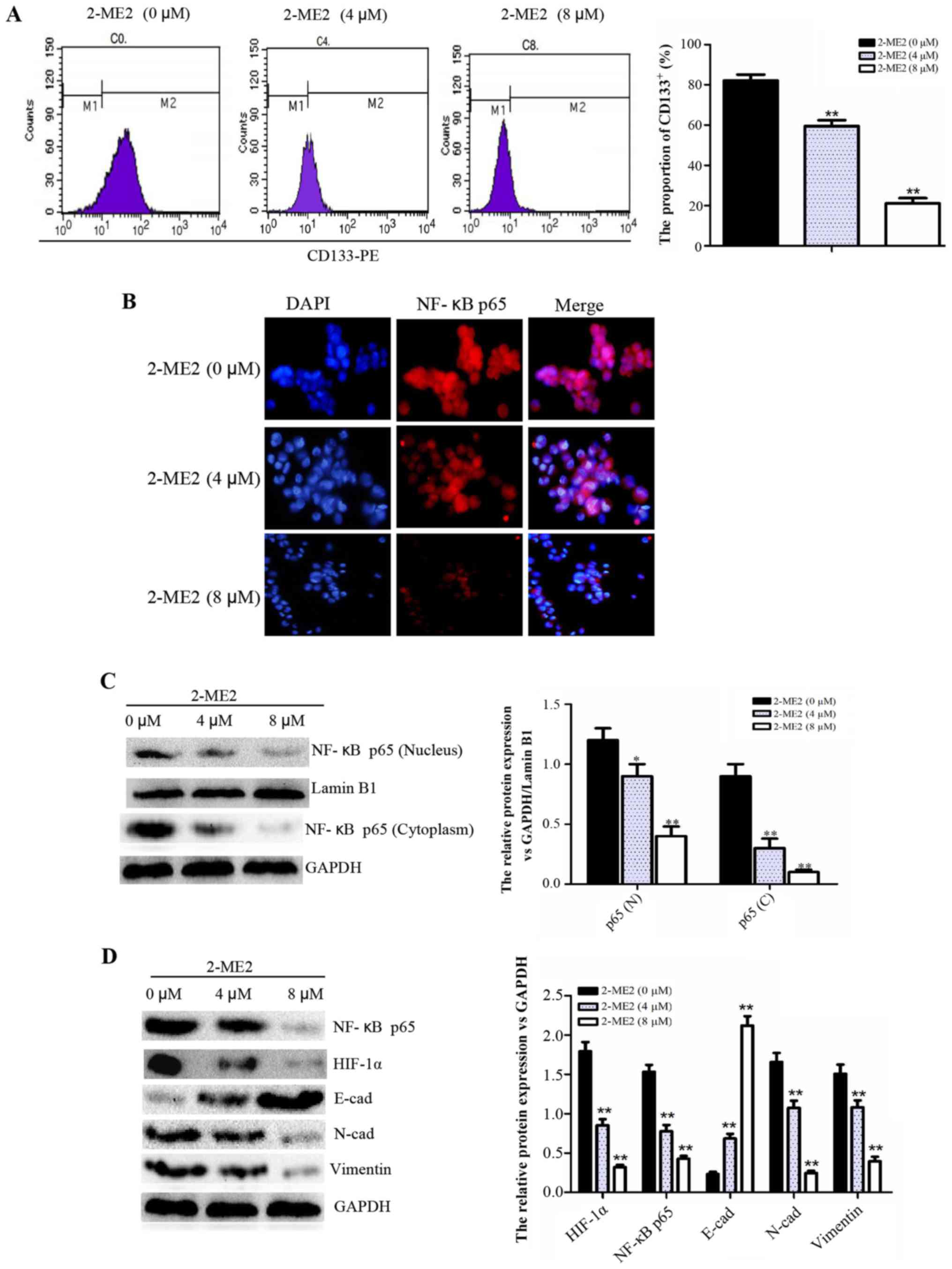
2-ME2 decreases the proportion of
CD133+ cells, inhibits the NF-κB p65/HIF-1α signaling
pathway, and reverses EMT in NPCSCs
To determine the molecular mechanism of 2-ME2
inhibition of NPCSC growth and migration and the reduction of NPCSC
radioresistance, FCM, cellular immunofluorescence and western blot
analyses were performed. The FCM results indicated that the
proportion of CD133+ cells decreased significantly in
the groups treated with higher concentrations of 2-ME2 (Fig. 3A), thus demonstrating that 2-ME2 may
decrease NPCSC stemness. According to cellular immunofluorescence,
the location of NF-κB p65 in the nucleus is representative of
significantly decreased activation (Fig. 3B). Furthermore, western blot
analysis revealed that the protein expression of NF-κB p65 in the
nucleus and cytoplasm and the protein expression of total HIF-1α
were reduced (Fig. 3C and D), which
indicated that the NF-κB/HIF-1 signaling pathway was inhibited.
Additionally, the expression of the EMT marker E-cad increased,
whereas the expression of N-cad and vimentin decreased (Fig. 3D), which indicated reversal of EMT.
Based on these results, we concluded that 2-ME2 decreased the cell
stemness, inhibited the NF-κB/HIF-1 signaling pathway, and reversed
EMT in NPCSCs.
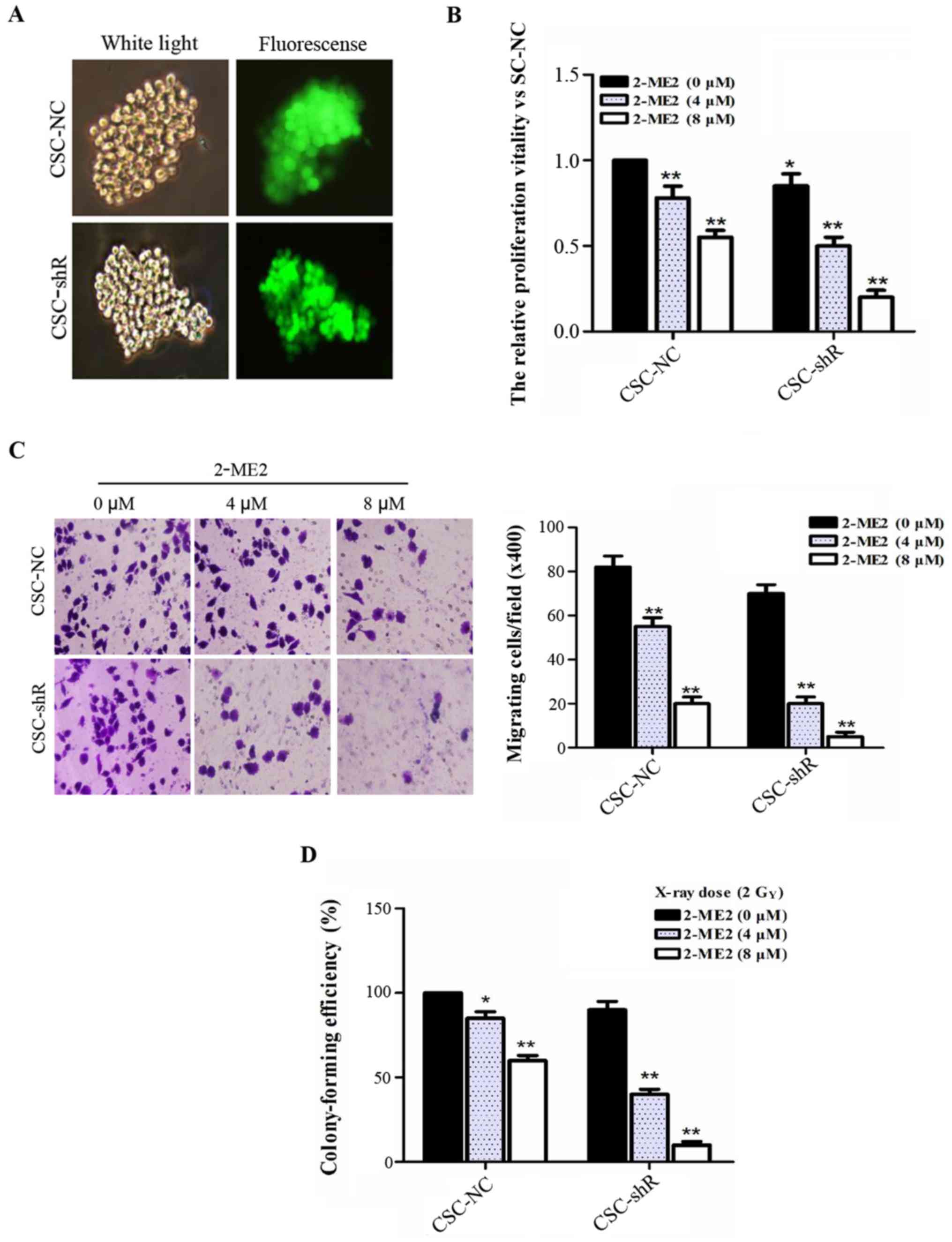
NF-κB p65 knockdown enhances the
effect of 2-ME2 on NPCSCs
Lentiviral infection was performed in NPCSCs using a
virus-shRNA-NF-κB p65 solution and stably transfected cell lines.
NPCSCs transfected with the lentivirus-mediated RNA targeting NF-κB
p65 (CSC-shR) and with the empty retroviral vector (CSC-NC) were
also established (Fig. 4A). To
investigate the role of NF-κB p65 in the effect of 2-ME2 on NPCSCs,
CCK-8, Transwell, and colony formation assays were performed. The
viability of the CSC-NC cells was significantly decreased in a
2-ME2 concentration-dependent manner. Additionally, NF-κB p65
knockdown enhanced the effect of 2-ME2 on NPCSCs (Fig. 4B). A Transwell assay was used to
evaluate the migration of NF-κB p65-silenced NPCSCs in
vitro. The number of CSC-shR cells that migrated to the other
side of the membrane decreased more significantly with increasing
concentrations of 2-ME2 compared to the CSC-NC (Fig. 4C). These results indicated that
silencing of NF-κB p65 enhanced the effect of 2-ME2 on the
proliferation and migration of NPCSCs. In addition, a colony
formation assay was used to examine whether silencing of NF-κB p65
affected the anti-radioresistance effect of 2-ME2 on NPCSCs. The
data showed that 2-ME2 reduced the radioresistance of NPCSCs and
that NF-κB p65 further enhanced the anti-radioresistance effects of
2-ME2 (Fig. 4D).
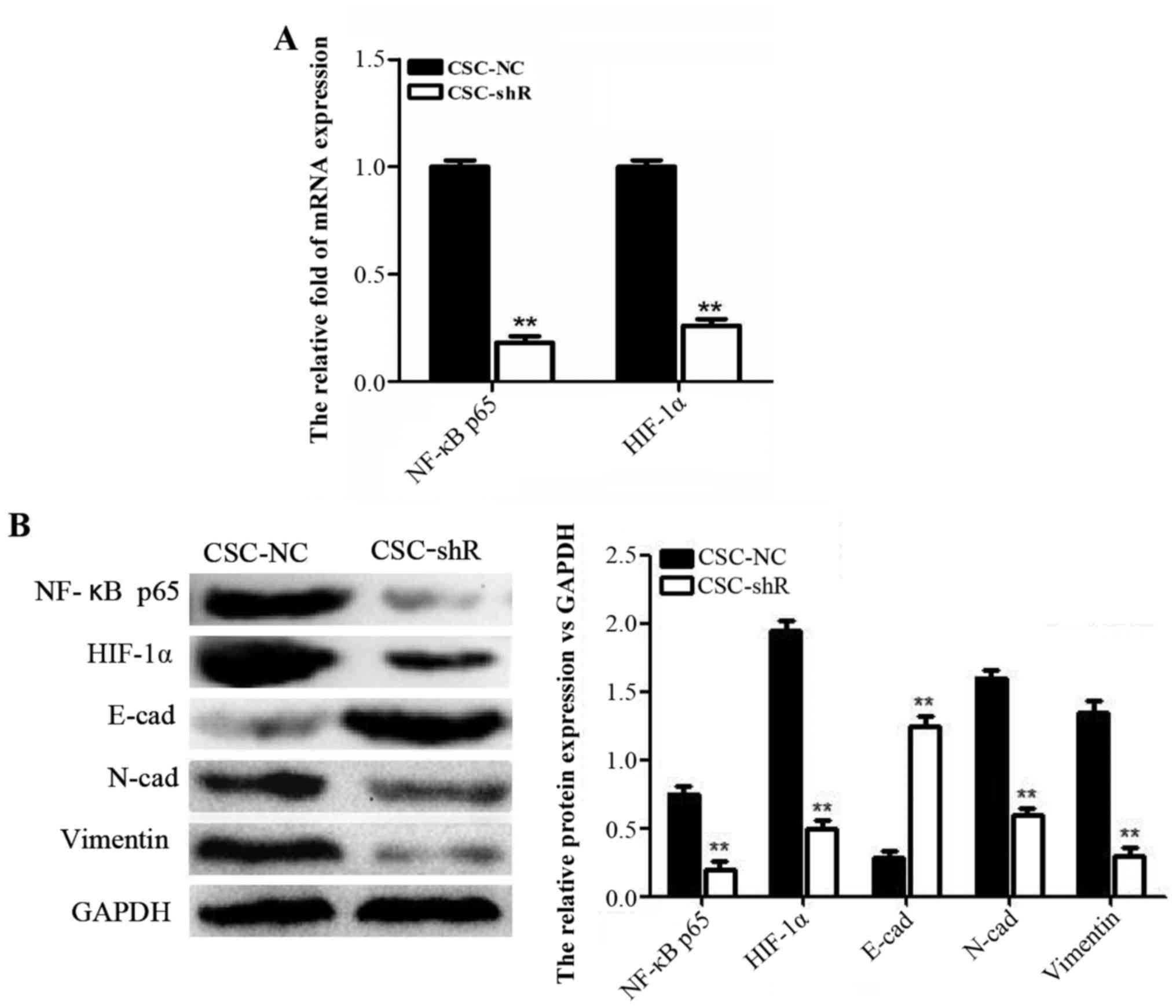
NF-κB p65 knockdown suppresses HIF-1α
mRNA and protein expression and reverses EMT in NPCSCs
RT-PCR and western blot analyses were used to
explore the molecular mechanism involved in the effect of NF-κB p65
on NPCSCs. After NF-κB p65 knockdown, the mRNA and protein levels
of p65 and HIF-1α were significantly decreased (Fig. 5A). The protein levels of the EMT
markers N-cad and vimentin were decreased, whereas the protein
level of E-cad was increased (Fig.
5B). These results suggested that NF-κB p65 knockdown inhibited
HIF-1α and reversed EMT in NPCSCs.
Discussion
Previous studies have confirmed that CSCs can be
collected quickly and efficiently in a serum-free culture system
(25,26). Therefore, a serum-free culture
system was used in this study. According to these stemness
identification results, compared with the parental cells, the
stem-like spherical cells presented suspended growth, limitless
self-renewal and re-differentiation abilities, overexpression of
stem-relevant genes, a higher proportion of CD133+
cells, stronger migration ability, and radioresistance. These
results indicated that these stem-like spherical cells possess
strong stemness, therefore, these cells were used in the
subsequently described experiments as NPCSCs.
In the human body, 2-ME2 is an estrogen metabolite.
The anticancer effect of 2-ME2 is currently being researched in
studies (14). Recent studies have
found that 2-ME2 regulates the cell cycle, apoptosis, transcription
factor (including NF-κB, HIF-1) activation, and mitosis and that
2-ME2 plays an important role in the therapy resistance of
malignant prostatic cancer, glioma, and head and neck squamous cell
carcinoma (15–18,23,27,28).
However, the effect of 2-ME2 on NPCSCs remains unclear. Our study
indicates that 2-ME2 inhibits NPCSC growth and migration and
reduces NPCSC radioresistance.
NF-κB is overexpressed in almost all cancer cells
and mediates multiple signaling pathways to contribute to cell
proliferation and to treatment resistance (29–31).
However, the role of NF-κB regulated by 2-ME2 in cancer progression
is complex. 2-ME2 displays different effects on NF-κB activation in
different types of cells. For example, 2-ME2 increased NF-κB
activity in LNCaP cells, in contrast, 2-ME2 slightly decreased
NF-κB activity in PC3 cells. 2-ME2 can increase NF-κB activity in
tumor cell lines, however, whether this increase stimulates or
antagonizes apoptosis appears to be dependent on the specific type
of tumor cell (17). 2-ME2
modulation of the downregulation of glucocorticoid receptor (GR)
levels is accompanied by NF-κB activation in 2-ME2-responsive, but
not in 2-ME2-resistant, pancreatic cancer cells (32). 2-ME inhibits the proliferation and
induces the apoptosis of leukemia K562 cells via inhibition of
NF-κB activity (15). To determine
the mechanism of the anticancer effect of 2-ME2 on NPCSCs, we
evaluated the effect of 2-ME2 on NF-κB p65. The western blot
analysis and cellular immunofluorescence results revealed that
2-ME2 (0–8 µM) reduced the nuclear and cytoplasmic protein
expression and nuclear localization of NF-κB p65 at 24 h, which
indicated that 2-ME2 inhibited NF-κB p65 activation in NPCSCs. We
also found that HIF-1α protein expression was decreased in a 2-ME2
concentration-dependent manner. In addition, evidence suggests that
2-ME2 can depolymerize microtubules and inhibit the nuclear
accumulation and transcription activity of HIF-1α (33,34).
2-ME2 has been shown to decrease the activity of HIF-1α and affect
the expression of downstream genes in head and neck squamous cell
carcinoma (23). The
radiosensitization effects of 2-ME2 may be partially dependent on
HIF-1α inhibition in various tumors (35). Therefore, we speculated that 2-ME2
inhibits the NF-κB/HIF-1 signaling pathway.
For further study, we knocked down NF-κB p65 using
gene interference technology. We found that NF-κB p65 knockdown
enhanced the antitumor effect of 2-ME2 on NPCSCs and that the
protein and mRNA levels of HIF-1α decreased along with NF-κB p65,
which implied that positive regulation of NF-κB p65 to HIF-1α
existed in NPCSCs, corresponding with previous evidence that NF-κB
can regulate HIF-1α at the mRNA level (36,37).
Our previous study determined that NF-κB p65 knockdown reduced the
chemoresistance of NPCSCs via EMT reversal (24). Our subsequent experiments determined
that NF-κB p65 knockdown inhibited EMT and reduced the
radioresistance of NPCSCs. Based on the above evidence, we conclude
that NF-κB p65 knockdown may enhance the effect of 2-ME2 on NPCSCs
via HIF-1α downregulation and EMT reversal.
Overall, our findings indicate that 2-ME2 inhibits
NPCSC proliferation, migration and radioresistance possibly by
inhibiting the NF-κB/HIF-1 signaling pathway and reversing EMT.
2-ME2 has a potential use as a novel tumor therapy drug or
radiotherapy sensitization agent, however, additional research and
evidence are needed.
Acknowledgements
This study was supported by the General Program of
the National Natural Science Foundation of China (nos. 81171365 and
81560444).
References
|
1
|
Peng G, Wang T, Yang KY, Zhang S, Zhang T,
Li Q, Han J and Wu G: A prospective, randomized study comparing
outcomes and toxicities of intensity-modulated radiotherapy vs.
conventional two-dimensional radiotherapy for the treatment of
nasopharyngeal carcinoma. Radiother Oncol. 104:286–293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang R, Wu F, Lu H, Wei B, Feng G, Li G,
Liu M, Yan H, Zhu J, Zhang Y, et al: Definitive intensity-modulated
radiation therapy for nasopharyngeal carcinoma: Long-term outcome
of a multicenter prospective study. J Cancer Res Clin Oncol.
139:139–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang CF, Peng LX, Huang TJ, Yang GD, Chu
QQ, Liang YY, Cao X, Xie P, Zheng LS, Huang HB, et al: Cancer
stem-like cell characteristics induced by EB virus-encoded LMP1
contribute to radioresistance in nasopharyngeal carcinoma by
suppressing the p53-mediated apoptosis pathway. Cancer Lett.
344:260–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang WJ, Wu SP, Liu JB, Shi YS, Huang X,
Zhang QB and Yao KT: MYC regulation of CHK1 and CHK2 promotes
radioresistance in a stem cell-like population of nasopharyngeal
carcinoma cells. Cancer Res. 73:1219–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei P, Niu M, Pan S, Zhou Y, Shuai C, Wang
J, Peng S and Li G: Cancer stem-like cell: A novel target for
nasopharyngeal carcinoma therapy. Stem Cell Res Ther. 5:442014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Liu H, Xu L, An H, Liu W, Liu Y,
Lin Z and Xu J: p21-activated kinase 1 determines stem-like
phenotype and sunitinib resistance via NF-κB/IL-6 activation in
renal cell carcinoma. Cell Death Dis. 6:e16372015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vinogradov S and Wei X: Cancer stem cells
and drug resistance: The potential of nanomedicine. Nanomedicine
(Lond). 7:597–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meijer TW, Kaanders JH, Span PN and
Bussink J: Targeting hypoxia, HIF-1, and tumor glucose metabolism
to improve radiotherapy efficacy. Clin Cancer Res. 18:5585–5594.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scholz CC, von Kriegsheim A, Tambuwala MM,
Hams E, Cheong A, Bruning U, Fallon PG, Cummins EP and Taylor CT:
Prolyl Hydroxylase 1 (PHD1) and Factor Inhibiting HIF (FIH)
regulate IL-1β-induced NF-κB activity linking key hypoxic and
inflammatory signaling pathways. FASEB J. 27:717–719. 2013.
|
|
10
|
Wong JH, Lui VW, Umezawa K, Ho Y, Wong EY,
Ng MH, Cheng SH, Tsang CM, Tsao SW and Chan AT: A small molecule
inhibitor of NF-kappaB, dehydroxymethylepoxyquinomicin (DHMEQ),
suppresses growth and invasion of nasopharyngeal carcinoma (NPC)
cells. Cancer Lett. 287:23–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kan R, Shuen WH, Lung HL, Cheung AK, Dai
W, Kwong DL, Ng WT, Lee AW, Yau CC, Ngan RK, et al: NF-κB p65
subunit is modulated by Latent Transforming Growth Factor-β Binding
Protein 2 (LTBP2) in nasopharyngeal carcinoma HONE1 and HK1 cells.
PLoS One. 10:e01272392015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL,
Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao D, Tang XF, Yang K, Liu JY and Ma XR:
Over-expression of integrin-linked kinase correlates with aberrant
expression of Snail, E-cadherin and N-cadherin in oral squamous
cell carcinoma: Implications in tumor progression and metastasis.
Clin Exp Metastasis. 29:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kambhampati S, Rajewski RA, Tanol M, Haque
I, Das A, Banerjee S, Jha S, Burns D, Borrego-Diaz E, Van
Veldhuizen PJ, et al: A second-generation 2-Methoxyestradiol
prodrug is effective against Barrett's adenocarcinoma in a mouse
xenograft model. Mol Cancer Ther. 12:255–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XY, Zhan R, Huang HB and Yang T:
Mechanism underlying 2-methoxyestradiol inducing apoptosis of K562
cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 17:340–344. 2009.(In
Chinese). PubMed/NCBI
|
|
16
|
Kumar AP, Garcia GE, Orsborn J, Levin VA
and Slaga TJ: 2-Methoxyestradiol interferes with NFκB
transcriptional activity in primitive neuroectodermal brain tumors:
Implications for management. Carcinogenesis. 24:209–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parrondo R, de las Pozas A, Reiner T, Rai
P and Perez-Stable C: NF-κB activation enhances cell death by
antimitotic drugs in human prostate cancer cells. Mol Cancer.
9:1822010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marie-Egyptienne DT, Lohse I and Hill RP:
Cancer stem cells, the epithelial to mesenchymal transition (EMT)
and radioresistance: Potential role of hypoxia. Cancer Lett.
341:63–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conley SJ, Baker TL, Burnet JP, Thiesen
RL, Lazarus D, Peters CG, Clouthier SG, Eliasof S and Wicha MS:
CRLX101, an investigational camptothecin-containing
nanoparticle-drug conjugate, reverses the HIF-1α-mediated increase
in cancer stem cells caused by bevacizumab in a preclinical model
of triple-negative breast cancer. Cancer Res. 75:(Suppl 15).
13842015. View Article : Google Scholar
|
|
21
|
Kazi AA, Shah P, Schech A, Sabnis G,
Chumsri S and Brodie A: Inhibition of non-hypoxic HIF-1 expression
in letrozole-resistant breast cancer cells reduces their cancer
stem cell characteristics. Cancer Res. 73:(Suppl 8). 952013.
View Article : Google Scholar
|
|
22
|
Gammon L and Mackenzie IC: Roles of
hypoxia, stem cells and epithelial-mesenchymal transition in the
spread and treatment resistance of head and neck cancer. J Oral
Pathol Med. 45:77–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ricker JL, Chen Z, Yang XP, Pribluda VS,
Swartz GM and Van Waes C: 2-methoxyestradiol inhibits
hypoxia-inducible factor 1α, tumor growth, and angiogenesis and
augments paclitaxel efficacy in head and neck squamous cell
carcinoma. Clin Cancer Res. 10:8665–8673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YJ, Wu SL, Lu SM, Chen F, Guo Y, Gan
SM, Shi YL, Liu S and Li SL: (−)-Epigallocatechin-3-gallate
inhibits nasopharyngeal cancer stem cell self-renewal and migration
and reverses the epithelial-mesenchymal transition via NF-κB p65
inactivation. Tumour Biol. 36:2747–2761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan X, Liu S, Su F, Pan Q and Lin T:
Effective enrichment of prostate cancer stem cells from spheres in
a suspension culture system. Urol Oncol. 30:314–318. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen SF, Chang YC, Nieh S, Liu CL, Yang CY
and Lin YS: Nonadhesive culture system as a model of rapid sphere
formation with cancer stem cell properties. PLoS One. 7:e318642012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang SH, Cho HT, Devi S, Zhang Z, Escuin
D, Liang Z, Mao H, Brat DJ, Olson JJ, Simons JW, et al: Antitumor
effect of 2-methoxyestradiol in a rat orthotopic brain tumor model.
Cancer Res. 66:11991–11997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chua YS, Chua YL and Hagen T: Structure
activity analysis of 2-methoxyestradiol analogues reveals targeting
of microtubules as the major mechanism of antiproliferative and
proapoptotic activity. Mol Cancer Ther. 9:224–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maier HJ, Schmidt-Strassburger U, Huber
MA, Wiedemann EM, Beug H and Wirth T: NF-κB promotes
epithelial-mesenchymal transition, migration and invasion of
pancreatic carcinoma cells. Cancer Lett. 295:214–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu M, Sakamaki T, Casimiro MC, Willmarth
NE, Quong AA, Ju X, Ojeifo J, Jiao X, Yeow WS, Katiyar S, et al:
The canonical NF-κB pathway governs mammary tumorigenesis in
transgenic mice and tumor stem cell expansion. Cancer Res.
70:10464–10473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Y, Bai L, Chen W and Xu S: The NF-κB
activation pathways, emerging molecular targets for cancer
prevention and therapy. Expert Opin Ther Targets. 14:45–55. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ray G, Dhar G, Van Veldhuizen PJ, Banerjee
S, Saxena NK, Sengupta K and Banerjee SK: Modulation of cell-cycle
regulatory signaling network by 2-methoxyestradiol in prostate
cancer cells is mediated through multiple signal transduction
pathways. Biochemistry. 45:3703–3713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siebert AE, Sanchez AL, Dinda S and
Moudgil VK: Effects of estrogen metabolite 2-methoxyestradiol on
tumor suppressor protein p53 and proliferation of breast cancer
cells. Syst Biol Reprod Med. 57:279–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pasquier E, André N and Braguer D:
Targeting microtubules to inhibit angiogenesis and disrupt tumour
vasculature: Implications for cancer treatment. Curr Cancer Drug
Targets. 7:566–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: Role of reoxygenation, free radicals, and stress granules.
Cancer Cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Walmsley SR, Print C, Farahi N,
Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM,
Cowburn AS, Johnson N, et al: Hypoxia-induced neutrophil survival
is mediated by HIF-1α-dependent NF-κB activity. J Exp Med.
201:105–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taylor CT: Interdependent roles for
hypoxia inducible factor and nuclear factor-κB in hypoxic
inflammation. J Physiol. 586:4055–4059. 2008. View Article : Google Scholar : PubMed/NCBI
|